Content uploaded by Matti Kiupel
Author content
All content in this area was uploaded by Matti Kiupel on Dec 31, 2013
Content may be subject to copyright.
http://vdi.sagepub.com/
Investigation
Journal of Veterinary Diagnostic
http://vdi.sagepub.com/content/18/2/147
The online version of this article can be found at:
DOI: 10.1177/104063870601800202
2006 18: 147J VET Diagn Invest
Hall
T. V. Baszler, M. Kiupel, E. S. Williams, deceased, B. V. Thomsen, T. Gidlewski, D. L. Montgomery, K. I. O'Rourke and S. M.
)Odocoileus Hemionus) and Mule Deer (Virginianus OdocoileusDomestic Sheep and Chronic Wasting Disease in North American White-Tailed Deer (
Comparison of Two Automated Immunohistochemical Procedures for the Diagnosis of Scrapie in
Published by:
http://www.sagepublications.com
On behalf of:
Official Publication of the American Association of Veterinary Laboratory Diagnosticians, Inc.
can be found at:Journal of Veterinary Diagnostic InvestigationAdditional services and information for
http://vdi.sagepub.com/cgi/alertsEmail Alerts:
http://vdi.sagepub.com/subscriptionsSubscriptions:
http://www.sagepub.com/journalsReprints.navReprints:
http://www.sagepub.com/journalsPermissions.navPermissions:
What is This?
- Mar 1, 2006Version of Record >>
by guest on May 29, 2012vdi.sagepub.comDownloaded from
Comparison of two automated immunohistochemical procedures for the
diagnosis of scrapie in domestic sheep and chronic wasting disease in
North American white-tailed deer (Odocoileus virginianus) and mule
deer (Odocoileus hemionus)
T. V. Baszler,
1
M. Kiupel, E. S. Williams, B. V. Thomsen, T. Gidlewski, D. L. Montgomery,
K. I. O’Rourke, S. M. Hall
Abstract. Two commercially available automated immunohistochemistry platforms, Ventana NexES and
DakoCytomation Autostainer Universal Staining System, were compared for diagnosing sheep scrapie and
cervid chronic wasting disease. Both automated platforms used the same antiprion protein monoclonal
primary antibodies, but different platform-specific linker and amplification reagents and procedures.
Duplicate sections of brainstem (at the level of the obex) and lymphoid tissue (retropharyngeal lymph node or
tonsil) from the same tissue block were immunostained for the comparison. Examination of 1,020 tissues from
796 sheep revealed 100%concordance of results between the Ventana NexES and DakoCytomation platforms
for diagnosing sheep scrapie from lymphoid tissue (103/103 positive; 405/405 negative) and brainstem (120/120
positive; 392/392 negative). Similarly, examination of 1,008 tissues from 504 white-tailed deer revealed 100%
concordance between the Ventana NexES and DakoCytomation platforms for diagnosing chronic wasting
disease from lymphoid tissue (104/104 positive; 400/400 negative) and brainstem (104/104 positive; 400/400
negative). Examination of 1,152 tissues from 482 mule deer revealed a concordance of 98.6%in lymphoid
tissue and 99.9%in brainstem between the Ventana NexES and DakoCytomation platforms for diagnosing
chronic wasting disease. The results indicate equivalence or near equivalence between the DakoCytomation
and Ventana NexES autostainer platforms for identification of the disease-associated prion protein (PrP
d
)-
positive and PrP
d
-negative brain and lymphoid tissues in sheep, white-tailed deer, and mule deer.
Key words: Scrapie; chronic wasting disease; prion; immunohistochemistry.
Introduction
Sheep scrapie and cervid chronic wasting disease
(CWD) are prion diseases of animals, transmissible
spongiform encephalopathies (TSEs), characterized
by spongiform lesions in the brain and the accumu-
lation of an abnormal isoform of host-encoded prion
protein (PrP).
9,20
The accurate diagnosis of TSEs
relies on a combination of clinical signs of disease,
microscopic examination of the brain, and demon-
stration of the insoluble, protease-resistant, disease-
associated form of host prion protein (PrP
d
). Dem-
onstrating the accumulation of PrP
d
in affected
animals has become routine for confirmatory di-
agnosis and surveillance of animal TSEs, mainly
because disease can exist in the absence of either
clinical signs of disease or spongiform lesions,
2,5
and
detecting PrP
d
in lymphoid tissues can provide
accurate antemortem diagnosis of scrapie and CWD
during preclinical disease.
2,10,13,19
Methods for PrP
d
detection include immunohistochemical (IHC) analy-
sis, immunoblotting, and enzyme-linked immunosor-
bent assay (ELISA).
