Available via license: CC BY 4.0
Content may be subject to copyright.
Citation: Svietlova, N.; Reichelt, M.;
Zhyr, L.; Majumder, A.; Scholz, S.S.;
Grabe, V.; Krapp, A.; Oelmüller, R.;
Mithöfer, A. The Beneficial Fungus
Mortierella hyalina Modulates Amino
Acid Homeostasis in Arabidopsis
under Nitrogen Starvation. Int. J. Mol.
Sci. 2023,24, 16128. https://doi.org/
10.3390/ijms242216128
Academic Editor: Jiangyun Gao
Received: 9 October 2023
Revised: 30 October 2023
Accepted: 3 November 2023
Published: 9 November 2023
Copyright: © 2023 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
International Journal of
Molecular Sciences
Article
The Beneficial Fungus Mortierella hyalina Modulates Amino
Acid Homeostasis in Arabidopsis under Nitrogen Starvation
Nataliia Svietlova 1, Michael Reichelt 2, Liza Zhyr 1, Anindya Majumder 1, Sandra S. Scholz 3,
Veit Grabe 4, Anne Krapp 5, Ralf Oelmüller 3and Axel Mithöfer 1,*
1
Research Group Plant Defense Physiology, Max Planck Institute for Chemical Ecology, 07745 Jena, Germany;
nsvietlova@ice.mpg.de (N.S.); yzhyr@ice.mpg.de (L.Z.); amajumder@bot.uni-kiel.de (A.M.)
2Department of Biochemistry, Max Planck Institute for Chemical Ecology, 07745 Jena, Germany;
reichelt@ice.mpg.de
3Department of Plant Physiology, Matthias-Schleiden-Institute, Friedrich-Schiller-University, 07743 Jena,
Germany; s.scholz@uni-jena.de (S.S.S.); ralf.oelmueller@uni-jena.de (R.O.)
4Microscopic Imaging Service Group, Max Planck Institute for Chemical Ecology, 07745 Jena, Germany;
vgrabe@ice.mpg.de
5Institut Jean-Pierre Bourgin (IJPB), AgroParisTech, INRAE, UniversitéParis-Saclay, 78000 Versailles, France;
anne.krapp@inrae.fr
*Correspondence: amithoefer@ice.mpg.de; Tel.: +49-(0)-3641-571263
Abstract:
Non-mycorrhizal but beneficial fungi often mitigate (a)biotic stress-related traits in host
plants. The underlying molecular mechanisms are mostly still unknown, as in the interaction between
the endophytic growth-promoting soil fungus Mortierella hyalina and Arabidopsis thaliana. Here,
abiotic stress in the form of nitrogen (N) deficiency was used to investigate the effects of the fungus
on colonized plants. In particular, the hypothesis was investigated that fungal infection could
influence N deficiency via an interaction with the high-affinity nitrate transporter NRT2.4, which
is induced by N deficiency. For this purpose, Arabidopsis wild-type nrt2.4 knock-out and NRT2.4
reporter lines were grown on media with different nitrate concentrations with or without M. hyalina
colonization. We used chemical analysis methods to determine the amino acids and phytohormones.
Experimental evidence suggests that the fungus does not modulate NRT2.4 expression under N
starvation. Instead, M. hyalina alleviates N starvation in other ways: The fungus supplies nitrogen
(
15
N) to the
N-starved
plant. The presence of the fungus restores the plants’ amino acid homeostasis,
which was out of balance due to N deficiency, and causes a strong accumulation of branched-chain
amino acids. We conclude that the plant does not need to invest in defense and resources for
growth are maintained, which in turn benefits the fungus, suggesting that this interaction should be
considered a mutualistic symbiosis.
Keywords:
nitrate/nitrogen deficiency; nitrate transporters (NRTs); free amino acids; Mortierella
hyalina; plant–fungus interactions; endophytic fungi
1. Introduction
The uptake of nitrogen via the roots is essential for plant growth. Nitrogen plays a
special role in plant growth and productivity and is a crucial nutrient for plants, incorpo-
rated as the main building block of amino acids, proteins and many secondary metabolites.
Plants efficiently acquire nitrogen and distribute it from source to sink organs under various
environmental conditions [
1
,
2
]. It is usually absorbed in inorganic form, as ammonium
or nitrate (NO
3−
). The latter is the most important source of nitrogen. While ammonium
can be directly assimilated into glutamine in the root, nitrate is first transported to the
shoot. Nitrate is then reduced into ammonium in various enzymatic steps, transferred
to the amino acid glutamine using glutamine synthetase and further introduced into the
metabolism by aminotransferases. From inorganic nitrate acquisition to organic nitrogen
Int. J. Mol. Sci. 2023,24, 16128. https://doi.org/10.3390/ijms242216128 https://www.mdpi.com/journal/ijms
Int. J. Mol. Sci. 2023,24, 16128 2 of 16
translocation and distribution, plants have evolved different strategies and systems [
3
–
11
].
An interesting aspect in this context is the initial uptake of nitrate, especially under ni-
trate deficiency. In order to survive in soil environments with different amounts of nitrate
present, plants have evolved different transport systems to take up nitrate, which have been
described in detail [
3
–
6
]. Briefly, two classes of transporter systems are involved in nitrate
uptake: nitrate transport systems with high affinity, called High-Affinity Transporter Sys-
tems (HATS), and with low affinity called Low-Affinity Transporter Systems (LATS) [
3
,
9
,
12
].
So far, four nitrate transporter families have been identified, those are NRT1/PTR (NPF,
nitrate transporter 1/peptide transporter family), NRT2 (nitrate transporter), CLC (chloride
channels) and SLAC1/SLAH (slow anion channel-associated 1 homologs) [
5
,
8
]. Among
these, only NRTs are involved in nitrate uptake from the soil, while NRT1 transporters
are mainly LATS with different intracellular localizations. Most NRT2 transporters belong
to HATS and are often localized in the plasma membrane [
5
]. The NRT2 family has a
major contribution to the nitrate influx into roots. NRT2.1, NRT2.2, NRT2.4 and NRT2.5 are
important for plants to survive with nitrate limitation. Here, NRT2.1 and NRT2.4 play a
major role in the maintenance of optimal plant growth under different nitrate conditions.
While NRT2.1 is a main component of HATS both under low nitrate conditions and with
a nitrate supply, expression of NRT2.4 was observed only in lateral roots and younger
parts of the main root under nitrogen starvation. This revealed that NRT2.4 is specifically
involved in the response to nitrate starvation [12].
