Content uploaded by Stephen G Brickley
Author content
All content in this area was uploaded by Stephen G Brickley on Jun 07, 2017
Content may be subject to copyright.
Available via license: CC BY-NC-SA 4.0
Content may be subject to copyright.
Neurobiology of Disease
GABA Transporter Deficiency Causes Tremor, Ataxia,
Nervousness, and Increased GABA-Induced Tonic
Conductance in Cerebellum
Chi-Sung Chiu,
1,6
Stephen Brickley,
2
Kimmo Jensen,
3,4
Amber Southwell,
1
Sheri Mckinney,
1
Stuart Cull-Candy,
5
Istvan Mody,
3
and Henry A. Lester
1
1
Division of Biology, California Institute of Technology, Pasadena, California 91125,
2
Biophysics Section, Imperial College London, London SW7 2AZ,
United Kingdom,
3
Departments of Neurology and Physiology, School of Medicine, University of California Los Angeles, Los Angeles, California 90095-1769,
4
Department of Physiology, University of Aarhus, DK-8000 Aarhus C, Denmark,
5
Department of Pharmacology, University College London, London WC1E
6BT, United Kingdom, and
6
Department of Neurobiology, Merck Research Laboratories, West Point, Pennsylvania 19486
GABA transporter subtype 1 (GAT1) knock-out (KO) mice display normal reproduction and life span but have reduced body weight
(female, ⫺10%; male, ⫺20%) and higher body temperature fluctuations in the 0.2–1.5/h frequency range. Mouse GAT1 (mGAT1) KO
mice exhibit motor disorders, including gait abnormality, constant 25–32 Hz tremor, which is aggravated by flunitrazepam, reduced
rotarod performance, and reduced locomotor activity in the home cage. Open-field tests show delayed exploratory activity, reduced
rearing, and reduced visits to the central area, with no change in the total distance traveled. The mGAT1 KO mice display no difference in
acoustic startle response but exhibit a deficiency in prepulse inhibition. These open-field and prepulse inhibition results suggest that the
mGAT1 KO mice display mild anxiety or nervousness. The compromised GABA uptake in mGAT1 KO mice results in an increased GABA
A
receptor-mediated tonic conductance in both cerebellar granule and Purkinje cells. The reduced rate of GABA clearance from the synaptic
cleft is probably responsible for the slower decay of spontaneous IPSCs in cerebellar granule cells. There is little or no compensatory
change in other proteins or structures related to GABA transmission in the mGAT1 KO mice, including GAT1-independent GABA uptake,
number of GABAergic interneurons, and GABA
A
-, vesicular GABA transporter-, GAD65-, and GAT3-immunoreactive structures in
cerebellum or hippocampus. Therefore, the excessive extracellular GABA present in mGAT1 KO mice results in behaviors that partially
phenocopy the clinical side effects of tiagabine, suggesting that these side effects are inherent to a therapeutic strategy that targets the
widely expressed GAT1 transporter system.
Key words: tiagabine; epilepsy; flunitrazepam; cerebellum; inhibition; tremor
Introduction
GABA is the principal inhibitory neurotransmitter in the mam-
malian brain, where it activates GABA
A
, GABA
B
, and GABA
C
receptors. GABA released from presynaptic terminals is removed
from the vicinity of the synaptic cleft by GABA transporters, and
this action is believed to be a key event in terminating synaptic
currents. GABA transporters are also involved in maintaining a
low extracellular GABA concentration throughout the brain, pre-
venting excessive tonic activation of synaptic and extrasynaptic
receptors. GABA transporters may also play a role in replenishing
the supply of presynaptic transmitter. Furthermore, GABA trans-
porters may reverse, under both normal and pathological cir-
cumstances, to release GABA (Richerson and Wu, 2003, 2004).
Of the three GABA transporters identified in the CNS, GABA
transporter subtype 1 (GAT1) is highly expressed in the olfactory
bulb, neocortex, cerebellum, superior colliculus, and substantia
nigra, where it is predominantly found in axons, presynaptic ter-
minals, and glial cells. GAT2 is weakly expressed throughout the
brain, primarily in arachnoid and ependymal cells. GAT3 expres-
sion is densest in the olfactory bulb, midbrain regions, and deep
cerebellar nuclei, where it is found predominantly on glial cells
(Radian et al., 1990; Ikegaki et al., 1994; Itouji et al., 1996; Yan et
al., 1997; Engel and Wu, 1998; Barakat and Bordey, 2002; Chiu et
al., 2002).
The GAT1 inhibitor tiagabine is a clinically useful antiepilep-
tic drug with few cognitive side effects (Aldenkamp et al., 2003),
but it also causes tremor (its major side effect), ataxia, dizziness,
asthenia, somnolence (sedation), and nonspecific nervousness
(Adkins and Noble, 1998; Pellock, 2001; Schachter, 2001). It is
important to know whether these side effects arise directly from
increased extracellular concentration of GABA in the CNS or,
instead, from actions on unintended targets. For instance, GAT1
Received Aug. 16, 2004; revised Jan. 25, 2005; accepted Jan. 25, 2005.
This research was supported by National Institutes of Health Grants DA-01921, NS-11756, MH-49176, NS-
030549, and DA-010509, National Science Foundation Grant 0119493, the Wellcome Trust, a Royal Society-Wolfson
Award (S.C.-C.), and a Della Martin Fellowship (C.-S.C.). We are indebted to members of Caltech and University of
California Los Angeles groups for advice, Limin Shi and Paul Patterson for use and help with the startle system, and
J. Crawley for comments on this manuscript.
Correspondence should be addressed to Henry A. Lester, Division of Biology, California Institute of Technology,
156-29, 1201 East California Boulevard, Pasadena, CA 91125. E-mail: lester@caltech.edu.
DOI:10.1523/JNEUROSCI.3364-04.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/253234-12$15.00/0
3234 •The Journal of Neuroscience, March 23, 2005 •25(12):3234 –3245
inhibitors may also inhibit GABA
A
receptors (Overstreet et al.,
2000; Jensen et al., 2003). If the latter mechanism holds, then a
more selective GAT1 inhibitor could be a more effective
antiepileptic.
To address this question, we examined the phenotype of the
ultimate GAT1-specific inhibitor: genetic interruption of GAT1
function. The homozygous and heterozygous mouse GAT1
(mGAT1) knock-out (KO) strain is viable and fertile, with a nor-
mal life span. Its hippocampal electrophysiology has been studied
previously (Jensen et al., 2003), but this is the first report of
several other phenotypes, including motor behavior, general
mood, cerebellar electrophysiology, and thermoregulation. We
emphasize measurements on the cerebellum, where GAT1 is
heavily expressed and has been quantified (Chiu et al., 2002).
GABA influences circadian rhythm (Liu and Reppert, 2000).
Because tiagabine-treated patients show dizziness, asthenia, and
somnolence, we determined whether the GAT1 KO mice display
altered activity in their habituated home cage. We also monitored
body temperature rhythm, which is synchronized with daily ac-
tivity (Weinert and Waterhouse, 1999). We found that the
mGAT1 KO mouse does phenocopy some effects of tiagabine,
which, in turn, suggests that the various clinical side effects of this
drug result, directly or indirectly, from its blockade of GAT1. We
measure altered synaptic physiology, deriving from increased
and prolonged extracellular [GABA], which provides a plausible
physiological basis for these effects.
Materials and Methods
GAT1 knock-out strain. The mGAT1 KO strain, previously termed
“intron-14-neo-mGAT1,” carries an intact neomycin selection marker
in intron 14. The details of the targeting construct, homologous recom-
bination, and genotyping were described previously (Chiu et al., 2002).
Synaptosomal GABA uptake assay. Details of synaptosomal prepara-
tion and GABA uptake assay were described previously (Chiu et al.,
2002). Briefly, mice were anesthetized with halothane (2-bromo-2-
chloro-1,1,1-trifluorothane), and brains were dissected and collected on
ice. The cerebellum (⬃50 mg) was homogenized in 20⫻(w/v) medium I
(0.32 Msucrose, 0.1 mMEDTA, and 5 mMHEPES, pH 7.5; 1 ml) (Nagy
and Delgado-Escueta, 1984). The P2 fraction (synaptosome fraction) was
suspended with 1 ml of medium I. Protein concentrations were analyzed
by using the Coomassie Plus kit (Pierce, Rockford, IL).