1,3,7
In most diagnostic laboratory
settings, IHC analysis has advantages over immuno-
blotting or ELISA because: 1) sampling methods are
routine for histologic examination (formalin-fixed
tissue), 2) histologic lesions can be spatially correlated
with PrP
d
accumulation,
9,16
3) the distribution pattern
of PrP
d
can be identified (disease phenotype),
4,15,17
4) depending on specific procedures, IHC analysis
may be more sensitive than immunoblotting,
6
and
From the Washington Animal Disease Diagnostic Laboratory
and Department of Veterinary Microbiology and Pathology,
College of Veterinary Medicine, Washington State University,
Pullman, WA (Baszler); and the Department of Veterinary
Pathobiology and Diagnostic Investigation, College of Veterinary
Medicine, Michigan State University, East Lansing, MI (Kiupel);
Department of Veterinary Sciences, College of Agriculture,
University of Wyoming, Laramie, WY (Williams); Texas Veter-
inary Medical Diagnostic Laboratory, Amarillo, TX (Montgom-
ery); National Veterinary Services Laboratories, US Department
of Agriculture, Animal and Plant Health Inspection Service,
Ames, IA (Gidlewski, Hall, Thomsen); and Agricultural Research
Service, US Department of Agriculture, Animal Disease Research
Unit, Pullman, WA (O’Rourke). Current address (Montgomery):
Department of Veterinary Sciences, College of Agriculture,
University of Wyoming, Laramie, WY. E. S. Williams is deceased.
1
Corresponding Author: Tim Baszler, Washington Animal
Disease Diagnostic Laboratory, Washington State University, PO
Box 2037 College Station, Pullman, WA 99165-2037l; e-mail:
baszlert@vetmed.wsu.edu
J Vet Diagn Invest 18:147–155 (2006)
147
by guest on May 29, 2012vdi.sagepub.comDownloaded from
5) automated IHC equipment and procedures are
readily available, fast, and simple.
Several manual and automated immunohistochem-
ical methods have been successfully applied to detect
PrP
d
for the diagnosis of sheep scrapie and cervid
CWD. Most techniques include steps to differentiate
PrP
d
from normal prion protein in host cells (PrP
C
),
4
epitope unmasking (usually by use of heat-induced
antigen retrieval),
8,15
and use of antibodies identifying
conserved PrP epitopes.
10,11,14
Scrapie testing and
CWD IHC analysis carried out by the Veterinary
Services of the United States Department of Agricul-
ture (USDA), and Animal and Plant Health In-
spection Service (APHIS) or its contract laboratories
is largely performed by use of the Ventana NexES
a
autostainer with proprietary immunostaining re-
agents. Results of a recent survey by the American
Association of Veterinary Laboratory Diagnosticians
indicated that many diagnostic laboratories have
proprietary automated immunostainers other than
the Ventana NexES; the DakoCytomation Autostain-
er Universal Staining System
b
was the most common
of those.
12
Because different IHC reagents and
procedures can affect the outcome of immunoreac-
tivity, equivalency testing was carried out, using the
same previously validated anti-PrP monoclonal anti-
bodies, between the Ventana NexES platform and the
DakoCytomation Autostainer Universal Staining
System for diagnosing sheep scrapie and cervid
CWD. Testing of brain and lymphoid tissues from
1,300 sheep and white-tailed deer revealed near 100%
concordance between the 2 automated immunostain-
ing platforms. Equivalency between the platforms
was slightly less in 500 mule deer tested for CWD.
Materials and methods
Animal tissues
Scrapie. Sheep of the study originated from privately
owned flocks located in 30 states representing all major
geographic regions of the United States. The sheep were
identified for the National Scrapie Eradication Program
because they were index scrapie cases (based on presence of
clinical neurologic disease and pathologic changes),
positive flock depopulations, trace-backs from positive
flocks, or slaughter surveillance. Sheep age ranged from 1
to 9 years. Breed identifications included Suffolk,
Hampshire, cross-breed, white face, black face, and
mottled face. A total of 1,020 brain or lymphoid tissues
were obtained from 796 sheep (Table 1). Lymphoid tissues
collected and examined (508 samples) included either tonsil
or retropharyngeal lymph node. Brain samples collected
and examined for the purposes of the study (512 samples)
included the brainstem at the level of the obex (containing
the dorsal motor nucleus of the vagus nerve). The lymphoid
and brain sample sets contained good- and poor-quality
(autolyzed or frozen then thawed) tissue specimens.
Ventana NexES
a
immunostaining was performed at the
National Veterinary Services Laboratories in Ames, IA and
USDA, Animal Disease Research Unit in Pullman, WA.
DakoCytomation
b
immunostaining was performed at the
Washington Animal Disease Diagnostic Laboratory at
Washington State University.
Chronic wasting disease. Tissues for CWD testing were
obtained from captive and free-ranging cervids (white-
tailed deer, and mule deer). Samples from free-ranging
cervids were obtained during hunter-kill CWD surveillance
programs in Wisconsin, Michigan, Nebraska, Wyoming,
and Colorado. Samples from captive cervids were obtained
from privately owned cervid farms as part of CWD
surveillance programs. A total of 2,160 brain or lymphoid
tissues were obtained from 986 cervids. Lymphoid tissues
collected and examined (1,174 samples) included either
tonsil or retropharyngeal lymph node. Brain collected and
examined for the purposes of the study (986 samples)
included the brainstem at the level of the obex (containing
the dorsal motor nucleus of the vagus nerve). The lymphoid
and brain sample sets contained good- and poor-quality
(autolyzed or frozen then thawed) tissue specimens.
Ventana NexES
a
immunostaining was performed at the
National Veterinary Services Laboratories in Ames, IA and
the USDA Animal Disease Research Unit in Pullman, WA.
DakoCytomation
b
immunostaining for white-tailed deer
and mule deer was performed at the Department of
Veterinary Pathobiology and Diagnostic Investigation at
Michigan State University, and at the Department of
Veterinary Sciences, University of Wyoming respectively.