Of course, such nitrate or nitrogen deficiencies must be recognized and managed
by the plant [
13
]. The ability to monitor the cellular N status is essential for maintaining
metabolic homeostasis, growth and development in plants. Candidates that are consid-
ered for the role of N sensory systems and further signaling to appropriate physiological
responses include the target of rapamycin (TOR) signaling pathway, the general control
non-derepressible 2 (GCN2) pathway, the family of glutamate-like receptors (GLRs) and
the plastidic P
II
-dependent pathway [
14
]. All these putative candidates have in common
a hypothesized role in binding amino acids. Strikingly, the widely distributed P
II
is non-
functional in Brassicaceae, including Arabidopsis [
15
]. However, despite recent progress in
understanding the function and in part the mode of action of these signaling systems, there
is still lacking knowledge concerning to what extent they contribute to the process of N
status monitoring in plants [14].
It is already known that a number of beneficial microorganisms, in particular endo-
phytic fungi, can positively influence the growth of many plants under stress. Endophytic
fungi are facultative symbionts of plants. Depending on the particular host plant, develop-
mental stage, nutrition and other environmental factors, they may interact with their host
as mutualistic symbionts, as commensals or as latent pathogens [
16
]. Unlike mycorrhizal
fungi, their growth is not synchronized with the development of their hosts [
17
]. Plants col-
onized by endophytic fungi often show improved growth, better productivity and induced
resistance against biotic attackers [
18
–
23
]. For example, co-cultivation with beneficial fungi
such as Mortierella hyalina can promote the growth of Arabidopsis thaliana [
24
,
25
]. M. hyalina
is an endophytic fungus belonging to the order Mortierellales, the largest genus within
Mucoromycota [
26
]. Dominant fungal communities in natural ecosystems harbor various
members of the order Mortierellales [
27
], including the non-pathogenic genus Mortierella.
Species of Mortierella live as saprotrophs in soil, on decaying leaves and other organic
material. In addition, many of those colonize roots of a wide variety of plant species and
stimulate growth and biomass production in the aerial parts of plants [
23
]. However, very
often, the underlying molecular mechanisms are still unknown.
The positive influence of beneficial fungi on stressed plants is a well-known phe-
nomenon. This also applies to the positive effect of beneficial fungi on plants under N
starvation. Although the role of nitrate transporters in nitrate deficiency has been demon-
strated [28], a possible influence of beneficial fungi on such NRTs has not yet been shown.
Therefore, the aim of this study was to investigate and understand the putative role of a
beneficial fungus, M. hyalina, on nitrate uptake by a high-affinity nitrate transporter such as
Int. J. Mol. Sci. 2023,24, 16128 3 of 16
NRT2.4 and furthermore on nitrogen metabolism, exemplified by amino acid metabolism,
in colonized Arabidopsis plants under N starvation. It is shown here for the first time
that the fungus does not affect the expression of NRT.2.4. Instead, M. hyalina can supply
nitrogen to N-starved plants. Furthermore, we show that the presence of M. hyalina can
restore the amino acid homeostasis disturbed by nitrogen deficiency in both the shoots and
roots of the host plant.
2. Results
2.1. Effect of Mortierella hyalina Colonization on Fresh Weight of Arabidopsis Plants under
Nitrogen Starvation
A first study of the root growth features in the different Arabidopsis lines showed that
even under a high NO
3−
concentration (7 mM), the total length of the main and lateral
roots was reduced on the 6th and even more on the 10th day of incubation in the NRT2.4
knock-out (ko) mutant lines (nrt2.4-1 and nrt2.4-2) compared to the wild type (WT, Col-0).
The fresh weight (FW) of the shoots and roots in all these lines was detected to be more
dependent on the nitrate concentration than on the genotype (Figure 1).
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 3 of 17
benecial fungus, M. hyalina, on nitrate uptake by a high-anity nitrate transporter such
as NRT2.4 and furthermore on nitrogen metabolism, exemplied by amino acid metabo-
lism, in colonized Arabidopsis plants under N starvation. It is shown here for the rst time
that the fungus does not aect the expression of NRT.2.4. Instead, M. hyalina can supply
nitrogen to N-starved plants. Furthermore, we show that the presence of M. hyalina can
restore the amino acid homeostasis disturbed by nitrogen deciency in both the shoots
and roots of the host plant.
2. Results
2.1. Eect of Mortierella hyalina Colonization on Fresh Weight of Arabidopsis Plants under
Nitrogen Starvation
A rst study of the root growth features in the dierent Arabidopsis lines showed
that even under a high NO3− concentration (7 mM), the total length of the main and lateral
roots was reduced on the 6th and even more on the 10th day of incubation in the NRT2.4
knock-out (ko) mutant lines (nrt2.4-1 and nrt2.4-2) compared to the wild type (WT, Col-
0). The fresh weight (FW) of the shoots and roots in all these lines was detected to be more
dependent on the nitrate concentration than on the genotype (Figure 1).
A
B
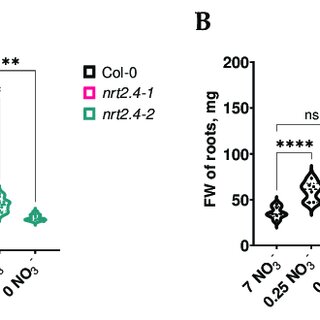
Figure 1. Fresh weight of shoots (A) and roots (B) of Arabidopsis thaliana WT and ko mutant plants
during NO3− starvation. Two-week-old seedlings pre-grown on full NO3− (7 mM NO3−) medium were
further incubated on a dierent NO3− medium (N-free, 0 mM NO3−; N-low, 0.25 mM NO3−; N-com-
plete, 7 mM NO3−) for another 10 d. Each replicate represents a sum of 3 seedlings. Two-way ANOVA
with Dunne’s multiple comparison test; n = 6–10; *** p < 0.001; **** p < 0.0001; ns: not signicant.
In the WT and mutant lines, 10 d of nitrate deciency reduced the FW in the shoots,
and the lower the nitrate concentration, the lower the weight (Figure 1A). In contrast, the
roots’ FW was not negatively aected (Figure 1B). Upon infection with M. hyalina, the
shoots and roots showed the same trend as the non-infected plants, but with a clearly
lower FW. These results suggest that the fungus somehow competes with the plant for
nitrogen, resulting in a lower FW for the plant. The reduction in root FW was most pro-
nounced on complete nitrate media, which is reected in the shoot/root ratio (Figure 2).
There was no dierence in shoot/root ratio between the non-infected ko mutants and WT
plants, only a slight reduction due to the nitrate concentrations (Figure 2A). However,
upon M. hyalina colonization, on complete nitrate media, the WT plants showed a signi-
cantly higher shoot/root ratio compared with the mutant lines (Figure 2B). However, the
shoot/root ratio in the mutant lines grown with M. hyalina is almost on the same level as
in the non-infected control plants.