GABA uptake assays were performed by mixing 20
l of the suspen-
sion with 280
l of uptake buffer (in mM: 128 NaCl, 2.4 KCl, 3.2 CaCl
2
,
1.2 MgSO
4
, 1.2 KH
2
PO
4
, 10 glucose, 25 HEPES, pH 7.5) and then incu-
bated at 37°C for 10 min (Lu et al., 1998). GABA and [
3
H]GABA
in various concentrations (100
l) were added to the synaptosome sus-
pension and incubated for 10 min (final radioactive concentrations were
2.2– 8.8
Ci/ml). Uptake was terminated by placing the samples
in an ice-cold bath, followed by two washes with uptake buffer contain-
ing the same concentration of cold GABA at 10,000 ⫻g. The GABA
uptake inhibitor 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-
tetrahydro-3-pyridinecarboxylic acid hydrochloride (NO711) (final
concentration, 30
M) was included to measure the non-GAT1 uptake
activity; the NO711-sensitive fraction accounted for 75– 85% of wild-
type (WT) activity.
Tremor measurements. The mouse was placed ina2Lpolyethylene
freezer container. A piezoelectric transducer (LDT0 – 028K; Measure-
ment Specialties, Fairfield, NJ) was taped to the bottom of a 7.5 ⫻10 cm
plastic board (8 g), and this board was loosely attached to the bottom of
the container with a loop of paper tape. The mice were placed directly on
the board. The signal from the sensor was low-pass filtered at 200 Hz,
amplified by 100 (model 902; Frequency Devices, Haverhill, MA), and
led to the analog-to-digital inputs on an Axon DigiData 1200 interface
(Axon Instruments, Union City, CA). The signals were collected using
Clampex Gap-Free recording, and power spectra were computed in
ClampFit. We verified that the resonant frequency of this instrument was
far from the tremor frequency by replacing the mouse with 20 g of mass,
and the response of the instrument to constant-frequency mechanical
stimulation varied, with frequency, by ⬍40% between 20 and 32 Hz.
Benzodiazepine modulation of the tremor. Mice were tested for baseline
tremor as described previously. They were then injected intraperitoneally
with either flunitrazepam in 20% FreAmine HBC (B Braun Medical,
Bethlehem, PA) or vehicle. After 15 min, the tremor was measured.
Footprint. Hindpaws were painted with black India ink, and mice were
placed in a cardboard box (90 ⫻12 ⫻12 cm) with a 75-cm-long white
paper floor. Paw angle is the hindpaw central axis relative to its walking
direction.
Rotarod. Mice were tested on a motorized rotarod (Ugo Basile, Com-
erio, Italy) consisting of a grooved metal roller (3 cm in diameter) and
separated 11-cm-wide compartments elevated 16 cm. The acceleration
rate was set at 0.15 rpm/s. Mice were placed on the roller, and the time
they remained on it during rotation was measured. The rotarod has an
increment of 4 rpm/step. Tests were performed for fixed speed at either
12 or 20 rpm and for accelerating speed. A maximum of 120 s was allowed
per animal for fixed speed tests.
Exploratory locomotor activity. An individual mouse was placed in a
novel environment of a square open field (50 ⫻50 cm), the floor of
which was divided into 25 smaller squares (5 ⫻5) by painted lines.
Within 10 cm of the chamber walls is termed the periphery (16 squares),
and the central region indicates the central nine squares. The animal
behavior in the open field was recorded by videotaping for 10 min and
analyzed subsequently. The measurements include delayed exploratory
activity (measuring the time required for mice to walk the first 50 cm),
frequency of visits to the central area, dwell time in the inner field, num-
ber of rearing events, total distance traveled, and walking speed. Mice
usually made short walks interrupted by brief stops. To make meaningful
walking-speed measurements, we chose uninterrupted walking for ⬎25
cm and averaged 3–12 such walking-speed measurements for each ani-
mal. All animals were tested in a particular behavioral assay on the same
day during the light part of the light/dark cycle.
Elevated plus maze. Mice were allowed to habituate to the testing room
for 2 h. The maze consisted of two opposing open arms (40 ⫻10 cm) and
two opposing closed arms (40 ⫻10 cm, with 40 cm walls) on a platform
50 cm above the ground. Mice were placed in the center square (10 ⫻10
cm) facing an open arm and videotaped during a 5 min exploration. Arm
entries and duration were scored when all four paws entered the arm.
Partial arm entries were scored when one to three paws entered the
arm. Head dipping was scored when the head was dipped over the edge of
the maze. All animals were tested on the same day during the light part of
the light/dark cycle.
Home-cage activity. Mice were housed individually in cages with bed-
ding, food, and water. To assess activity, beam breaks were collected for
42 h with a photo beam system (San Diego Instruments, San Diego, CA).
Plots show the number of beam breaks for each 5 min interval.
Benzodiazepine hyperlocomotor activity. Mice were allowed to habitu-
ate to activity cages for 2 h. They were then injected intraperitoneally with
either flunitrazepam in 20% FreAmine HBC (5 or 15 mg/kg) or vehicle.
Activity (single beam breaks) and ambulation (successive beam breaks)
data were then collected for 1 h and plotted for each 5 min interval.
Acoustic startle and prepulse inhibition. Animals were tested in a Startle
Response system (SR-LAB; San Diego Instruments) consisting ofa5cm
Plexiglas cylinder mounted on a Plexiglas platform in a ventilated,
lighted, sound-attenuated chamber. Acoustic stimuli were presented by a
high-frequency loudspeaker mounted 28 cm above the cylinder. A piezo-
electric accelerometer attached to the Plexiglas base was used to detect
movement of the animals within the cylinder. Animal movement was
scored in arbitrary numbers between 0 and 1000. Ambient background
noise of 68 dB was maintained throughout each testing session. Each
session was initiated with a 5 min acclimation period followed by six 120
dB trials and concluded with another six 120 dB trials. These first and last
sets of six 120 dB pulses were not included in the analysis. For acoustic
startle-response (ASR) testing, seven different levels of acoustic startle
pulse (73, 78, 83, 85, 100, 110, and 120 dB) were presented along with a
trial containing only the background noise for 40 ms each in random
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3235
order with variable intertrial intervals of 10 –20
s. At the onset of stimulus, 65 startle-amplitude
readings were taken for 1 ms each. Ten trials of
each decibel level were performed, and the av-
erage startle amplitude was determined. The
session used for prepulse inhibition (PPI) test-
ing consisted of five different trials presented 10
times each in random order. These include 120
dB startle pulse alone, 120 dB startle pulse pre-
ceded by a prepulse of 73, 78, or 83 dB (5, 10,
and 15 dB above background), and a trial con-
taining only the background noise. The per-
centage of prepulse inhibition was calculated as
follows: 100 ⫻[(average 120 dB startle pulse ⫺
average prepulse ⫹120 dB startle pulse)/aver-
age 120 dB startle pulse].
Temperature measurements. Mini Mitter
(Sunriver, OR) ER-4000 telemetric tempera-
ture probes were used in 3- to 6-month-old
male mGAT1 KO mice. For implantation, mice
were anesthetized with halothane, anda1cm
incision was made at the back of the neck.
Probes were inserted subcutaneously into the
back. The incision was sealed with surgical glue.
The mice were housed with ad libitum water
and food at 24 ⫾2.5°C. Lights were on between
6:00 A.M. and 6:00 P.M. for 7–10 d after im-
plantations and then off for the period of data
collection. Temperature and activity data were
acquired using Vital View software (Mini-
Mitter) and analyzed (including fast Fourier
transforms) in Origin.
Seizure tests. Pentylenetetrazole (PTZ) was
solubilized in 0.9% NaCl saline solution, and
bicuculline was dissolved in 0.1N HCl, pH ad-
justed to 5.5 with 0.1N NaOH (Pericic and Bu-
jas, 1997). Animals were injected intraperitone-
ally with either PTZ or bicuculline. For PTZ,
animals were injected with either subthreshold
(40 mg/kg) or suprathreshold (70 mg/kg)
doses. For bicuculline, animals were injected
with 3, 4, or 5 mg/kg.