Immunohistochemical methods
DakoCytomation autostainer universal staining system.
Pretreatment prior to IHC analyis for PrP
d
was performed
using heat-induced antigen retrieval and formic acid as
described.
10,15
Prior to paraffin embedding, lymphoid
tissue was placed en bloc in 96%formic acid
for 60 minutes, rinsed in distilled water, and returned
to neutral-buffered 10%formalin. Sections were
deparaffinized in xylene and graded ethanols, incubated
5 minutes with 96%formic acid, and rinsed in Tris-
buffered saline (TBS; 0.05 M Tris-HCl, 0.15 M NaCl,
pH 7.6) to neutralize acidity. Heat-induced antigen
retrieval consisted of heating sections to 121uCfor
20 minutes at 15 to 20 pounds per square inch in
modified citrate buffer (Target Retrieval Solution,
pH 6.1
b
) using either a standard tabletop autoclave or
commercially available decloaking device.
c
Table 1. Number of animals and tissues tested for scrapie or
chronic wasting disease (CWD) by immunohistochemistry using
the Ventana NexES Autostainer System and the DakoCytomation
Autostainer Universal Staining System.
Sheep White-tailed deer Mule deer
No. of animals tested 796 504 482
Lymphoid tissue 508 504 670
Brain 512 504 482
Total tissues tested 1020 1008 1152
148 Baszler et al.
by guest on May 29, 2012vdi.sagepub.comDownloaded from
After antigen retrieval, all incubations were performed at
room temperature. Blocking steps included incubation for
15 minutes with 3%H
2
O
2
in methanol to block endoge-
nous peroxidase, and in Serum-free Protein Block
b
for
5 minutes. Critical immunostaining steps associated with
the autostainer included incubation of sections with anti-
PrP primary antibody, biotinylated linker antibody, de-
tection reagent, and chromogen, interceded by washes with
IHC buffer (TBS containing 0.2%Tween 20
b
). Anti-PrP
primary antibodies consisted of F89.160.1.5
d
(F89), a mono-
clonal antibody to ruminant PrP targeting a conserved
epitope at residues 142–145 and validated for diagnosis of
scrapie,
10,11
and F99.97.6.1
d
(F99), a monoclonal antibody
to ruminant PrP targeting a conserved epitope at residues
200–225 and validated for diagnosis of scrapie and
CWD.
10,14
For scrapie assays (because of rare variation
in the F89 epitope in some sheep breeds
10
), a cocktail
containing 3.4 mg each of F89 and F99/ml was incubated
on tissue sections for 10 minutes; F99 alone at concentra-
tion of 3.4 mg/ml for a 10-minute incubation was used for
CWD assays. Biotinylated goat antimouse/rabbit IgG
linker reagent,
b
horse radish perioxidase-conjugated stre-
pavidin-biotin complex detection reagent (LSAB-HRP
Tertiary Reagent
b
), and 3-amino-9-ethyl-carbazole (AEC)
chromogen
b
were incubated on sections for 10 minutes
each. After AEC incubation, sections were rinsed with
deionized water and counterstained manually with Mayer’s
hematoxylin
b
; then coverslips were mounted by use of
aqueous mounting medium, and slides were examined by
light microscopy.
Positive-control tissue, consisting of brain or lymph node
from a known scrapie-positive sheep or CWD-positive
deer, was included in each run to confirm immunoreactivity
of the appropriate pattern and intensity in lymphoid follicle
germinal centers and in the neuropil of the brain.
11,15
Negative antibody control, consisting of either Universal
negative mouse serum
b
(DakoCytomation) or an irrelevant
isotype-matched primary antibody (anti-Neospora caninum
mAb Nc-5B6-25, 3.4 mg/ml
d
) reacted with each test slide to
ensure the lack of non-specific binding by linker or signal
amplification reagents to tissue sections. Results for test
slides were classified as positive or negative for PrP
d
. Slides
classified as PrP
d
positive had immunoreactivity in a pattern
consistent with that of positive-control slides and of that in
previous publications.
10,11,17,18
Slides classified as PrP
d
negative did not have specific immunoreactivity. Only
optimal sections were classified; if a sectioning artifact such
as detached sections was seen, the sections were recut and
tested again.
Ventana NexES. Pretreatment antigen retrieval,
antiprion primary antibodies, and positive and negative
controls for Ventana NexES IHC were identical to the
aforementioned procedures used for the DakoCytomation
Autostainer.
For automated immunostaining on the Ventana NexES
platform, all incubations were performed at 37uC using the
AEC Detection System
a
according to manufacturer’s
recommendations. Critical immunostaining steps associat-
ed with the autostainer included incubation of sections with
inhibitor (to block nonspecific binding), anti-PrP primary
antibody, biotinylated linker antibody, detection reagent,
chromogen, counterstain, and bluing reagent, interceded by
washes with IHC buffer. Anti-PrP primary antibodies, F99
for CWD and F89 plus F99 for scrapie, were the same as
those used for the Dako autostainer, but antibody
concentration was 5 mg/ml. Primary antibodies were di-
luted in proprietary antibody diluent,
a
incubation time was
32 minutes, and incubation temperature was 37uC. Bioti-
nylated linker reagent (consisting of biotinylated goat
antimouse IgG,
a
detection reagent (consisting of horse
radish perioxidase-labeled strepavidin-biotin complex
a
),
Enhancer reagent,
a
and AEC chromogen
a
were each
incubated on sections for 8 minutes. Sections were counter-
stained with Gill’s hematoxylin
a
and bluing reagent,
a
then
coverslips were mounted with aqueous mounting medium.