7 NO3-
0.25 NO3-
0 NO3-
7 NO3-
0.25 NO3-
0 NO3-
7 NO3-
0.25 NO3-
0 NO3-
0
50
100
150
200
FW of shoots, mg
Col-0
nrt2.4-1
nrt2.4-2
✱✱✱✱
✱✱✱✱
✱✱✱✱
✱✱✱✱
✱✱✱✱
✱✱✱✱
7 NO3-
0.25 NO3-
0 NO3-
7 NO3-
0.25 NO3-
0 NO3-
7 NO3-
0.25 NO3-
0 NO3-
0
50
100
150
200
FW of roots, mg
Col-0
nrt2.4-1
nrt2.4-2
✱✱✱✱
ns
✱✱✱
ns
ns
ns
Figure 1.
Fresh weight of shoots (
A
) and roots (
B
) of Arabidopsis thaliana WT and ko mutant plants
during NO
3−
starvation. Two-week-old seedlings pre-grown on full NO
3−
(7 mM NO
3−
) medium
were further incubated on a different NO
3−
medium (N-free, 0 mM NO
3−
; N-low, 0.25 mM NO
3−
;
N-complete, 7 mM NO
3−
) for another 10 d. Each replicate represents a sum of 3 seedlings. Two-
way ANOVA with Dunnett’s multiple comparison test; n = 6–10; *** p< 0.001; **** p< 0.0001;
ns: not significant.
In the WT and mutant lines, 10 d of nitrate deficiency reduced the FW in the shoots,
and the lower the nitrate concentration, the lower the weight (Figure 1A). In contrast, the
roots’ FW was not negatively affected (Figure 1B). Upon infection with M. hyalina, the
shoots and roots showed the same trend as the non-infected plants, but with a clearly lower
FW. These results suggest that the fungus somehow competes with the plant for nitrogen,
resulting in a lower FW for the plant. The reduction in root FW was most pronounced on
complete nitrate media, which is reflected in the shoot/root ratio (Figure 2). There was no
difference in shoot/root ratio between the non-infected ko mutants and WT plants, only a
slight reduction due to the nitrate concentrations (Figure 2A). However, upon M. hyalina
colonization, on complete nitrate media, the WT plants showed a significantly higher
shoot/root ratio compared with the mutant lines (Figure 2B). However, the shoot/root ratio
in the mutant lines grown with M. hyalina is almost on the same level as in the non-infected
control plants.
2.2. Effect of Mortierella hyalina Colonization on Phytohormones in Arabidopsis Plants under
Nitrogen Starvation
M. hyalina has been described as a beneficial fungus. In principle, however, the plant
could also recognize the infection with M. hyalina as an attack. To investigate this possibility,
defense and stress-related phytohormones (salicylic acid, SA; jasmonate, JA; abscisic acid,
ABA) were analyzed (Figure 3). It became clear that in all Arabidopsis genotypes, the roots
(Figure 3B) and not shoots (Figure 3A) showed a significant increase in SA under nitrate
deficiency stress, but not under M. hyalina infection. JA was also found to have an effect on
Int. J. Mol. Sci. 2023,24, 16128 4 of 16
the phytohormone content. A higher JA content was found in the roots and shoots of WT
plants under nitrate deficiency; this effect was attenuated by infection with M. hyalina. In
both nrt2.4 mutant lines, the JA-reducing effect of the fungus was also visible (Figure 3).
ABA was also weakly but significantly induced in the shoots but not in the roots when
nitrate was deficient. The presence of M. hyalina slightly increased the ABA levels in all
cases (Figure 3). These results clearly show that the plant neither recognized M. hyalina as a
pathogen nor activated any defense mechanism against the fungus.
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 4 of 17
A
B
Figure 2. Shoot/root ratio of Arabidopsis thaliana WT and ko mutant plants without (A) or with (B)
Mortierella hyalina co-cultivation during NO3− starvation. Two-week-old seedlings pre-grown on full
NO3− (7 mM NO3−) medium were further incubated on a dierent NO3− medium (N-free, 0 mM NO3−;
N-low, 0.25 mM NO3−; N-complete, 7 mM NO3−) and were not (A) or were (B) co-cultivated with M.
hyalina for another 10 d. Each replicate represents the sum of three seedlings. Two-way ANOVA
with Dunne’s multiple comparison test; n = 6–10; *** p < 0.001; **** p < 0.0001; ns: not signicant.
2.2. Eect of Mortierella hyalina Colonization on Phytohormones in Arabidopsis Plants under
Nitrogen Starvation
M. hyalina has been described as a benecial fungus. In principle, however, the plant
could also recognize the infection with M. hyalina as an aack. To investigate this possi-
bility, defense and stress-related phytohormones (salicylic acid, SA; jasmonate, JA; ab-
scisic acid, ABA) were analyzed (Figure 3). It became clear that in all Arabidopsis geno-
types, the roots (Figure 3B) and not shoots (Figure 3A) showed a signicant increase in SA
under nitrate deciency stress, but not under M. hyalina infection. JA was also found to
have an eect on the phytohormone content. A higher JA content was found in the roots
and shoots of WT plants under nitrate deciency; this eect was aenuated by infection
with M. hyalina. In both nrt2.4 mutant lines, the JA-reducing eect of the fungus was also
visible (Figure 3). ABA was also weakly but signicantly induced in the shoots but not in
the roots when nitrate was decient. The presence of M. hyalina slightly increased the ABA
levels in all cases (Figure 3). These results clearly show that the plant neither recognized
M. hyalina as a pathogen nor activated any defense mechanism against the fungus.
A
B
7 NO3-0.25 NO3-0 NO3-
0
5
10
15
Shoot/Root Ratio
Col-0
nrt2.4-1
nrt2.4-2
ns
ns
ns
ns
ns
ns
7 NO3-0.25 NO3-0 NO3-
0
5
10
15
Shoot/Root Ratio
Col-0
nrt2.4-1
nrt2.4-2
✱✱✱
✱✱✱✱
ns
ns
ns
ns
Figure 2.
Shoot/root ratio of Arabidopsis thaliana WT and ko mutant plants without (
A
) or with
(
B
)Mortierella hyalina co-cultivation during NO
3−
starvation. Two-week-old seedlings pre-grown
on full NO
3−
(7 mM NO
3−
) medium were further incubated on a different NO
3−
medium (N-free,
0 mM NO
3−
; N-low, 0.25 mM NO
3−
; N-complete, 7 mM NO
3−
) and were not (
A
) or were (
B
) co-
cultivated with M. hyalina for another 10 d. Each replicate represents the sum of three seedlings.