Brain slice electrophysiology. Cerebellar slices
were prepared using standard procedures
(Brickley et al., 1996). The brain was rapidly
dissected and submerged in cold slicing solu-
tion (⬃4°C), which contained the following (in
mM): 125 NaCl, 2.5 KCl, 1 CaCl
2
, 4 MgCl
2
,25
NaHCO
3
, 1.25 NaH
2
PO
4
, and 25 glucose. All
extracellular solutions were bubbled with 95%
O
2
and 5% CO
2
, pH 7.4. After cutting on a
moving-blade microtome, slices were main-
tained at 32°C for 60 min before transfer to a
recording chamber. For granule cell recordings,
slices were constantly perfused (1.5 ml/min)
with recording solution containing the follow-
ing (in mM): 125 NaCl, 2.5 KCl, 2 CaCl
2
,1
MgCl
2
, 26 NaHCO
3
, 1.25 NaH
2
PO
4
, and 25 glucose. For Purkinje cell
recordings, slices were perfused with the following (in mM): 126 NaCl, 2.5
KCl, 2 CaCl
2
, 2 MgCl
2
, 26 NaHCO
3
, 1.25 NaH
2
PO
4
, 10 glucose, 0.2
L-ascorbic acid, 1 pyruvic acid, and 3 kynurenic acid. All experiments
were performed at room temperature, and whole-cell voltage-clamp re-
cordings were made using Axopatch 1D or 200B amplifiers (Axon Instru-
ments). The pipette solution contained the following (in mM): 140 CsCl,
4 NaCl, 0.5 CaCl
2
, 10 HEPES, 5 EGTA, 2 Mg-ATP, adjusted to pH 7.3
with CsOH.
Currents were filtered at 2–3 kHz and digitized at 10 kHz. The tonic
GABA
A
receptor-mediated conductance (G
GABA
) was measured from the
reduction in holding current recorded in the presence of the GABA
A
recep-
torantagonist 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)-pyrida-
zinium bromide (SR95531) (⬎100
M). All-points histograms were con-
structed from sections of data not containing synaptic currents and mean
values calculated from a Gaussian fit to the histogram. Spontaneous IPSCs
(sIPSCs) were detected with amplitude- and kinetics-based criteria (events
were accepted when they exceeded a threshold of 6 – 8 pA for 0.5 ms) using
custom-written LabView-5.1-based software (National Instruments, Aus-
tin, TX). All IPSCs were also inspected visually, and sweeps were rejected or
accepted manually. Individual spontaneously occurring IPSCs were then
aligned on their initial rising phase, and average IPSC waveforms were con-
structed from those events that exhibited a clear monotonic rise and re-
turned to baseline before the occurrence of later sIPSCs. The decay of average
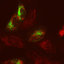
Figure 1. mGAT1 KO cerebellar images, synaptosomal GABA uptake, and body weight. A, Fluorescent image of an mGAT1-GFP
knock-in mouse cerebellar cortex, showing typical GAT1 expression pattern. B, Fluorescent image of GAT1 KO showing no obvious
GAT1 expression pattern. C, WT mouse shows no obvious fluorescence. Band Cwere exposed to ⬎20-fold greater photo power
thanA.GL,Granulecelllayer;ML,molecularlayer; P, Purkinje cell. Scale bar, 50
m.D,QuantificationofGAD65,vGAT,andGABA
A
receptor-containing boutons in WT and KO mice based on the immunocytochemical staining (see supplemental figure for the
actual images, available at www.jneurosci.org as supplemental material). E, The NO711-sensitive synaptosomal [
3
H]GABA up-
take activities among the three genotypes (mean ⫾SEM; triplicate assays from each of two experiments with all three geno-
types).E,DecreasedbodyweightoftheGAT1KOmouse.Data measured from 11 litters of het/het matings between the ages of 50
and 66 d are shown. Compared with WT littermates, male homozygotes weigh ⬃20% less, whereas female homozygotes weigh
⬃10% less. M, Male; F, female. WT, Het, KO: n⫽8, 17, 15 for males; n⫽11, 14, 8 for females. Differences from WT at *p⬍0.05
and **p⬍0.01.
3236 •J. Neurosci., March 23, 2005 •25(12):3234 –3245 Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice
sIPSC waveforms was quantified as a weighted
value calculated from the
charge transfer of normalized averages (
integral
).
Immunocytochemistry. Detailed procedures for immunocytochemis-
try were described previously (Chiu et al., 2002; Jensen et al., 2003). Mice
were anesthetized with halothane and perfused with 4% paraformalde-
hyde in PBS, pH adjusted to 7.6 with Na
2
HPO
4
. Brains were dissected
and kept in 4% paraformaldehyde for1hin4°Candthen incubated in
30% sucrose in PBS for ⬃20 h. The brains were embedded in OCT
medium (Tissue-Tek; Miles, Elkhart, IN) for either horizontal or sagittal
sections and sliced by cryostat at 35
m. Brain slices were stored in a
solution containing the following (in mM): 11 NaH
2
PO
4
,20Na
2
HPO
4
,
30% ethylene glycol, and 30% glycerol, pH 7.5, at ⫺20°C.
Sections were incubated for2hatroom temperature in a blocking
solution (10% normal goat serum and 0.3% Triton X-100 in PBS, pH
7.6), followed by incubation with the primary antibody for2dat4°C
with rotational mixing. Primary antibodies and their dilutions were rab-
bit anti-GAT3 (1:200 dilution; Chemicon, Temecula, CA), rabbit anti-
GABA
A
receptor
␣
1 (1:100; Upstate Biotechnology, Lake Placid, NY),
rabbit anti-glutamate decarboxylase 65 (GAD65) (1:1000; Chemicon),
and rabbit anti-vesicular GABA transporter (vGAT) (1:100; Synaptic
Systems, Goettingen, Germany). The brain slices were first washed with
PBS containing 0.5% Triton X-100 followed by two additional washes
with PBS. The slices were then incubated in solutions containing the
appropriate rhodamine red-x-conjugated secondary antibodies. These
secondary antibodies include goat anti-rabbit, goat anti-guinea pig, or
donkey anti-goat secondary antibodies (1:200; Jackson ImmunoRe-
search, West Grove, PA). After three washes with PBS, slices were rinsed
with PBS, mounted with Vectashield (Vector Laboratories, Burlingame,
CA), and subjected to confocal microscope imaging.
Results
Evidence for functional knock-out of GAT1
The knock-in mouse strain studied here, previously termed
intron-14-neo-intact-mGAT1, harbors a neomycin resistance
cassette (neo) in intron 14 as well as a green
fluorescent protein (GFP) moiety fused to
the C terminus of the mGAT1 coding re-
gion in exon 14 (Jensen et al., 2003). This
strain was originally constructed as a ge-
netic intermediate in the eventual con-
struction of a neo-deleted mGAT1-GFP
knock-in strain that has also been de-
scribed previously (Chiu et al., 2002).
However, we found that the present strain
appears to have essentially no functional
mGAT1 (Jensen et al., 2003), presumably
because the neo sequences interfere with
mRNA or protein. Figure 1 shows addi-
tional evidence on this point in the cere-
bellum, for which we later provide electro-
physiological data. First, the GFP moiety at
the C terminus of the GAT1 construct pro-
vides a fluorescent label for the level of
GAT1 expression (Chiu et al., 2002). The
mGAT1 KO strain shows ⬍2% as much
fluorescence as the mGAT1-GFP strain
(Chiu et al., 2002) and no more fluores-
cence than WT mice (Fig. 1A–C). Second,
to measure mGAT1 function, we per-
formed GABA uptake assays on cerebellar
synaptosomes. The NO711-sensitive
GABA uptake activity from mutant mice
synaptosomes was ⬍2% of that of WT lit-
termates, whereas heterozygotes displayed
intermediate GABA uptake activity (Fig.
1E), indicating that mutant mice have little
or no functional presynaptic GAT1 activity. mGAT1-deficient mice
also display reduced body weight, ⬃20 and ⬃10% less than WT for
males and females, respectively (Fig. 1F).
Cerebellar immunocytochemistry
To test whether the mGAT1 KO mouse has abnormalities in the
GABAergic system, we performed immunocytochemistry on sev-
eral proteins related to GABA function. Immunocytochemistry
using antibodies against GAD65, the GABA
A
␣
1 subunit and the
vGAT indicated that mGAT1 KO mice do not change GABAergic
synapse densities and related receptor expression in the molecu-
lar layer of the cerebellum (summarized in Fig. 1D) (based on
images in the supplemental figure, available at www.jneurosci.
org as supplemental material). We also found no qualitative dif-
ferences in GABA
A
␣
1 subunit staining in the granule cell layer
(data not shown). These data agree with previous data on the
hippocampus (Jensen et al., 2003). Also, the expression pattern
for GAT3 is not changed, suggesting that no compensatory
changes occurred because of the GAT1 deficit (see supplemental
figure, available at www.jneurosci.org as supplemental material).