Results for test slides were classified as positive or negative
for PrP,
a
as described previously for slides stained by the
DakoCytomation platform. Positive slides had immunore-
activity in a pattern consistent with that of positive-control
slides and previous publications.
14,15
Only optimal sections
were classified; if sectioning artifact such as de-attached
sections was seen, the sections were recut and tested again.
Immunohistochemical comparative analysis. Scrapie and
CWD IHC testing of all samples was initially completed
using the Ventana NexES, the standard protocol used at
the National Veterinary Services Laboratories (NVSL) by 3
of the authors (BVT, TG, SMH). A random subset of
negative and positive samples (glass slides or paraffin
blocks) of brain and lymphoid tissue from sheep, white-
tailed deer, and mule deer (Table 1) were sent to authors at
participating diagnostic laboratories (Michigan State
University [MK], Washington State University [TVB],
and University of Wyoming [ESW] for subsequent
equivalency testing using the DakoCytomation
Autostainer Universal Staining System
b
). The
participating laboratories were blinded to the disease
status of the tissue specimens and used identical
immunostaining procedures (as described previously) for
scrapie and CWD testing with the DakoCytomation
Autostainer Universal Staining System.
b
Results of scrapie and CWD testing using the DakoCy-
tomation Autostainer Universal Staining System,
b
were
forwarded to the authors at USDA-NVSL in Ames, IA
(BVT, TG, SMH) or USDA-ARS-ARU in Pullman, WA
(KIO) for comparison of test results with those of the
Ventana NexES.
a
Discordant results were retested using
a ‘‘sandwich’’ technique to verify or resolve differences. The
sandwich technique consists of immunostaining 3 consec-
utive serial sections from the paraffin blocks of the
specimens of interest. The first and third sections were
immunostained using Ventana NexES, and the second
(sandwich) section was immunostained using the DakoCy-
tomation Autostainer. The sandwich technique was neces-
sary to avoid false discordant results due to sectioning
artifact between initial testing with the Ventana NexES and
subsequent testing with the DakoCytomation Autostainer,
which occurred months later and after paraffin blocks were
sealed, requiring ‘‘refacing’’ of the paraffin blocks. Indeed,
it is possible that sectioning through a small immunoreac-
tive focus in the obex or lymph node of a weakly positive
Comparing IHC methods for scrapie and CWD diagnosis 149
by guest on May 29, 2012vdi.sagepub.comDownloaded from
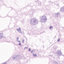
Figure 1. Retropharyngeal lymph node; sheep No. 1. Minimal multifocal granular immunoreactivity with anti-prion monoclonal
antibody (MAb) cocktail in cells of the germinal center (arrow). Ventana NexES IHC system. 3-Amino-9-ethyl-carbazole (AEC)
chromogen (red). Gill’s hematoxylin counterstain. Bar 590 mm.
Figure 2. Retropharyngeal lymph node; sheep No. 1. Minimal multifocal granular immunoreactivity with anti-prion MAb
cocktail in cells of the same serially sectioned germinal center as that in Figure 1 (arrow). DakoCytomation IHC system. AEC
150 Baszler et al.
by guest on May 29, 2012vdi.sagepub.comDownloaded from
sample could be interpreted as positive results on initial
testing and negative results on later testing. After initial
testing and retesting of discordant samples, comparisons of
results were tabulated as percentage concordance or
percentage discordance between the immunostaining plat-
forms for all species tested.
A random subset of 104 CWD-positive lymph nodes and
84 CWD-positive brains from white-tailed deer were
numerically classified to estimate semi-quantitative immu-
nostaining differences between the Ventana and DakoCy-
tomation platforms. The examiner (TG) was blinded to the
status of each slide. Because the number of total follicles
per section varied much between individual animals
examined, lymph nodes were classified semi-quantitatively
as: 1) minimal immunoreactivity in only a few follicles; 2)
obvious immunoreactivity, but only in a few follicles; 3)
obvious immunoreactivity in a moderate number of
follicles; 4) obvious and sometimes intense immunoreactiv-
ity in many follicles; and 5) intense immunoreactivity in
nearly all follicles. Brainstems were classified as de-
scribed,
15
namely: 1) partial immunoreactivity in the dorsal
motor nucleus of the vagus nerve; 2) total immunoreactiv-
ity of the dorsal motor nucleus of the vagus nerve; 3)
immunoreactivity extending from the dorsal motor nucleus
of the vagus nerve into the immediately adjacent neuropil;
4) moderate immunoreactivity throughout entire brainstem
section; and 5) intense immunoreactivity throughout entire
brainstem section.