Two-way ANOVA with Dunnett’s multiple comparison test; n = 6–10; *** p< 0.001; **** p< 0.0001;
ns: not significant.
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 4 of 17
A
B
Figure 2. Shoot/root ratio of Arabidopsis thaliana WT and ko mutant plants without (A) or with (B)
Mortierella hyalina co-cultivation during NO3− starvation. Two-week-old seedlings pre-grown on full
NO3− (7 mM NO3−) medium were further incubated on a dierent NO3− medium (N-free, 0 mM NO3−;
N-low, 0.25 mM NO3−; N-complete, 7 mM NO3−) and were not (A) or were (B) co-cultivated with M.
hyalina for another 10 d. Each replicate represents the sum of three seedlings. Two-way ANOVA
with Dunne’s multiple comparison test; n = 6–10; *** p < 0.001; **** p < 0.0001; ns: not signicant.
2.2. Eect of Mortierella hyalina Colonization on Phytohormones in Arabidopsis Plants under
Nitrogen Starvation
M. hyalina has been described as a benecial fungus. In principle, however, the plant
could also recognize the infection with M. hyalina as an aack. To investigate this possi-
bility, defense and stress-related phytohormones (salicylic acid, SA; jasmonate, JA; ab-
scisic acid, ABA) were analyzed (Figure 3). It became clear that in all Arabidopsis geno-
types, the roots (Figure 3B) and not shoots (Figure 3A) showed a signicant increase in SA
under nitrate deciency stress, but not under M. hyalina infection. JA was also found to
have an eect on the phytohormone content. A higher JA content was found in the roots
and shoots of WT plants under nitrate deciency; this eect was aenuated by infection
with M. hyalina. In both nrt2.4 mutant lines, the JA-reducing eect of the fungus was also
visible (Figure 3). ABA was also weakly but signicantly induced in the shoots but not in
the roots when nitrate was decient. The presence of M. hyalina slightly increased the ABA
levels in all cases (Figure 3). These results clearly show that the plant neither recognized
M. hyalina as a pathogen nor activated any defense mechanism against the fungus.
A
B
7 NO3-0.25 NO3-0 NO3-
0
5
10
15
Shoot/Root Ratio
Col-0
nrt2.4-1
nrt2.4-2
ns
ns
ns
ns
ns
ns
7 NO3-0.25 NO3-0 NO3-
0
5
10
15
Shoot/Root Ratio
Col-0
nrt2.4-1
nrt2.4-2
✱✱✱
✱✱✱✱
ns
ns
ns
ns
Figure 3. Cont.
Int. J. Mol. Sci. 2023,24, 16128 5 of 16
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 5 of 17
Figure 3. Phytohormone contents in shoots (A) and roots (B) of Arabidopsis thaliana WT and ko mu-
tant plants with and without M. hyalina co-cultivation during NO3− starvation. Two-week-old seed-
lings pre-grown on full NO3− (7 mM NO3−) medium were further incubated on dierent NO3− me-
dium (N-free, 0 mM NO3−; N-low, 0.25 mM NO3−; N-complete, 7 mM NO3−) and co-cultivated with-
out and with M. hyalina for another 10 d. The line from the box’s ends extends from the rst and the
third quartile, the line in the middle represents median. Two-way ANOVA with Tukey’s post hoc
test, n = 5–6 (data were transformed when needed). Dierent leers indicate signicant dierences
(p < 0.05) across groups.
2.3. Mortierella hyalina Colonization Can Provide Nitrogen to Arabidopsis Plants under
Nitrogen Starvation
Next, we investigated whether and to what extent the fungus may provide nitrogen
to the plant. Compared with the incubation with the unlabeled fungus, much higher 15N
levels were detected in the Arabidopsis shoots (Table 1). The lower the nitrate concentra-
tion in the medium, the higher the uptake of fungus-provided 15N. A 15N level up to 9895
times higher was found in shoots growing without nitrate, 829 times higher with 0.25 mM
nitrate and 52 times higher with 7 mM nitrate. This clearly indicted a transport of 15N from
the fungal hyphae to the plant roots and from the roots to the shoots. This eect is much
more intense if no NO3− is available in the medium, suggesting an exchange of nutrients
only when it is necessary.
Table 1. δ15N level in shoots of Arabidopsis thaliana Col-0 colonized by 15N-labeled or unlabeled
Mortierella hyalina fungi and the impact of dierent NO3− concentrations in the growth medium.
Unlabeled Fungi
Labeled Fungi
Delta vs. Air-corr.
29/28
%N corr. Concen-
tration
Delta vs. Air-corr.
29/28
%N corr. Concen-
tration
7 NO3−
4.70 ± 0.51
6.14 ± 0.12
242.04 ± 50.57
5.64 ± 0.24
0.25 NO3−
1.37 ± 0.38
1.49 ± 0.03
1134.98 ±259.92
1.55 ± 0.03
0 NO3−
0.21 ± 1.37
1.52 ± 0.06
2077.79 ± 330.63
1.45 ± 0.05
Two-week-old seedlings pre-grown on full NO3− (7 mM NO3−) medium were further incubated on
dierent NO3− media (N-free (0 mM NO3−), N-low (0.25 mM NO3−) and N-complete (7 mM NO3−))
and co-cultivated with M. hyalina for another 10 d. M. hyalina was pre-grown for 10 d either on 15N-
labeled or unlabeled amino acids.
2.4. Eect of Nitrogen Starvation on NTR2.4 Gene Induction with/without Mortierella hyalina
Colonization
In order to nd out whether M. hyalina somehow aects the high-anity nitrate up-
take system (HATS), the ProNRT2.4:GFP reporter line was employed (Figure 4). While u-
orescence was detectable rapidly upon transfer in seedlings grown without nitrate with a
peak after 4–6 d, with 7 mM nitrate in the medium, no induction occurred. Strikingly, the
presence of M. hyalina had no obvious eect on the N-deciency-induced expression of
NRT2.4. This suggests that M. hyalina does not inuence NRT2.4-mediated nitrate uptake.
Figure 3.
Phytohormone contents in shoots (
A
) and roots (
B
) of Arabidopsis thaliana WT and ko mutant
plants with and without M. hyalina co-cultivation during NO
3−
starvation. Two-week-old seedlings
pre-grown on full NO
3−
(7 mM NO
3−
) medium were further incubated on different NO
3−
medium
(N-free, 0 mM NO
3−
; N-low, 0.25 mM NO
3−
; N-complete, 7 mM NO
3−
) and co-cultivated without
and with M. hyalina for another 10 d. The line from the box’s ends extends from the first and the
third quartile, the line in the middle represents median. Two-way ANOVA with Tukey’s post hoc
test,
n = 5–6
(data were transformed when needed). Different letters indicate significant differences
(p< 0.05) across groups.