Immunocytochemistry using antibodies against GABAergic,
interneuron-specific, calcium-binding proteins showed no
changes in GABAergic interneuron density in the hippocampus
and in the cerebellum.
Behavioral characterizations of GAT1 KO mice
Tremor
The mGAT1 KO mice display readily observable, nearly contin-
uous tremor in the limbs and tail. Measured by a simple instru-
ment (Fig. 2A) (see Materials and Methods), the tremor fre-
Figure 2. Characterization of mGAT1 KO tremor. A, Recordings from the vibration transducer. Arrows (higher amplitude)
indicate activities when forepaws were raised. B, Power spectrum of the transducer signal for all genotypes. All genotypes shared
a minor peak at ⬃80 Hz; however, only the KO showed a significant tremor at 25–32 Hz. C, Modulation of tremor frequency and
amplitude by flunitrazepam. Error bars represent SEM.
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3237
quency is 25–32 Hz (Fig. 2B). In addition,
KO and WT share an additional lower am-
plitude tremor at ⬃80 Hz (Fig. 2B)(n⫽
6). Vibrations in both frequency ranges are
highest during rearing episodes (Fig. 2A,
arrows). Acute high-dose NO711 treat-
ment caused complete sedation in WT
mice, vitiating any observations on tremor
in NO711-treated WT mice.
Flunitrazepam treatment decreased the
frequency and increased the amplitude of
the tremor in mGAT1 KO mice (Fig. 2C) but
had very little effect on the power spectrum
of WT mice (data not shown). These effects
in KO mice were both significant for 15
mg/kg flunitrazepam; both effects were in-
termediate for 10 mg/kg flunitrazepam, but
only the frequency change was significant for
10 mg/kg flunitrazepam.
Ataxia
Ataxia is associated with cerebellar defects
in many strains of mice (Mullen et al.,
1976; Watanabe et al., 1998; Rico et al.,
2002). The mGAT1 KO mice walk with an
abnormally large paw angle relative to the
direction of walking: 23 ⫾0.7 versus
12.5 ⫾1.4° for KO and WT, respectively
(Fig. 3E,F). In another indication of
ataxia, mGAT1 KO mice display flattened
stance and lowered hip on the rotarod
(Fig. 3B). The mGAT1 KO mice show re-
duced time on the rotarod in both fixed
speed (Fig. 3C) and accelerating speed
(Fig. 3D) tests, indicating ataxia. Both WT
and mutant mice improved performance
on the rotarod after training; however, the
difference of latency to fall remained sig-
nificant between WT and KO mice (data
not shown). The mGAT1 KO and WT
mice displayed equal muscle strength in
hanging-wire activity tests (data not
shown).
Mild anxiety: flexor contraction,
exploratory activity, elevated plus maze,
and startle
When suspended by the tail, the mGAT1-
deficient mice display trembling and
flexor contraction (front paws held to-
gether and rear paws flexed) (Fig. 3A).
This gesture resembles typical mouse
models for anxiety. WT littermates dis-
played normal extension without trem-
bling (Fig. 3A). The flexor contraction was
also observed in WT mice treated with a high dose of NO711
(10 – 40 mg/kg; data not shown).
The open field was used as an additional test of anxiety-like
behavior (Fig. 4) (Prut and Belzung, 2003). Because an open field
is a novel environment, rodents tend to prefer the periphery of
the apparatus, later exploring the central parts of the open field.
We observed several aspects of behavior in this apparatus. The
mGAT1 KO mice tend to remain longer in the corner of the open
field (Fig. 4A) and then tend to walk slowly along the wall; thus,
there was markedly reduced frequency of visits to the central area
(Fig. 4B), reduced dwell time in the central area (Fig. 4C), and
reduced rearing activity (Fig. 4D). These results may signify anx-
iety of the mGAT1 KO mice. There was modestly reduced walk-
ing speed (Fig. 4E). Although WT and heterozygotes walk faster
than KO mice, they spend more time in rearing; as a consequence,
all three genotypes traveled about the same distance (Fig. 4F).
Several of the observations in the open-field test suggest that the
Figure 3. mGAT1 KO displays abnormal motor behavior. A, WT (left) and mGAT1 KO (right) mice showed different gestures
whenhungbytheirtails.WT mice showed a typical extensor gesture, whereas KO mice showed flexor contraction. B,StanceofWT
and KO mice on the rotarod. The KO mice show flattened and lowered hips, and their paws move more slowly than WT mice. C,
Mice were tested at fixed speed (either 12 or 20 rpm) on the rotarod. n⫽6, 5, and 8 (WT, Het, and KO, respectively). D, Mice were
tested at accelerating speeds. KO mice fell significantly sooner than WT mice. E, Abnormal gait. Hindpaw footprint pattern of WT,
heterozygotes, and homozygotes is shown. The hindpaws of mGAT1 KO mice show a wider angle with respect to the direction of
walking. The KO mouse seems to waddle. F, Comparison of the average paw angles among WT (n⫽8), Het (n⫽9), and KO (n⫽
17) mice. The paw angle of the KO mouse is approximately twice as large as that of WT and heterozygotes (23 ⫾1 vs 12.5 ⫾1°).
Differences from WT at *p⬍0.05 and **p⬍0.01.
3238 •J. Neurosci., March 23, 2005 •25(12):3234 –3245 Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice
heterozygote is the least anxious phenotype; we did not explore
this observation systemically.
We observed the mGAT1 KO mice in the elevated plus maze,
another test of anxiety (Fig. 5A,B). The mGAT1 KO mice dis-
played increased partial arm entries (Fig. 5A) and time spent in
the central square (Fig. 5B) compared with WT mice. Homozy-
gous mutant mice showed reduced open-arm entries and re-
duced total time spent in the open arms (data not shown). There
was a trend toward reduced closed-arm entries; however, this
difference was not statistically significant compared with WT.
Mutant mice spent the majority of the testing time in the central
square engaging in partial-arm entries, indicating no reduction in
locomotor activity. No difference was seen in head dipping. Thus,
the elevated plus maze provided some additional evidence for
anxiety.
Startle is a fast twitch of facial and body muscles evoked by
sudden and intense tactile, visual, or acoustic stimulations. Many
anxious mouse strains display both enhanced ASR and reduced
PPI. The mGAT1-deficient mice display normal ASR (Fig. 5C)
but reduced PPI (Fig. 5D), compared with their WT littermates.
The baseline movement of the mGAT1 mutant in the absence of
acoustic stimulation was elevated above the WT. This most likely
reflects the constant tremor of these mice.
Ambulation activity
The mGAT1 KO mice show reduced ambulation in their cages; as
a consequence, the 24 h activity cycle becomes less obvious (Fig.
6A). The total ambulation activities were 2425 ⫾395 versus
965 ⫾146 times during 42 h for WT and mGAT1 KO mice,
respectively (Fig. 6B) (mean ⫾SEM). Flunitrazepam treatment
caused hyperlocomotor activity in KO an-
imals and sedation in WT animals (data
not shown).
Autonomic regulation: body
temperature fluctuations
The mGAT1 KO mice display a striking
pattern of abnormal temperature regula-
tion (Fig. 7A,B). There is a normal circa-
dian temperature rhythm, but in addition,
there are many fluctuations, primarily hy-
perthermic episodes on a time scale of sev-
eral minutes to ⬃2 h. To quantify these
fluctuations, we computed and averaged
the power spectral density of temperature
fluctuations in WT or KO mice (Fig. 7C).
The data have been normalized to the peak
at 0.0416 h
⫺1
(corresponding to the circa-
dian rhythm). It is clear that mGAT1 KO
mice display increased relative noise
power in the frequency range from 0.2 to
1.5 h
⫺1
. The mGAT1 hyperthermic epi-
sodes are larger, especially during high ac-
tivity (i.e., higher body temperature), but
no more frequent than in WT mice (Fig.
7B). Two additional animals in each group
provided similar data, but these animals
were not included in the averaged power
spectra because of differences in sample
rate.
Sensitivity to convulsants
The mGAT1 KO mouse is slightly more
sensitive than the WT mouse to PTZ-induced seizures, but there
is no obvious change in bicuculline-induced seizure susceptibil-
ity. Bicuculline (i.p.) at 5 mg/kg kills WT and mGAT1 KO mice,
whereas at 3 and 4 mg/kg, both WT and KO mice survived with
moderate seizure (n⫽2 each). PTZ at a subthreshold dose (40
mg/kg, i.p.) decreased observable activity in WT and heterozy-
gotes while causing preconvulsive states and mild seizures in
mGAT1 KO mice (n⫽3 each). At a suprathreshold dose (70
mg/kg), all WT and heterozygotes survived with severe seizures,
whereas mGAT1 KO mice showed severe seizures, and one of
three died (n⫽3 each).