Results
Sheep scrapie
A total of 1,020 tissues from 796 sheep were
examined by use of prion IHC analysis with the
Ventana NexES and DakoCytomation immunostain-
ing platforms (Table 1). Of the 508 lymphoid tissues
examined (tonsil or retropharyngeal lymph node), 103
were IHC positive and 405 were IHC negative by
both the Ventana NexES and DakoCytomation
platforms (Table 2). Of the 512 brainstems (obex)
examined, 120 were IHC positive and 392 were IHC
negative by both the Ventana NexES and DakoCy-
tomation platforms (Table 2). Initial examination
identified 5 discordant samples (4 brainstem and 1
lymph node), which on re-testing using the aforemen-
tioned serial section sandwich technique, indicated
concordant results between the 2 platforms. All
samples initially discordant were tissues that had
weak, focal immunoreactivity on initial testing using
the Ventana NexES platform and were IHC negative
Table 2. Comparison of scrapie and CWD immunohisto-
chemistry results using the Ventana NexES Autostainer System
and the DakoCytomation Autostainer Universal Staining System.
Animal Tissue (status) Ventana NexES DakoCytomation
Sheep Lymphoid
(positive)*
103 103
Lymphoid
(negative){
405 405
Brain (positive){120 120
Brain (negative)1392 392
White-tailed
deer
Lymphoid
(positive)*
104 104
Lymphoid
(negative){
400 400
Brain (positive){104 104
Brain (negative)1400 400
Mule deer Lymphoid
(positive)*
171 167
Lymphoid
(negative){
499 503
Brain (positive){102 101
Brain (negative)1380 381
* Lymphoid positive: PrPSc immunoreactivity in at least 1
germinal center.
{Lymphoid negative: no PrPSc immunoreactivity in any
germinal center (at least 10 present in sample).
{Brain positive: PrPSc immunoreactivity at least in dorsal
motor nucleus of the vagus nerve.
1Brain negative: no PrPSc immunoreactivity (dorsal motor
nucleus of vagus nerve present in sample).
r
chromogen (red). Mayer’s hematoxylin counterstain. Bar 590 mm.
Figure 3. Retropharyngeal lymph node; sheep No. 2. Moderate asymmetric granular immunoreactivity with anti-prion MAb
cocktail in cells of the germinal center. Ventana NexES IHC system. AEC chromogen (red). Gills’s hematoxylin counterstain. Bar 5
90 mm.
Figure 4. Retropharyngeal lymph node; sheep No. 2. Moderate asymmetric granular immunoreactivity with antiprion MAb
cocktail in cells of the same serially sectioned germinal center as that in Figure 3. DakoCytomation IHC system. AEC chromogen (red).
Mayer’s hematoxylin counterstain. Bar 590 mm.
Figure 5. Brainstem (obex); sheep No. 3. Immunoreactivity with anti prion MAb cocktail in the dorsal motor nucleus of the vagus
nerve (oval). Ventana NexES IHC system. AEC chromogen (red). Gill’s hematoxylin counterstain. Bar 5800 mm.
Figure 6. Brainstem (obex); sheep No. 3. Immunoreactivity with antiprion MAb cocktail in a serial section of the same dorsal
motor nucleus of the vagus nerve as that in Figure 5 (oval). DakoCytomation IHC system. AEC chromogen (red). Mayer’s hematoxylin
counterstain. Bar 5800 mm.
Figure 7. Brainstem (obex); sheep No. 3. Higher magnification of Figure 5. Ventana NexES IHC system. AEC chromogen (red).
Gill’s hematoxylin counterstain. Bar 5160 mm.
Figure 8. Brainstem (obex); sheep No. 3. Higher magnification of Figure 6. DakoCytomation IHC system. AEC chromogen (red).
Mayer’s hematoxylin counterstain. Bar 5160 mm.
Comparing IHC methods for scrapie and CWD diagnosis 151
by guest on May 29, 2012vdi.sagepub.comDownloaded from
Figure 9. Retropharyngeal lymph node; white-tailed deer No. 1. Moderate multifocal granular immunoreactivity with antiprion
MAb in cells of the germinal center. Ventana NexES IHC system. AEC chromogen (red). Gill’s hematoxylin counterstain. Bar 5
110 mm.
Figure 10. Retropharyngeal lymph node; white-tailed deer No. 1. Moderate multifocal granular immunoreactivity with antiprion
MAb in cells of the same serially sectioned germinal center as that in Figure 9. DakoCytomation IHC system. AEC chromogen (red).
152 Baszler et al.
by guest on May 29, 2012vdi.sagepub.comDownloaded from
using the DakoCytomation platform, which was
performed at a later date after the paraffin blocks
were resealed. On re-testing using serial sections, the
tissues were IHC negative by use of both platforms.
Thus, there was 100%concordance between the
Ventana NexES and DakoCytomation immunostain-
ing platforms in identifying PrP
d
-positive or PrP
d
-
negative brain and lymphoid tissues in sheep.
Overall, the quality of prion immunoreactivity was
equivalent between the Ventana NexES and Dako-
Cytomation immunostaining platforms (Figs. 1–8). In
lymphoid tissue, immunoreactivity was granular and
multifocal in germinal centers, a pattern consistent
with PrP
Sc
accumulation in lymphoid tissue (Figs. 1–
4).
10,17
Use of both platforms identified weakly
positive germinal centers, although the signal in-
tensity was slightly stronger for the Ventana NexES
platform (Figs. 1, 2). In brain tissue, the signal-to-
noise ratio was slightly higher in the Ventana NexES,
compared with the DakoCytomation platform
(Figs. 5–8). However, use of both platforms yielded
an immunoreactivity pattern typical of PrP
d
in sheep
with scrapie (i.e., plaques, cellular processes, peri-
neuronal rims, and perivascular accumulations,
11,18
and both platforms identified sheep early in disease,
with PrP
d
immunoreactivity restricted to focal regions
of the dorsal motor nucleus of the vagus nerve
(Figs. 5, 6).