2.3. Mortierella hyalina Colonization Can Provide Nitrogen to Arabidopsis Plants under
Nitrogen Starvation
Next, we investigated whether and to what extent the fungus may provide nitrogen
to the plant. Compared with the incubation with the unlabeled fungus, much higher
15
N
levels were detected in the Arabidopsis shoots (Table 1). The lower the nitrate concentration
in the medium, the higher the uptake of fungus-provided
15
N. A
15
N level up to 9895 times
higher was found in shoots growing without nitrate, 829 times higher with 0.25 mM nitrate
and 52 times higher with 7 mM nitrate. This clearly indicted a transport of
15
N from the
fungal hyphae to the plant roots and from the roots to the shoots. This effect is much more
intense if no NO
3−
is available in the medium, suggesting an exchange of nutrients only
when it is necessary.
Table 1. δ15
N level in shoots of Arabidopsis thaliana Col-0 colonized by
15
N-labeled or unlabeled
Mortierella hyalina fungi and the impact of different NO3−concentrations in the growth medium.
Unlabeled Fungi Labeled Fungi
Delta vs.
Air-corr. 29/28
%N corr.
Concentration
Delta vs.
Air-corr. 29/28
%N corr.
Concentration
7 NO3−4.70 ±0.51 6.14 ±0.12 242.04 ±50.57 5.64 ±0.24
0.25 NO3−1.37 ±0.38 1.49 ±0.03 1134.98 ±259.92 1.55 ±0.03
0 NO3−0.21 ±1.37 1.52 ±0.06 2077.79 ±330.63 1.45 ±0.05
Two-week-old seedlings pre-grown on full NO
3−
(7 mM NO
3−
) medium were further incubated on different
NO
3−
media (N-free (0 mM NO
3−
), N-low (0.25 mM NO
3−
) and N-complete (7 mM NO
3−
)) and co-cultivated
with M. hyalina for another 10 d. M. hyalina was pre-grown for 10 d either on
15
N-labeled or unlabeled amino acids.
2.4. Effect of Nitrogen Starvation on NTR2.4 Gene Induction with/without Mortierella
hyalina Colonization
In order to find out whether M. hyalina somehow affects the high-affinity nitrate
uptake system (HATS), the ProNRT2.4:GFP reporter line was employed (Figure 4). While
fluorescence was detectable rapidly upon transfer in seedlings grown without nitrate with
a peak after 4–6 d, with 7 mM nitrate in the medium, no induction occurred. Strikingly,
the presence of M. hyalina had no obvious effect on the N-deficiency-induced expression of
NRT2.4. This suggests that M. hyalina does not influence NRT2.4-mediated nitrate uptake.
Int. J. Mol. Sci. 2023,24, 16128 6 of 16
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 6 of 17
A
B
Figure 4. Relative uorescence intensity (RFI) (A) and confocal microscopy images (B) of roots of
Arabidopsis thaliana ProNRT2.4:GFP transgenic plants. (A) Two-week-old seedlings pre-grown on
full NO3− (7 mM NO3−) medium were further incubated on dierent NO3− media (N-free (0 mM
NO3−), N-low (0.25 mM NO3−) and N-complete (7 mM NO3−)) and were or were not co-cultivated
with M. hyalina for the indicated time. Two-way ANOVA with Dunne’s multiple comparison test;
n = 8. M. hyalina: Mixed-eects model (REML) with Dunne’s multiple comparison test; n = 4–8; the
error bars indicate standard error (SE); * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns: not
signicant. (B) Two-week-old seedlings pre-grown on full NO3− (7 mM NO3−) medium were further
incubated on N-complete (7 mM NO3−, left) or N-free (0 mM NO3−, right) for 2 d. STEDYCON mi-
croscopy and STEDYCON processing software (exc. laser 488 nm, detector 526 nm). To compare the
uorescence intensity in these variants, the raw images were aligned by photon count to the same
level in the two images.
Additional qPCR experiments supported the NRT2.4 gene induction over time under
nitrate deciency in the Arabidopsis Col-0 roots (Figure S2A). A 12.2-fold increase of
NRT2.4 transcripts was detected after 2 d on the media without nitrate and still a 3.4-fold
increase on 0.25 mM nitrate, all compared to the controls grown on 7 mM nitrate. After 10
d, NRT2.4 gene induction did not increase anymore and even decreased without nitrate,
suggesting an early but transient induction of this transporter (Figure S2A). Strikingly, an
interesting nding was that the expression of the high-anity nitrate transporter NRT2.5
under nitrate starvation was regulated dierently. In contrast to NRT2.4, the NRT2.5 ex-
pression was lower at d 2 than at d 10 (from 66.7- to 255-fold, respectively) on the media
with and without nitrate (Figure S2B).
2.5. Eect of Mortierella hyalina Colonization on Amino Acid Pools in Arabidopsis Plants under
Nitrogen Starvation
Since the rst organic compounds that carry absorbed N are amino acids, composi-
tions and changes in the amino acid pools in the dierent Arabidopsis lines were analyzed
individually in both the shoots and roots, depending on the given nitrate level in the me-
dium, representing N starvation, and the presence/absence of M. hyalina (Figure 5). Look-
ing deeper into the amino acid results, it is interesting to note that in all Arabidopsis lines,
nitrate depletion had a particularly strong eect on the accumulation of branched-chain
aliphatic proteinogenic amino acids (BCAA) such as leucine (Leu), isoleucine (Ile) and va-
line (Val) in the shoots (Figures 5A and 6). All three amino acids were signicantly accu-
mulated at higher concentrations correlating with increasing nitrate deciency. The pres-
ence of M. hyalina completely abolished this eect (Figures 5B and 6).
5 10
0
5,000
10,000
15,000
Control
Days
RFI
7 NO3-
0.25 NO3-
0 NO3-
**
***
***
***
****
****
****
****
****
***
5 10
0
5,000
10,000
15,000
M. hyalina
Days
RFI
7 NO3-
0.25 NO3-
0 NO3-
***
**
**
*
***
*** ****
*** ****
****
Figure 4.