Cerebellar slice electrophysiology
GABA
A
receptor-mediated currents, recorded from wild-type
mice, are similar to those reported previously (Brickley et al.,
2001) (Fig. 8). Granule cells dialyzed with high-internal Cl
⫺
and
voltage clamped at ⫺70 mV (see Materials and Methods) exhibit
sIPSCs, with a frequency of 0.8 ⫾0.6 Hz (n⫽4). In addition, a
tonic GABA
A
receptor-mediated conductance (G
GABA
) is clearly
present in all recordings. The phasic and tonic conductances are
both blocked by the GABA
A
receptor antagonist SR95531 (⬎100
M) (Fig. 8A). The magnitude of G
GABA
(84.2 ⫾50.4 pS/pF) is
similar to previous reports for animals of this age, as are the peak
amplitude (388.5 ⫾143.3 pS/pF) and kinetics (
integral
⫽17.6 ⫾
3.3 ms) of average sIPSCs (Brickley et al., 2001).
Recordings from mGAT1 KO cerebellar granule cells reveal
marked differences in both the tonic and phasic conductances,
consistent with the removal of a GABA transporter. In all seven
recordings from mGAT1 KO mice, G
GABA
is significantly in-
creased (Fig. 8B) to an average value of 318.9 ⫾65.6 pS/pF ( p⬍
Figure 4. Characterization of mGAT1 KO exploratory activity in the open field. A, The time required for mice to walk the first 50
cm in open field. Most WT and Het mice take ⬍10 s, whereas KO mice spend 13–240 s. Points show mean ⫾SEM. B,C, The KO
mouse shows reduced frequency (B) and reduced duration (C) visiting the central area in the open-field test. Total visits to the
central area were 15 ⫾2, 25 ⫾3, and 7 ⫾3, and total time to stay in the central area were 67 ⫾11, 106 ⫾23, and 21 ⫾7s
for WT, Het, and KO, respectively. D, The GAT1 KO mouse showed reduced frequencies of rearing (73 ⫾2, 87 ⫾9, and 29 ⫾10
for WT, Het, and KO, respectively). E, The average walking speeds for WT, Het, and KO mice were 16.2 ⫾0.5, 19.4 ⫾0.6, and
12.3 ⫾0.6 cm/s, respectively. F, mGAT1 KO mice showed no obvious difference in total walking distance within 10 min (2000 ⫾
210, 2840 ⫾320, and 2190 ⫾300 for WT, Het, and KO, respectively). E,n⫽7, 12, and 10 (WT, Het, and KO, respectively). For all
other panels, n⫽6, 8, and 8 (WT, Het, and KO, respectively). *p⬍0.05; **p⬍0.01.
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3239
0.05) (Fig. 8C). Conventional sIPSCs (Fig. 8D,E) are still detect-
able within the current record, albeit at an apparently lower av-
erage frequency (0.4 ⫾0.2 Hz). The increased current variance
associated with G
GABA
(Fig. 8E, histogram) made resolution of
small sIPSCs more difficult. Nevertheless, it appears that the av-
erage peak amplitude is not significantly different in the mGAT1
KO recordings (270.5 ⫾31.5 pS/pF). However, as shown in Fig-
ure 8F, the decay of sIPSCs is slower in the mGAT1 KO cells
(
integral
⫽36.9 ⫾5.7 ms compared with 17.6 ⫾3.3 ms in wild-
type granule cells). Therefore, in mature cerebellar granule cells,
we observed an ⬃300% increase in the magnitude of G
GABA
and
a 100% increase in the decay time of sIPSCs after the removal of
GAT1.
In Purkinje cell recordings, a standing inward GABA
A
receptor-mediated conductance, defined by sensitivity to the
GABA
A
receptor antagonist SR95531 (⬎100
M), was observed
4
Figure 5. Additional anxiety-related behaviors: elevated plus maze and acoustic startle.
mGAT1KOmicedisplay increased partial arm entries(A)andtime spent in the central square(B)
compared with WT mice. *p⬍0.01; n⫽4 mice in each group. C, Acoustic startle response of
mutant (open circle; n⫽4) and WT (filled square; n⫽4) measured in arbitrary units. D,
Prepulse inhibition of mutant (open column; n⫽7) and WT (filled column; n⫽7). The 5, 10,
and 15 under the x-axis refer to prepulses at 5, 10, and 15 dB, respectively, above the back-
ground level of 68 dB. The difference between KO and WT is significant at *p⬍0.05 and **p⬍
0.01. Error bars represent SEM.
Figure 6. GAT1 KO mice showed reduced ambulation in home cages. A, Profiles of ambula-
tion activity of WT (top) and KO (bottom) mice over a 42 h recording period. WT displays a 24 h
rhythm, whereas KO shows lower activity. B, Total ambulation activity of KO and WT mice
(2425 ⫾395 and 965 ⫾146 counts). Error bars represent SEM.
3240 •J. Neurosci., March 23, 2005 •25(12):3234 –3245 Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice
in mGAT1 KO mice, which is much larger than in WT mice (75 ⫾
19 pS/pF, n⫽10 vs 13 ⫾5 pS/pF, n⫽9, respectively) (Fig. 9).
However, the high frequency of sIPSCs consistently observed in
Purkinje cells (⬎10 Hz) indicates that a comparison of sIPSC
kinetics between WT and mGAT KO mice is not possible in this
cell type because of the considerable superimposition of events.
Moreover, it is not feasible to selectively analyze the tonic and phasic
components of the GABA
A
-mediated conductance in a similar man-
ner to the granule cell recordings. Nonetheless, as shown in Figure 9,
it is clear that in Purkinje cell recordings, the magnitude of a standing
inward GABA
A
receptor-mediated conductance is significantly in-
creased in mGAT1 KO mice.
Discussion
The mGAT1 KO mouse as a model for tiagabine side effects
The distinct phenotype of mGAT1 KO mice includes ataxia,
tremor, sedation, nervousness (mild anxiety), increased fre-
quency and amplitude of body temperature fluctuations, and
reduced body weight. Similar behavioral patterns were also ob-
served in WT mice treated with either tiagabine or NO711, both
GAT1 inhibitors (Nielsen et al., 1991; Suzdak et al., 1992; Suzdak,
1994). Epileptic patients treated with Tiagabine display similar
side effects, including dizziness, asthenia, somnolence (sedation),
nonspecific nervousness, tremor, and ataxia (Adkins and Noble,
1998). The fact that the mGAT1 KO mice phenocopy many ef-
fects of both mice and humans treated with GAT1 inhibitors
suggests that the clinical side effects might be expected from any
systemically administered drug that tar-
gets GAT1, no matter how selective.
Synaptic basis of the tremor
GAT1 inhibition causes elevated extracel-
lular [GABA] and therefore generates an
increased tonic GABA
A
-mediated con-
ductance, perhaps primarily by acting at
areas that typically express high-affinity,
nondesensitizing GABA
A
receptors
(Brickley et al., 1996; Wall and Usowicz,
1997; Hamann et al., 2002; Jensen et al.,
2003). Our data for cerebellar granule (Fig.
8) and Purkinje (Fig. 9) cells support these
ideas. Previous studies also report a pro-
longation of the evoked GABA
A
receptor-
mediated synaptic decay after block of
GABA transporters (Dingledine and Korn,
1985; Roepstorff and Lambert, 1992, 1994;
Thompson and Gahwiler, 1992; Draguhn
and Heinemann, 1996; Rossi and Ha-
mann, 1998; Overstreet et al., 2000). This
phenomenon is not observed after action
potential-independent release (Thomp-
son and Gahwiler, 1992; Isaacson et al.,
1993), suggesting that GAT1 transporters
are likely to be more important in limiting
the GABA profile after multivesicular re-
lease. However, the use of GABA transport
blockers in previous assays may be compli-
cated by the fact that GAT1 inhibitors are
also competitive antagonists of GABA
A
re-
ceptors (Overstreet et al., 2000; Jensen et
al., 2003).