Chronic wasting disease in white-tailed deer
A total of 1,008 tissues from 504 white-tailed deer
were examined by use of prion IHC analysis with the
Ventana NexES and DakoCytomation immunostain-
ing platforms (Table 1). Of the 504 lymphoid tissues
examined (tonsil or retropharyngeal lymph node), 104
were IHC positive and 400 were IHC negative by
both platforms (Table 2). Of the 504 brainstems
(obex) examined, 104 were IHC positive and 400 were
IHC negative by both platforms (Table 2). Thus,
there was 100%concordance between the Ventana
NexES and DakoCytomation immunostaining plat-
forms in identifying PrP
d
-positive or PrP
d
-negative
brain and lymphoid tissues in white-tailed deer.
There were no obvious semiquantitative differences
in immunoreactivity between the Ventana NexES and
DakoCytomation platforms using the aforemen-
tioned 1–5 numerical grading system to classify
a random subset of 104 CWD-positive lymph nodes
and 84 CWD- positive brainstems. Numbers of lymph
node specimens classified within each numerical grade
were: grade 1 513, grade 2 528, grade 3 534, grade
4529, and grade 5 50. Numbers of brainstem
samples classified within each numerical grade were:
grade 1 518, grade 2 522, grade 3 525, grade 4 5
15, and grade 5 54. The majority of brainstems
(86%; 72/84) were classified identically between the 2
immunostaining platforms. Similarly, most lymph
nodes (90%; 94/104) were classified identically be-
tween the 2 immunostaining platforms. The lymph
nodes and brainstems classified differently for in-
tensity of immunoreactivity varied by only 1 numer-
ical category (i.e., 4 vs. 5 or 1 vs. 2). Neither platform
had consistently higher scoring than the other.
Overall, the quality of prion immunoreactivity was
equivalent between the 2 immunostaining platforms
(Figs. 9–16). In lymphoid tissue, immunoreactivity
was granular and multifocal in germinal centers,
a pattern consistent with PrP
d
accumulation in
lymphoid tissue (Figs. 9–12).
14
Both platforms
identified weakly positive germinal centers, although
the signal intensity was slightly stronger for the
Ventana platform (Figs. 9, 10). In brain tissue, the
signal-to-noise ratio was slightly higher for the
Ventana, compared with the DakoCytomation plat-
form (Figs. 13–16). However, use of both platforms
yielded an immunoreactivity pattern typical of PrP
d
in
r
Mayer’s hematoxylin counterstain. Bar 5110 mm.
Figure 11. Retropharyngeal lymph node; white-tailed deer No. 2. Moderate asymmetric granular immunoreactivity with antiprion
MAb in cells of the germinal center. Ventana NexES IHC system. AEC chromogen (red). Gill’s hematoxylin counterstain. Bar 590 mm.
Figure 12. Retropharyngeal lymph node; white-tailed deer No. 2. Moderate asymmetric granular immunoreactivity with antiprion
MAb in cells of the same serially sectioned germinal center as that in Figure 11. DakoCytomation IHC system. AEC chromogen (red).
Mayer’s hematoxylin counterstain. Bar 590 mm.
Figure 13. Brainstem (obex); white-tailed deer No. 3. Immunoreactivity with antiprion MAb cocktail in the dorsolateral region of
the dorsal motor nucleus of the vagus nerve (oval). Ventana NexES IHC system. AEC chromogen (red). Gill’s hematoxylin counterstain.
Bar 5600 mm.
Figure 14. Brainstem (obex); white-tailed deer No. 3. Immunoreactivity with antiprion MAb cocktail in a serial section of the
same dorsal motor nucleus of the vagus nerve as that in Figure 13 (oval). DakoCytomation IHC system. AEC chromogen (red). Mayer’s
hematoxylin counterstain. Bar 5600 mm.
Figure 15. Brainstem (obex); white-tailed deer No. 3. Higher magnification of Figure 13. Ventana NexES IHC system. AEC
chromogen (red). Gill’s hematoxylin counterstain. Bar 5190 mm.
Figure 16. Brainstem (obex); white-tailed deer No. 3. Higher magnification of Figure 14. DakoCytomation IHC system. AEC
chromogen (red). Mayer’s hematoxylin counterstain. Bar 5190 mm.
Comparing IHC methods for scrapie and CWD diagnosis 153
by guest on May 29, 2012vdi.sagepub.comDownloaded from
deer with CWD (i.e., plaques, cellular processes,
perineuronal rims, and perivascular accumulations
(Figs. 15, 16),
11,18
and use of both platforms identified
deer early in disease, with PrP
d
immunoreactivity
restricted to the dorsal motor nucleus of the vagus
nerve (Figs. 13, 14).
There was slightly more nonspecific background
reactivity in the deer tissues tested for CWD than in
the sheep tissues tested for scrapie. Some deer tissues
stained by use of the Ventana NexES platform had
fine diffuse reactivity of the neuropil and intracyto-
plasmic reactivity of lymphocytes in lymph nodes.