Relative fluorescence intensity (RFI) (
A
) and confocal microscopy images (
B
) of roots of
Arabidopsis thaliana ProNRT2.4:GFP transgenic plants. (
A
) Two-week-old seedlings pre-grown on
full NO
3−
(7 mM NO
3−
) medium were further incubated on different NO
3−
media (N-free (0 mM
NO
3−
), N-low (0.25 mM NO
3−
) and N-complete (7 mM NO
3−
)) and were or were not co-cultivated
with M. hyalina for the indicated time. Two-way ANOVA with Dunnett’s multiple comparison test;
n = 8. M. hyalina: Mixed-effects model (REML) with Dunnett’s multiple comparison test; n = 4–8;
the error bars indicate standard error (SE); * p< 0.05; ** p< 0.01; *** p< 0.001; **** p< 0.0001; ns: not
significant. (
B
) Two-week-old seedlings pre-grown on full NO
3−
(7 mM NO
3−
) medium were further
incubated on N-complete (7 mM NO
3−
, left) or N-free (0 mM NO
3−
, right) for 2 d. STEDYCON
microscopy and STEDYCON processing software (exc. laser 488 nm, detector 526 nm). To compare
the fluorescence intensity in these variants, the raw images were aligned by photon count to the same
level in the two images.
Additional qPCR experiments supported the NRT2.4 gene induction over time under
nitrate deficiency in the Arabidopsis Col-0 roots (Figure S2A). A 12.2-fold increase of NRT2.4
transcripts was detected after 2 d on the media without nitrate and still a 3.4-fold increase
on 0.25 mM nitrate, all compared to the controls grown on 7 mM nitrate. After 10 d, NRT2.4
gene induction did not increase anymore and even decreased without nitrate, suggesting
an early but transient induction of this transporter (Figure S2A). Strikingly, an interesting
finding was that the expression of the high-affinity nitrate transporter NRT2.5 under nitrate
starvation was regulated differently. In contrast to NRT2.4, the NRT2.5 expression was
lower at d 2 than at d 10 (from 66.7- to 255-fold, respectively) on the media with and without
nitrate (Figure S2B).
2.5. Effect of Mortierella hyalina Colonization on Amino Acid Pools in Arabidopsis Plants under
Nitrogen Starvation
Since the first organic compounds that carry absorbed N are amino acids, compositions
and changes in the amino acid pools in the different Arabidopsis lines were analyzed
individually in both the shoots and roots, depending on the given nitrate level in the
medium, representing N starvation, and the presence/absence of M. hyalina (Figure 5).
Looking deeper into the amino acid results, it is interesting to note that in all Arabidopsis
lines, nitrate depletion had a particularly strong effect on the accumulation of branched-
chain aliphatic proteinogenic amino acids (BCAA) such as leucine (Leu), isoleucine (Ile)
and valine (Val) in the shoots (Figures 5A and 6). All three amino acids were significantly
accumulated at higher concentrations correlating with increasing nitrate deficiency. The
presence of M. hyalina completely abolished this effect (Figures 5B and 6).
Int. J. Mol. Sci. 2023,24, 16128 7 of 16
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 7 of 17
A B
Figure 5. Heat map of amino acid level in shoots and roots in A. thaliana WT and ko mutants co-
cultivated without (A) or with (B) Mortierella hyalina during NO3− starvation. Two-week-old seed-
lings pre-grown on full NO3− (7 mM NO3−) medium were further incubated on dierent NO3− media
(N-free, 0 mM NO3−; N-low, 0.25 mM NO3−; N-complete, 7 mM NO3−) and were not (A) or were co-
cultivated with M. hyalina (B). Amino acid proles were identied 10 d after treatment. Data are
given as the percentage of full NO3− (7 mM NO3−) medium; n = 5–6.
Figure 5.
Heat map of amino acid level in shoots and roots in A. thaliana WT and ko mutants
co-cultivated without (
A
) or with (
B
)Mortierella hyalina during NO
3−
starvation. Two-week-old
seedlings pre-grown on full NO
3−
(7 mM NO
3−
) medium were further incubated on different NO
3−
media (N-free, 0 mM NO
3−
; N-low, 0.25 mM NO
3−
; N-complete, 7 mM NO
3−
) and were not (
A
) or
were co-cultivated with M. hyalina (
B
). Amino acid profiles were identified 10 d after treatment. Data
are given as the percentage of full NO3−(7 mM NO3−) medium; n = 5–6.
Moreover, a principle component analysis (PCA) of the amino acid composition in the
roots revealed clear separation between the full medium (7 mM nitrate) on the one hand
and the media with low and no nitrate (0.25 mM and 0 mM), respectively, on the other hand
(Figure 7A). Here, the confidence areas (95%) of the data for no and low nitrate overlap
almost completely. A similar clustering as in the roots was found for the shoots. The PCA
of the amino acid composition in Arabidopsis roots and shoots that were and were not
colonized by M. hyalina showed a cluster representing colonized plants, which is almost a
sub-cluster of the non-colonized plants but clearly distinguishable (Figure 7B). This is even
more obvious in the shoots. Interestingly, in the shoots, the sub-cluster of colonized plants
also contains the non-colonized plants growing on 7 mM nitrate. This indicates that at least
in the shoots, the fungus supports the plants so that their amino acid level is similar to if
Int. J. Mol. Sci. 2023,24, 16128 8 of 16
they were growing on the full medium. Strikingly, the Arabidopsis lines (Col-0, nrt2.4-1,
nrt2.4-2) had no obvious impact. The two principal components, PC1 and PC2, explain in
the roots 81.6% and in the shoots 69.1% of all observed variances.
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 8 of 17
A
B
C
Figure 6. Branched-chain amino acids (BCAA) in shoots of A. thaliana Col-0 (A) and the ko mutants
nrt2.4-1 (B) and nrt 2.4-2 (C) co-cultivated without (left) or with (right) Mortierella hyalina during
NO3− starvation. Two-week-old seedlings pre-grown on full NO3− (7 mM NO3−) medium were fur-
ther incubated on dierent NO3− media (N-free, 0 mM NO3−; N-low, 0.25 mM NO3−; N-complete, 7
mM NO3−) and co-cultivated without (control) or with M. hyalina. Amino acids were measured 10 d
after treatment. Two-way ANOVA with Dunne’s multiple comparison test (data were transformed
when needed); n = 5–6; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns: not signicant.
Moreover, a principle component analysis (PCA) of the amino acid composition in
the roots revealed clear separation between the full medium (7 mM nitrate) on the one
hand and the media with low and no nitrate (0.25 mM and 0 mM), respectively, on the
other hand (Figure 7A). Here, the condence areas (95%) of the data for no and low nitrate
overlap almost completely. A similar clustering as in the roots was found for the shoots.
The PCA of the amino acid composition in Arabidopsis roots and shoots that were and
were not colonized by M. hyalina showed a cluster representing colonized plants, which
is almost a sub-cluster of the non-colonized plants but clearly distinguishable (Figure 7B).