The cerebellar glomerulus, like the bas-
ket cell–Purkinje cell pinceau synapse and
the chandelier cell–pyramidal cell car-
tridge of cortex, is a highly organized synaptic structure that con-
tains many synaptic contacts produced by just a few presynaptic
inhibitory axons (Jakab and Hamori, 1988) and features a dense
level of GAT1 expression (Chiu et al., 2002). The dramatically
prolonged granule cell IPSC waveforms in mGAT1 KO mice are
certainly consistent with the idea that GAT1 plays a more impor-
tant role in clearing GABA after multivesicular release in struc-
tures such as the glomerulus, where diffusion is limited (Nielsen
et al., 2004). This may explain the greater prolongation of sIPSCs
we observe in mGAT1 KO granule cells (Fig. 8) than previously
observed in hippocampus (Jensen et al., 2003). The unchanged
level of GAD65 (Fig. 1 D), vGAT (Fig. 1D), the GABA receptor
␣
1
subunit (Fig. 1D), GABA
B
receptors (Jensen et al., 2003), and
GAT3 (see supplemental figure, available at www.jneurosci.org
as supplemental material) in the mGAT1 KO mice argues against
some classes of compensatory changes in response to the chron-
ically elevated [GABA]. Furthermore, the NO711-insensitive cer-
ebellar synaptosomal GABA uptake was only 15–25% of the total
activity in WT, and the absolute value of NO711-insensitive
GABA uptake activity showed no difference between WT and
mGAT1 KO. However, we cannot rule out other changes such as
altered subunit composition of GABA
A
receptors or an altered
waveform of synaptically released [GABA]. Whatever the under-
lying synaptic mechanisms, the distorted inhibitory waveform
observed in granule cells suggests that inhibition in one or more
motor control nuclei provides a reasonable, although not quan-
Figure 7. mGAT1-deficient mice display more body temperature fluctuations in the 0.2–1.5/h frequency range than WT mice.
A, Raw traces of body temperature fluctuation from one WT (top) and one mutant (bottom) mouse. Mutant mice display multiple
hyperthermic episodes, especially during periods of higher activity (i.e., higher body temperature). B, Expanded traces from A.C,
Powerspectrumanalysis.They-axisrepresentstheaveragepower (n⫽4 KO, 3 WT) normalized to the peak at 24 h cycle as 100%.
The x-axis represents the frequency (inverse hours).
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3241
titative, explanation for the tremor that we
observed in the mGAT1 KO mouse. An
oscillation between excitation and inhibi-
tion underlies many neuronal pacemak-
ers, and in mGAT KO mice, this oscillation
is apparently timed in part by the accentu-
ated inhibitory phase that results from in-
creased and prolonged [GABA]. Fluni-
trazepam, an allosteric activator of GABA
A
receptors, increased the period and in-
creased the amplitude of the tremor (Fig.
2C), consistent with the idea that one
phase of the oscillation is governed by the
waveform of GABA
A
-mediated inhibition.
Which inhibitory synapse(s) domi-
nates the tremor? We do not imply that the
oscillation is solely determined by the tim-
ing of a cerebellar inhibitory synapse such
as the Golgi cell– granule cell contact.
The removal of GAT1 presumably alters
characteristics of GABA-mediated trans-
mission in many nuclei. GABA
A
receptor
␣
1 subunit knock-out mice tremble at
⬃18 Hz (Kralic et al., 2002), suggesting
that a tremor can arise from either too
little or too much GABAergic transmis-
sion throughout the brain. However, the
tremor in mGAT1 KO mice is inconsis-
tent with the low-frequency tremors
generally associated with basal ganglia
and midbrain pathology. The tremor
also has a higher frequency (25–32 Hz)
(Fig. 2) than most previously reported
mouse tremors but equal to that of mice
expressing the hypofunctional glycine
receptor (GlyR) oscillator
␣
1 subunit
(Simon, 1997) or a human hyper-
ekplexia-related GlyR mutant (Becker et
al., 2002). Glycine transporter 2 knock-
out mice also display 15–25 Hz tremor
(Gomeza et al., 2003). These observa-
tions on the glycinergic system suggest
that the tremor is primarily spinal in
origin.
Ataxia
The ataxia exhibited by mGAT1-deficient mice (i.e., rotarod def-
icits) (Fig. 3C,D; broader paw angle in E,F) is more likely to
originate from a specific cerebellar defect, because ablation of
GABAergic neurons in the cerebellum also causes ataxia in sev-
eral classic mouse mutants. Overall, these results illustrate that
normal motor control depends on maintaining appropriate lev-
els of both phasic and tonic GABA
A
receptor-mediated inhibition
in the cerebellum.
Nervousness versus anxiety
Nervousness describes the clinical side effects of tiagabine (Do-
drill et al., 1997, 1998, 2000; Adkins and Noble, 1998). In the
absence of an accepted test for nervousness in rodents, we as-
sumed that it can be assessed as a mild form of anxiety. The GAT1
KO mice show such a phenotype. In the open-field test, mGAT1-
deficient mice display delayed exploratory activity and decreased
frequency of visits to the central area (Fig. 4A–C). However, the
reduced rearing (Fig. 4D) could be caused by simply the de-
creased motor ability that leads to the lowered stance (Fig. 3B);
there was only moderately reduced walking speed (Fig. 4E) and
no reduction in total distance traveled (Fig. 4F). Furthermore,
mutant mice show no difference in acoustic startle response com-
pared with WT (Fig. 5C), but they display a dramatic decrease in
prepulse inhibition of the acoustic startle response (Fig. 5D). The
mGAT1 KO displays reduced home-cage activity, but the modest
decrement in open-field walking speed suggests that mutant mice
remain active when encountering novel environments, whereas
they display reduced activity in a habituated environment (Fig.
5A,B). In contrast, 5-HT transporter null mice exhibit a classical
pattern of increased anxiety-like behavior in the elevated plus
maze, in light– dark exploration and emergence tests, and in
open-field tests (Holmes et al., 2003).
It is also true that many classical anxiolytic drugs operate by
increasing the activity of GABA
A
receptors. Likewise, reduced
GABA also causes anxiety; for example, GAD65 knock-out mice
exhibit increased anxiety-like behavior in both the open-field and
elevated-zero maze assays (Kash et al., 1999).
Figure 8. mGAT1 KO cerebellar granule cells are characterized by an increased tonic GABA
A
-mediated conductance and pro-
longedIPSCs.A,B,Continuous current records from typical wild-type (A) andmGAT1KO(B) internal granule cells voltage clamped
at ⫺70 mV. The horizontal line indicates the 0 current level in each recording. There is an increased inward current in mGAT1 KO
cells and a substantial increase in the current variance associated with this conductance. This increased tonic conductance is
completely blocked by the GABA
A
receptor antagonist SR95531 (gabazine). C, The bar graph illustrates that, on average, G
GABA
in
GAT1 KO granule cells was 319 ⫾65 pS/pF (n⫽7) compared with 84 ⫾50 pS/pF (n⫽4) in control littermates. This resembles
the 98 ⫾20 pS/pF G
GABA
recorded previously in the C57BL/6 strain (Brickley et al., 2001). Therefore, the tonic conductance tripled
after the removal of GAT1, indicating a raised concentration of ambient GABA in the slice preparation. D,E, Two average sIPSC
waveforms recorded from a wild-type (D) and an mGAT1 KO (E) granule cell are shown on the same scale. The waveforms have
similar peak amplitudes but very different decays. The histograms also illustrate the peak amplitude distribution of all sIPSCs
recorded in these cells. The open histograms were constructed from periods of baseline noise. As shown by the increase in the
width of the baseline histogram for mGAT1 KO, the increased current variance associated with mGAT1 KO recordings does
complicateinterpretationofpeakamplitudemeasurements. It is possible that we are missing a significant fraction of small events
in the mGAT1 KO, because they would be unresolved in the noisy mGAT1 KO recordings. However, this possible artifact does not
affect the decay estimates, because the decay of sIPSCs is not correlated with peak amplitude in granule cells (data not shown). F,
The significant increase in the decay of sIPSC recorded from mGAT1 KO granule cells. The decay was defined as
integral
(see
Materials and Methods). The
integral
of control littermates was 13 ⫾5ms(n⫽4) compared with 37 ⫾6ms(n⫽5) in the
mGAT1 KO animals. In contrast, there was no significant difference between the average peak amplitudes recorded in the two
strains. Error bars represent SEM.