Some deer tissues stained by use of the DakoCytoma-
tion platform had intraneuronal reactivity in the
brain, and in lymph nodes, accentuation of germinal
centers with orange brown reactivity was apparent.
However, the increased background visualized in
some deer tissues did not affect classification of
tissues as CWD positive or CWD negative.
Chronic wasting disease in mule deer
A total of 1,152 tissues from 482 mule deer were
examined by use of prion IHC analysis with the
Ventana NexES and DakoCytomation immunostain-
ing platforms (Table 1). Similar to tissues from white-
tailed deer tested for CWD, the quality of prion
immunoreactivity was equivalent between the 2
immunostaining platforms (data not shown). Of the
670 lymphoid tissues examined (tonsil or retrophar-
yngeal lymph node), 171 were IHC positive and 499
were IHC negative by use of the Ventana NexES
platform, and 167 were IHC positive and 503 were
IHC negative by use of the DakoCytomation
platform (Table 2), calculating to a 98.8%overall
concordance ([662/670] 3100). Of the 482 brainstems
(obex), examined, 102 were IHC positive and 380
were IHC negative by use of the Ventana NexES
platform, and 101 were IHC positive and 381 were
IHC negative by use of the DakoCytomation
platform (Table 2), calculating to a 99.6%overall
concordance ([480/482] 3100). For all of the
discordant samples, the DakoCytomation results
were repeatable in the individual testing laboratory
of one of the authors (ESW). However, due to
logistical problems retrieving archived tissue blocks,
the discordant results were not retested by both the
DakoCytomation and Ventana NexES systems using
the aforementioned sandwich technique. All discor-
dant samples were PrP
d
positive using the Ventana
NexES platform and were PrP
d
negative using the
DakoCytomation Universal platform. Thus, in total,
there was slightly less concordance between the
Ventana NexES and DakoCytomation immunostain-
ing platforms in identifying PrP
d
-positive or PrP
d
-
negative brain and lymphoid tissues in mule deer.
Discussion
Comparative testing of 2 automated immunostain-
ing platforms, Ventana NexES
a
Autostainer System
and DakoCytomation Autostainer Universal Staining
System,
b
revealed 100%concordance in correctly
classifying scrapie-positive and scrapie-negative
sheep, and CWD-positive and CWD-negative white-
tailed deer. Concordance was slightly ,100%for
mule deer with CWD. Use of both immunostaining
systems produced sections that were easily interpreted
and readily classified as PrP
d
positive or PrP
d
negative. The comparative testing was done in 5
laboratories, state and federal, carried out by
different technical personnel, and interpreted by
different pathologists at the respective institutions.
The results illustrate the reliability of either immu-
nostaining platform to detect scrapie and CWD, and
endorse the official use of both platforms in
laboratories approved for TSE testing.
The reason for the minimal discordance between
the Ventana NexES and DakoCytomation immuno-
staining platforms (1.2%for lymphoid tissue and
0.4%for brainstem) when classifying tissues as PrP
d
positive or PrP
d
negative from mule deer with
suspected CWD was not clearly evident from the
study. It is possible the Ventana NexES immuno-
staining platform was more sensitive with this subset
of tissue, perhaps due to the higher primary antibody
concentration used in the assay (5 mg/ml), compared
with that of the DakoCytomation platform (3.4 mg/
ml). However, the authors cannot differentiate with
certainty whether the Ventana NexES was more
sensitive or less specific than the DakoCytomation
platform (all the discordant samples were positive
using the Ventana NexES platform and were negative
using the DakoCytomation platform). Since the
discordant mule deer samples could not be retested
using the ‘‘sandwich’’ technique (because archived
blocks could not be retrieved), it is possible that
a small focus of immunoreactivity that was identified
using the Ventana NexES platform on initial screen-
ing was no longer present in the block when sections
were made for testing by the DakoCytomation
platform. It is possible there was no discordance at
all between the 2 systems in diagnosing CWD in the
mule deer samples tested. Finally, since all samples
were formalin fixed, it was not possible to further
verify the status of the samples in question by using
other detection methods such as Western blotting or
ELISA.
The slight qualitative differences in prion immuno-
reactivity between the Ventana NexES
a
and Dako-
Cytomation
b
autostainers could be a matter of
personal preference or due to methodologic differ-
154 Baszler et al.
by guest on May 29, 2012vdi.sagepub.comDownloaded from
ences. For example, the proprietary AEC chromogen
and hematoxylin counterstain of the Ventana NexES
platform produced a slightly magenta-red signal
against an aqua-blue counterstain, compared with
the red-brown AEC and darker blue hematoxylin of
the DakoCytomation platform. Also, the apparent
increased signal strength in the Ventana NexES
platform could have been due to the higher concen-
tration and incubation time of the primary antiprion
monoclonal antibodies (5 mg/ml for 32 minutes at
37uC), compared with those of the DakoCytomation
platform (3.4 mg/ml for 10 minutes at ambient
temperature). Regardless of these technical differ-
ences, the end result, prion immunoreactivity, did not
significantly vary sufficiently to incorrectly classify
a given tissue regarding TSE status. Furthermore, the
equal performance of the DakoCytomation and
Ventana NexES platforms in semi-quantitative grad-
ing classification, even for samples with minimal PrP
d
immunoreactivity, strengthens the conclusion that
both systems perform similarly.