This is even more obvious in the shoots. Interestingly, in the shoots, the sub-cluster of
colonized plants also contains the non-colonized plants growing on 7 mM nitrate. This
indicates that at least in the shoots, the fungus supports the plants so that their amino acid
level is similar to if they were growing on the full medium. Strikingly, the Arabidopsis
lines (Col-0, nrt2.4-1, nrt2.4-2) had no obvious impact. The two principal components, PC1
and PC2, explain in the roots 81.6% and in the shoots 69.1% of all observed variances.
Val Ile Leu
0
1000
2000
3000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
ns
✱✱
✱✱
✱✱✱✱
ns
✱✱
Val Ile Leu
0
1000
2000
3000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
ns
ns
ns
ns
ns
ns
Val Ile Leu
0
1000
2000
3000
4000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
✱✱✱ ✱✱✱✱
✱✱✱✱
✱✱✱✱
✱✱✱✱
✱✱
Val Ile Leu
0
500
3000
4000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
ns
ns
ns
ns
ns
ns
Val Ile Leu
0
1000
2000
3000
4000
5000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
ns
✱✱✱✱✱✱✱
✱✱
ns
✱✱✱✱
Val Ile Leu
0
1000
2000
3000
4000
5000
Amino acid, nmol/g FW
7 NO3-
0.25 NO3-
0 NO3-
ns
ns
ns
ns
ns
ns
Figure 6. Branched-chain amino acids (BCAA) in shoots of A. thaliana Col-0 (A) and the ko mutants
nrt2.4-1 (
B
) and nrt 2.4-2 (
C
) co-cultivated without (left) or with (right) Mortierella hyalina during
NO
3−
starvation. Two-week-old seedlings pre-grown on full NO
3−
(7 mM NO
3−
) medium were
further incubated on different NO
3−
media (N-free, 0 mM NO
3−
; N-low, 0.25 mM NO
3−
; N-complete,
7 mM NO
3−
) and co-cultivated without (control) or with M. hyalina. Amino acids were measured 10 d
after treatment. Two-way ANOVA with Dunnett’s multiple comparison test (data were transformed
when needed); n = 5–6; ** p< 0.01; *** p< 0.001; **** p< 0.0001; ns: not significant.
Int. J. Mol. Sci. 2023,24, 16128 9 of 16
Int. J. Mol. Sci. 2023, 24, x FOR PEER REVIEW 9 of 17
A
Roots Shoots
B
Roots Shoots
Figure 7. Principal component analyses (PCA) of amino acid compositions in Arabidopsis thaliana
roots and shoots without or with Mortierella hyalina colonization. (A) The PCA score plot distin-
guishes the amino acid proles of plants grown under dierent treatments of nitrate starvation (7
mM, 0.25 mM, no nitrate). (B) The PCA score plot distinguishes the amino acid proles of plants
grown without or with M. hyalina colonization. Two-week-old seedlings pre-grown on full NO3− (7
mM NO3−) medium were further incubated on dierent NO3− media (N-free, 0 mM NO3−; N-low,
0.25 mM NO3−; or N-complete, 7 mM NO3−) and were or were not co-cultivated with M. hyalina.
Amino acid proles were separately analyzed after 10 d. Open symbols, non-inoculated; closed
symbols, M. hyalina-inoculated. The ellipses represent the multivariate normal distribution.
3. Discussion
Fungal endophytes are an important component of the rhizosphere’s microbial com-
munities. Many of these fungi are dened as commensalistic, with no or yet unknown
functions in plants. However, some fungi have been shown to have negative (pathogens)
or positive (mutualists) eects on their host plants. Typically, Mortierella species have a
saprophytic lifestyle but they are also able to interact with and colonize many dierent
plant species [23]. Similar to mycorrhizal fungi, some Mortierella spp. are supposed to sup-
port phosphate uptake into the plants, which may stimulate the biomass production of
the host [29]. M. hyalina has been described as a benecial fungus, promoting the growth
of aerial plant tissues at least in the non-mycorrhizal plant Arabidopsis thaliana [24,25]. In
addition, M. hyalina conferred tolerance against Alternaria brassicae infection [24]. How-
ever, whether or not and how M. hyalina, as with other benecial fungi, can also rescue
plants from abiotic stress such as nutrient deciencies have not been studied so far. Thus,
Figure 7.
Principal component analyses (PCA) of amino acid compositions in Arabidopsis thaliana roots
and shoots without or with Mortierella hyalina colonization. (
A
) The PCA score plot distinguishes the
amino acid profiles of plants grown under different treatments of nitrate starvation (7 mM, 0.25 mM,
no nitrate). (
B
) The PCA score plot distinguishes the amino acid profiles of plants grown without
or with M. hyalina colonization. Two-week-old seedlings pre-grown on full NO
3−
(7 mM NO
3−
)
medium were further incubated on different NO
3−
media (N-free, 0 mM NO
3−
; N-low, 0.25 mM
NO
3−
; or N-complete, 7 mM NO
3−
) and were or were not co-cultivated with M. hyalina. Amino
acid profiles were separately analyzed after 10 d. Open symbols, non-inoculated; closed symbols,
M. hyalina-inoculated. The ellipses represent the multivariate normal distribution.
3. Discussion
Fungal endophytes are an important component of the rhizosphere’s microbial com-
munities. Many of these fungi are defined as commensalistic, with no or yet unknown
functions in plants. However, some fungi have been shown to have negative (pathogens)
or positive (mutualists) effects on their host plants. Typically, Mortierella species have a
saprophytic lifestyle but they are also able to interact with and colonize many different
plant species [
23
]. Similar to mycorrhizal fungi, some Mortierella spp. are supposed to
support phosphate uptake into the plants, which may stimulate the biomass production of
the host [
29
]. M. hyalina has been described as a beneficial fungus, promoting the growth
of aerial plant tissues at least in the non-mycorrhizal plant Arabidopsis thaliana [
24
,
25
]. In
addition, M. hyalina conferred tolerance against Alternaria brassicae infection [
24
]. However,
whether or not and how M. hyalina, as with other beneficial fungi, can also rescue plants
from abiotic stress such as nutrient deficiencies have not been studied so far. Thus, the
Int. J. Mol. Sci. 2023,24, 16128 10 of 16
effect of M. hyalina colonization on Arabidopsis plants with and without one component of
the HATS for nitrate (NRT2.4 vs. nrt2.4) facing N starvation stress was investigated.