3242 •J. Neurosci., March 23, 2005 •25(12):3234 –3245 Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice
Reduced body weight
The reduced body weight of mGAT1 KO mice (Fig. 1 F) contrasts
with obesity of transgenic mice overexpressing mGAT1 under
nonspecific or pan-neuronal promoters (Ma et al., 2000). GABA-
related regulatory mechanism of feeding behavior in the ventro-
medial hypothalamus may be responsible for impaired responses
to glucoprivation in genetically obese rats (Tsujii and Bray, 1991).
Benzodiazepine-treated rats lose body weight, presumably via
activation of GABA
A
receptors (Blasi, 2000). Excess GABA in the
anterior piriform cortex region reduces feeding (Truong et al.,
2002). We believe that the reduced body weight and tremor is not
related to delayed (or retarded) development, because mGAT1
KO mice are reproductive at the same time as WT and display
muscle strength and balance similar to WT. Additional detailed
studies are required.
Thermoregulation and circadian rhythm
Thermoregulation is controlled by several brain regions, includ-
ing the horizontal limb of the diagonal band of Broca (HDB), the
basal forebrain, the preoptic area (POA), and the rostral part of
the raphe pallidus nucleus (rRPa). Many neurons in these areas
are GABAergic. In the HDB, muscimol reduces thermosensitivity
(Hays et al., 1999) and, in the rRPa, muscimol to rRPa blocks
fever and thermogenesis in brown adipose tissue induced by
intra-POA as well as by intracerebroventricular prostaglandin E2
applications (Nakamura et al., 2002).
We know of no clinical studies on temperature effects of tiaga-
bine. However, the higher amplitude of hyperthermic episodes in
the mGAT1 KO mouse (Fig. 7) clearly does not phenocopy the
acute hypothermic effects of tiagabine in rodents (Inglefield et al.,
1995). Interestingly, GABA
B
activation leads to hypothermia
(Schuler et al., 2001), but we found previously that the presynap-
tic GABA
B
response is diminished or lost in mGAT1 KO mice
(Jensen et al., 2003), which may explain the discrepancy.
Although GABA has been related to circadian rhythm in many
publications (Liu and Reppert, 2000; Wagner et al., 2001), the
mGAT1-deficient mice did not display obvious changes in circa-
dian rhythm during5doftesting either in constant dark or in a
12 h light/dark cycle environment. These results suggest that ex-
cess GABA does not affect circadian rhythm.
An additional use for knock-out mice strains
To the other useful information obtained from knock-out mouse
strains, we may add the decision regarding whether the clinical
side effects of a drug (in this case, tiagabine) arise from either
widespread expression of its target or nonselective actions on
other targets. Such information is particularly valuable when the
pleiotropic effects cannot readily be predicted from, but are cer-
tainly consistent with, the widespread and varied roles of the
target molecule. Of course, such a study is rather straightforward
when it is believed that the effects are mostly acute and subject to
straightforward neurological tests (as in the present case), rather
than delayed and primarily psychiatric (as for serotonin and per-
haps dopaminergic and noradrenergic transporters).
References
Adkins JC, Noble S (1998) Tiagabine. A review of its pharmacodynamic and
pharmacokinetic properties and therapeutic potential in the management
of epilepsy. Drugs 55:437–460.
Aldenkamp AP, De Krom M, Reijs R (2003) Newer antiepileptic drugs and
cognitive issues. Epilepsia 44 [Suppl 4]:21–29.
Barakat L, Bordey A (2002) GAT-1 and reversible GABA transport in Berg-
mann glia in slices. J Neurophysiol 88:1407–1419.
Becker L, von Wegerer J, Schenkel J, Zeilhofer HU, Swandulla D, Weiher H
(2002) Disease-specific human glycine receptor
␣
1 subunit causes hy-
perekplexia phenotype and impaired glycine- and GABA
A
-receptor trans-
mission in transgenic mice. J Neurosci 22:2505–2512.
Blasi C (2000) Influence of benzodiazepines on body weight and food intake
Figure 9. mGAT1 KO mice display higher tonic currents in cerebellar Purkinje cells. In both
WT and mGAT1 KO slices, sIPSCs were recorded in Purkinje cells by holding at ⫺70 mV. Zero
current levels are shown in the light trace. Injection of SR95531 into the bath (⬎100
M; heavy
trace) blocked tonic current and sIPSCs. Average tonic currents in mGAT1 KO cells are approxi-
mately six times larger than in WT cells [75 ⫾19 and 13 ⫾5 pS/pF for KO (n⫽10) and WT
(n⫽9), respectively]. Error bars represent SEM.
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3243
in obese and lean Zucker rats. Prog Neuropsychopharmacol Biol Psychi-
atry 24:561–577.
Brickley SG, Cull-Candy SG, Farrant M (1996) Development of a tonic
form of synaptic inhibition in rat cerebellar granule cells resulting from
persistent activation of GABA
A
receptors. J Physiol (Lond) 497:753–759.
Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M (2001) Adap-
tive regulation of neuronal excitability by a voltage-independent potas-
sium conductance. Nature 409:88–92.
Chiu CS, Jensen K, Sokolova I, Wang D, Li M, Deshpande P, Davidson N,
Mody I, Quick MW, Quake SR, Lester HA (2002) Number, density, and
surface/cytoplasmic distribution of GABA transporters at presynaptic
structures of knock-in mice carrying GABA transporter subtype 1-green
fluorescent protein fusions. J Neurosci 22:10251–10266.
Dingledine R, Korn SJ (1985)
␥
-Aminobutyric acid uptake and the termi-
nation of inhibitory synaptic potentials in the rat hippocampal slice.
J Physiol (Lond) 366:387–409.
Dodrill CB, Arnett JL, Sommerville KW, Shu V (1997) Cognitive and qual-
ity of life effects of differing dosages of tiagabine in epilepsy. Neurology
48:1025–1031.
Dodrill CB, Arnett JL, Shu V, Pixton GC, Lenz GT, Sommerville KW (1998)
Effects of tiagabine monotherapy on abilities, adjustment, and mood.
Epilepsia 39:33–42.
Dodrill CB, Arnett JL, Deaton R, Lenz GT, Sommerville KW (2000) Tiaga-
bine versus phenytoin and carbamazepine as add-on therapies: effects on
abilities, adjustment, and mood. Epilepsy Res 42:123–132.
Draguhn A, Heinemann U (1996) Different mechanisms regulate IPSC ki-
netics in early postnatal and juvenile hippocampal granule cells. J Neuro-
physiol 76:3983–3993.
Engel JE, Wu CF (1998) Genetic dissection of functional contributions of
specific potassium channel subunits in habituation of an escape circuit in
Drosophila. J Neurosci 18:2254–2267.
Gomeza J, Ohno K, Hulsmann S, Armsen W, Eulenburg V, Richter DW,
Laube B, Betz H (2003) Deletion of the mouse glycine transporter 2
results in a hyperekplexia phenotype and postnatal lethality. Neuron
40:797–806.
Hamann M, Rossi DJ, Attwell D (2002) Tonic and spillover inhibition of
granule cells control information flow through cerebellar cortex. Neuron
33:625–633.
Hays TC, Szymusiak R, McGinty D (1999) GABA
A
receptor modulation of
temperature sensitive neurons in the diagonal band of Broca in vitro.
Brain Res 845:215–223.
Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003) Mice lacking
the serotonin transporter exhibit 5-HT
1A
receptor-mediated abnormali-
ties in tests for anxiety-like behavior. Neuropsychopharmacology
28:2077–2088.
Ikegaki N, Saito N, Hashima M, Tanaka C (1994) Production of specific
antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and
their application to immunocytochemistry. Brain Res Mol Brain Res
26:47–54.
Inglefield JR, Perry JM, Schwartz RD (1995) Postischemic inhibition of
GABA reuptake by tiagabine slows neuronal death in the gerbil hip-
pocampus. Hippocampus 5:460–468.
Isaacson JS, Solis JM, Nicoll RA (1993) Local and diffuse synaptic actions of
GABA in the hippocampus. Neuron 10:165–175.
Itouji A, Sakai N, Tanaka C, Saito N (1996) Neuronal and glial localization
of two GABA transporters (GAT1 and GAT3) in the rat cerebellum. Brain
Res Mol Brain Res 37:309–316.
Jakab RL, Hamori J (1988) Quantitative morphology and synaptology of
cerebellar glomeruli in the rat. Anat Embryol (Berl) 179:81–88.
Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I (2003) GABA
transporter-1 (GAT1)-deficient mice: differential tonic activation of
GABA
A
versus GABA
B
receptors in the hippocampus. J Neurophysiol
90:2690–2701.