Acknowledgements
The authors wish to acknowledge the excellent technical
assistance provided by technical staff at Michigan State
University Diagnostic Center for Population and Animal
Health, University of Wyoming Department of Veterinary
Science, and USDA/APHIS/NVSL, Pam Robertson at the
Washington Animal Disease Diagnostic Laboratory, and
Tom Truscott at USDA/ARS/ADRU Pullman, WA.
Sources and manufacturers
a. Ventana Medical Systems, Tucson, AZ.
b. DakyCytomation Inc., Carpinteria, CA.
c. BioCare Medical, Walnut Creek, CA.
d. VMRD Inc., Pullman, WA.
References
1. Bennion BJ, Daggett V: 2002, Protein conformation and
diagnostic tests: the prion protein. Clin Chem 48:2105–2114.
2. Bradley R: 1997, Animal prion diseases. In: Prion diseases, ed.
Collinge J, Palmer MS, pp. 89–129. Oxford University Press,
Oxford, UK.
3. Farquhar CF, Somerville RA, Ritchie LA: 1989, Post-mortem
immunodiagnosis of scrapie and bovine spongiform enceph-
alopathy. J Virol Methods 24:215–221.
4. Foster JD, Wilson M, Hunter N: 1996, Immunolocalisation of
prion protein (PrP) in the brains of sheep with scrapie. Vet
Rec 139:512–515.
5. Fraser H: 1976, The pathology of natural and experimental
scrapie. In: Slow virus diseases of animals and man, ed.
Kimberlin RH, pp. 276–305. North-Holland, Amsterdam,
The Netherlands.
6. Herrmann LM, Baszler TV, Knowles DP, Cheevers WP: 2002,
PrP(Sc) is not detected in peripheral blood leukocytes of
scrapie-infected sheep: determining the limit of sensitivity by
immunohistochemistry. Clin Diagn Lab Immunol 9:499–502.
7. Ingrosso L, Vetrugno V, Cardone F, Pocchiari M: 2002,
Molecular diagnostics of transmissible spongiform encepha-
lopathies. Trends Mol Med 8:273–280.
8. Miller J, Jenny A, Taylor W, et al.: 1994, Detection of prion
protein in formalin-fixed brain by hydrated autoclaving
immunohistochemistry for the diagnosis of scrapie in sheep.
J Vet Diag Invest 6:366–368.
9. O’Rourke KI: 2001, Ovine scrapie. New tools for control of
an old disease. Vet Clin North Am Food Anim Pract
17:283–300, vi.
10. O’Rourke KI, Baszler TV, Besser TE, et al.: 2000, Preclinical
diagnosis of scrapie by immunohistochemistry of third eyelid
lymphoid tissue. J Clin Microbiol 38:3254–3259.
11. O’Rourke KI, Baszler TV, Miller JM, et al.: 1998, Mono-
clonal antibody F89/160.1.5 defines a conserved epitope on
the ruminant prion protein. J Clin Microbiol 36:1750–1755.
12. Ramos-Vara JA, Kiupel M, Miller MA: 2005, Immunohisto-
chemical testing in veterinary diagnostic laboratories: results
of a survey of 47 laboratories. J Histotechnol 28:19–23.
13. Schreuder BE, van Keulen LJ, Vromans ME, et al.: 1998,
Tonsillar biopsy and PrPSc detection in the preclinical
diagnosis of scrapie. Vet Rec 142:564–568.
14. Spraker TR, O’Rourke KI, Balachandran A, et al.: 2002,
Validation of monoclonal antibody F99/97.6.1 for immuno-
histochemical staining of brain and tonsil in mule deer
(Odocoileus hemionus) with chronic wasting disease. J Vet
Diagn Invest 14:3–7.
15. Spraker TR, Zink RR, Cummings BA, et al.: 2002,
Distribution of protease-resistant prion protein and spongi-
form encephalopathy in free-ranging mule deer (Odocoileus
hemionus) with chronic wasting disease. Vet Pathol
39:546–556.
16. Spraker TR, Zink RR, Cummings BA, et al.: 2002,
Comparison of histological lesions and immunohistochemical
staining of proteinase-resistant prion protein in a naturally
occurring spongiform encephalopathy of free-ranging mule
deer (Odocoileus hemionus) with those of chronic wasting
disease of captive mule deer. Vet Pathol 39:110–119.
17. van Keulen LJ, Schreuder BE, Meloen RH, et al.: 1995,
Immunohistochemical detection and localization of prion
protein in brain tissue of sheep with natural scrapie. Vet
Pathol 32:299–308.
18. van Keulen LJ, Schreuder BE, Meloen RH, et al.: 1996,
Immunohistochemical detection of prion protein in lymphoid
tissues of sheep with natural scrapie. J Clin Microbiol
34:1228–1231.
19. Wild MA, Spraker TR, Sigurdson CJ, et al.: 2002, Preclinical
diagnosis of chronic wasting disease in captive mule deer
(Odocoileus hemionus) and white-tailed deer (Odocoileus
virginianus) using tonsillar biopsy. J Gen Virol 83:2629–2634.
20. Williams ES, Miller MW: 2002, Chronic wasting disease in
deer and elk in North America. Rev Sci Tech 21:305–316.
Comparing IHC methods for scrapie and CWD diagnosis 155
by guest on May 29, 2012vdi.sagepub.comDownloaded from