Kash SF, Tecott LH, Hodge C, Baekkeskov S (1999) Increased anxiety and
altered responses to anxiolytics in mice deficient in the 65-kDa isoform of
glutamic acid decarboxylase. Proc Natl Acad Sci USA 96:1698–1703.
Kralic JE, O’Buckley TK, Khisti RT, Hodge CW, Homanics GE, Morrow AL
(2002) Deletion of GABA
A
receptor (GABA
A
-R)
␣
1 subunit alters ben-
zodiazepine (BZD) site pharmacology, function and related behavior. Soc
Neurosci Abstr 28:39.12.
Liu C, Reppert SM (2000) GABA synchronizes clock cells within the supra-
chiasmatic circadian clock. Neuron 25:123–128.
Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC (1998)
Pharmacological characterization of nicotinic receptor-stimulated GABA
release from mouse brain synaptosomes. J Pharmacol Exp Ther
287:648–657.
Ma YH, Hu JH, Zhou XG, Zeng RW, Mei ZT, Fei J, Guo LH (2000) Trans-
genic mice overexpressing gamma-aminobutyric acid transporter sub-
type I develop obesity. Cell Res 10:303–310.
Mullen RJ, Eicher EM, Sidman RL (1976) Purkinje cell degeneration, a new
neurological mutation in the mouse. Proc Natl Acad Sci USA 73:208 –212.
Nagy A, Delgado-Escueta AV (1984) Rapid preparation of synaptosomes
from mammalian brain using nontoxic isoosmotic gradient material
(Percoll). J Neurochem 43:1114–1123.
Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M
(2002) The rostral raphe pallidus nucleus mediates pyrogenic transmis-
sion from the preoptic area. J Neurosci 22:4600–4610.
Nielsen EB, Suzdak PD, Andersen KE, Knutsen LJS, Sonnewald U, Braestrup
C (1991) Characterization of Tiagabine (NO-328), a new potent and
selective GABA uptake inhibitor. Eur J Pharmacol 196:257–266.
Nielsen TA, DiGregorio DA, Silver RA (2004) Modulation of glutamate
mobility reveals the mechanism underlying slow-rising AMPAR EPSCs
and the diffusion coefficient in the synaptic cleft. Neuron 42:757–771.
Overstreet LS, Jones MV, Westbrook GL (2000) Slow desensitization regu-
lates the availability of synaptic GABA
A
receptors. J Neurosci
20:7914–7921.
Pellock JM (2001) Tiagabine (gabitril) experience in children. Epilepsia 42
[Suppl 3]:49–51.
Pericic D, Bujas M (1997) Sex differences in the response to GABA antago-
nists depend on the route of drug administration. Exp Brain Res
115:187–190.
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects
of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33.
Radian R, Ottersen OP, Strorm-Mathisen J, Castel M, Kanner BI (1990)
Immunocytochemical localization of the GABA transporter in rat brain.
J Neurosci 10:1319–1330.
Richerson GB, Wu Y (2003) Dynamic equilibrium of neurotransmitter
transporters: not just for reuptake anymore. J Neurophysiol
90:1363–1374.
Richerson GB, Wu Y (2004) Role of the GABA transporter in epilepsy. Adv
Exp Med Biol 548:76–91.
Rico B, Xu B, Reichardt LF (2002) TrkB receptor signaling is required for
establishment of GABAergic synapses in the cerebellum. Nat Neurosci
5:225–233.
Roepstorff A, Lambert JD (1992) Comparison of the effect of the GABA
uptake blockers, tiagabine and nipecotic acid, on inhibitory synaptic effi-
cacy in hippocampal CA1 neurones. Neurosci Lett 146:131–134.
Roepstorff A, Lambert JDC (1994) Factors contributing to the decay of the
stimulus-evoked IPSC in rat hippocampal CA1 neurons. J Neurophysiol
72:2911–2926.
Rossi DJ, Hamann M (1998) Spillover-mediated transmission at inhibitory
synapses promoted by high affinity
␣
6 subunit GABA
A
receptors and
glomerular geometry. Neuron 20:783–795.
Schachter SC (2001) Pharmacology and clinical experience with tiagabine.
Expert Opin Pharmacother 2:179–187.
Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid
J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M,
Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, et al. (2001) Epi-
lepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic
GABA
B
responses in mice lacking GABA
B(1)
. Neuron 31:47–58.
Simon ES (1997) Phenotypic heterogeneity and disease course in three mu-
rine strains with mutations in genes encoding for alpha 1 and beta glycine
receptor subunits. Mov Disord 12:221–228.
Suzdak PD (1994) Lack of tolerance to the anticonvulsant effects of tiagab-
ine following chronic (21 day) treatment. Epilepsy Res 19:205–213.
Suzdak PD, Frederiksen K, Andersen KE, Sorensen PO, Knutsen LJ, Nielsen
EB (1992) NNC-711, a novel potent and selective gamma-aminobutyric
acid uptake inhibitor: pharmacological characterization. Eur J Pharmacol
224:189–198.
Thompson SM, Gahwiler BH (1992) Effects of the GABA uptake inhibitor
tiagabine on inhibitory synaptic potentials in rat hippocampal slice cul-
tures. J Neurophysiol 67:1698–1701.
3244 •J. Neurosci., March 23, 2005 •25(12):3234 –3245 Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice
Truong BG, Magrum LJ, Gietzen DW (2002) GABA(A) and GABA(B) re-
ceptors in the anterior piriform cortex modulate feeding in rats. Brain Res
924:1–9.
Tsujii S, Bray GA (1991) GABA-related feeding control in genetically obese
rats. Brain Res 540:48–54.
Wagner S, Sagiv N, Yarom Y (2001) GABA-induced current and circadian
regulation of chloride in neurones of the rat suprachiasmatic nucleus.
J Physiol (Lond) 537:853–869.
Wall MJ, Usowicz MM (1997) Development of action potential-dependent
and independent spontaneous GABA
A
receptor-mediated currents in
granule cells of postnatal rat cerebellum. Eur J Neurosci 9:533–548.
Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R,
Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Koba-
yashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S (1998) Ablation
of cerebellar Golgi cells disrupts synaptic integration involving GABA
inhibition and NMDA receptor activation in motor coordination. Cell
95:17–27.
Weinert D, Waterhouse J (1999) Daily activity and body temperature
rhythms do not change simultaneously with age in laboratory mice.
Physiol Behav 66:605–612.
Yan XX, Cariaga WA, Ribak CE (1997) Immunoreactivity for GABA plasma
membrane transporter, GAT-1, in the developing rat cerebral cortex:
transient presence in the somata of neocortical and hippocampal neu-
rons. Brain Res Dev Brain Res 99:1–19.
Chiu et al. •Behavior and Electrophysiology of GAT1 KO Mice J. Neurosci., March 23, 2005 •25(12):3234 –3245 • 3245




![mGAT1 KO cerebellar images, synaptosomal GABA uptake, and body weight. A, Fluorescent image of an mGAT1-GFP knock-in mouse cerebellar cortex, showing typical GAT1 expression pattern. B, Fluorescent image of GAT1 KO showing no obvious GAT1 expression pattern. C, WT mouse shows no obvious fluorescence. B and C were exposed to 20-fold greater photo power than A. GL, Granule cell layer; ML, molecular layer; P, Purkinje cell. Scale bar, 50 m. D, Quantification of GAD65, vGAT, and GABA A receptor-containing boutons in WT and KO mice based on the immunocytochemical staining (see supplemental figure for the actual images, available at www.jneurosci.org as supplemental material). E, The NO711-sensitive synaptosomal [ 3 H]GABA uptake activities among the three genotypes (mean SEM; triplicate assays from each of two experiments with all three genotypes). E, Decreased body weight of the GAT1 KO mouse. Data measured from 11 litters of het/het matings between the ages of 50 and 66 d are shown. Compared with WT littermates, male homozygotes weigh 20% less, whereas female homozygotes weigh 10% less. M, Male; F, female. WT, Het, KO: n 8, 17, 15 for males; n 11, 14, 8 for females. Differences from WT at *p 0.05 and **p 0.01.](https://www.researchgate.net/profile/Stephen-Brickley/publication/7946672/figure/fig1/AS:486540213264402@1493011634966/mGAT1-KO-cerebellar-images-synaptosomal-GABA-uptake-and-body-weight-A-Fluorescent_Q320.jpg)