3.1. Growth Analysis of Arabidopsis Plants with/without Mortierella hyalina Colonization under
Nitrogen Starvation
Growth analysis of Col-0 and the two NRT2.4 ko lines showed no phenotypic ab-
normalities in the mutants at an optimal NO
3−
supply, but did at low and no NO
3−
concentrations (Figure 1A). According to the published data, no such difference in shoot
growth has previously been found in the nrt2.4-1 and nrt2.4-2 lines [
28
]. However, the
growth conditions in that study were different; actually, plants grew for 32 d on 0.5 mM
NO
3−
in short d and without added sugar. Interestingly, the strong dependency on nitrate
concentration was not detected in the roots (Figure 1B), suggesting that the loss of function
of NRT2.4 could be compensated for by other nitrate uptake systems. Indeed, NRT2.5
was strongly induced in the nitrate-depleted plants, even with a different kinetics. While
under low and no nitrate conditions, the NRT2.4 gene was transiently but already highly
expressed after 2 d, and NRT2.5 was induced as well but with a different kinetics, i.e., much
higher after 10 d than after 2 d of nitrate deficiency (Figure S2). This can explain why the
NRT2.4 ko mutant plants can survive even with strong N deficiency (Figure 1) and show
similar growth compared with the wild type. Nevertheless, the presence of M. hyalina has
an additional impact on the plants’ growth. As shown in Figure 2, a promoting shoot over
root growth effect was detectable in all plant lines, most pronounced in the wild type Col-0.
Why this effect was neutralized under N deficiency is still not clear. It is conceivable that
the fungus competes with the plant for the limited nitrogen and cannot promote plant
growth anymore. However, even under such stress conditions, the fungus did not change
its non-pathogenic nature and, thus, did not harm the plant.
3.2. Mortierella hyalina Mitigates the Arabidopsis Defense Responses
In any fungal plant colonization event, there is some potential for fungal virulence
to facilitate infection, while host plant defenses can limit the development of fungal dis-
eases [
30
]. A successful endophyte–host interaction involves a balance of the protagonists,
regardless of the infected plant organ. In order to keep this balance and to establish a
mutualistic relationship, the plant should not attack beneficial endophytic fungi during
colonization. Thus, suppression of root immunity forms an important and very likely
necessary background in the formation of plant-associated microorganisms’ communi-
ties [
31
]. In the interaction of M. hyalina with Arabidopsis roots, former studies described
an increase of jasmonates after 1 d of co-cultivation, indicating that the plants initiated
defense responses [
32
]. This increase appeared to be restricted to the early phase of in-
teraction. Such a jasmonate accumulation was not detected in the present experiments,
where jasmonates were measured after 10 days of colonization (Figure 3), confirming the
former results in M. hyalina-colonized plants at later time points [
24
]. Moreover, even the N
starvation-induced increase in jasmonates in the different Arabidopsis lines was reduced
by the presence of the fungus (Figure 3). No M. hyalina-induced SA increase or even impact
on the SA level was observed, neither in the former nor in this study. These results suggest
that the fungus is able to mitigate the defense response and/or is accepted as a symbiotic
partner by the host plant. Overall, fungal colonization initiated only a very weak defense
response, while the abiotic N starvation stress increased the stress-related phytohormone
levels, which the presence of M. hyalina attenuated mostly. This strengthens the view of a
beneficial interaction between this fungus and the host plant.
3.3. Mortierella hyalina Does Not Modulate the NRT2.4 Induction in Arabidopsis under
Nitrogen Starvation
The working hypothesis was that the presence of the fungus might support the plant
in taking up NO
3−
from the N-deficient media and, as a consequence, the well-known
N-starvation-induced expression of NRT2.4 [
6
,
12
] was not necessary. However, our data did
not support this hypothesis, as shown in Figure 4. In the presence or absence of M. hyalina,
Int. J. Mol. Sci. 2023,24, 16128 11 of 16
the GFP under the control of the NRT2.4 promotor was expressed under N deficiency at
almost the same level with similar kinetics (Figure 4A). Nevertheless, the fungus clearly
supplied the plants under N starvation with nitrogen; the less N was available in the
medium, the more N was provided from the fungal stores (Table 1). Obviously, the N
starvation sensory system of the plant did not recognize the fungal N supply very well,
which might be due to the chemistry of the N, that is, whether it is inorganic or in a bound
organic form, such as in amino acids.
3.4. Mortierella hyalina Restored the Amino Acid Homeostasis in Arabidopsis under
Nitrogen Starvation
After uptake, nitrate is reduced into nitrite and ammonium (NO
3−→
NO
2−→
NH
4+
),
while NH
4+
is further incorporated into the amino acid glutamate, forming glutamine, the
first organic compound that carries the nitrate-derived nitrogen. The enzyme glutamine
synthetase mediates this reaction. Subsequently, many different aminotransferases dis-
tribute the amino group within the various amino acids and later into the whole plant
metabolism. Thus, it was interesting to analyze the amino acid profile in the plants with
and without fungal colonization as also postulated for interactions with beneficial bac-
teria [
33
]. The results obtained show clearly the N starvation effect on the Arabidopsis
plants. Compared to the amino acid composition in plants growing without NO
3−
stress,
Col-0 as well as the ko mutant lines showed strong inconsistent changes in the amino acid
levels, some of which accumulate to much higher levels (Val, Ile, Leu, His, Tyr, Trp, Lys),
while others were strongly reduced (Arg, Asn, Gln) (Figure 5A). These effects were much
more pronounced in the shoots compared with the roots. A corresponding PCA analysis
that distinguished the amino acid profiles of plants grown under different levels of nitrate
starvation (7 mM, 0.25 mM, no nitrate) indicated that in both the roots and shoots, all plants
grown with full nitrate cluster together, as well as plants from low and no nitrate media
(Figure 7A). The genetic background (WT, nrt2.4-1,nrt2.4-2) was less important than the
nitrate concentration. Strikingly, when distinguishing between the amino acid profiles
of plants grown with/without M. hyalina colonization, it became clear that in the roots,
the colonized plants cluster together. In the shoots, colonized plants cluster together with
most non-colonized Arabidopsis plants grown on a full nitrate medium, again indepen-
dent of the genotype (Figure 7B). Based on these results, one can conclude that M. hyalina
manipulates Arabidopsis so that even plants grown under N starvation gained an amino
acid profile comparable with unstressed plants. The fungus restored the plants’ disturbed
amino acid homeostasis to normal. It is still not known how this works mechanistically,
whether or not the fungus provides selectively certain amino acids or has an impact on
protein degradation and amino acid synthesis. These questions need to be addressed in
further studies.
It is interesting to note that low nitrate stress had a clear effect on the accumulation
of BCAA such as Leu, Ile and Val in shoots (Figures 5A and 6). This finding has been
mentioned before for Arabidopsis seedlings by Huang and Jander (2017) [
34
], who also
described this phenomenon in response to drought, salt and osmotic stress, as well as
herbicide treatment. In Arabidopsis, BCAA accumulation is primarily the result of protein
degradation [
34
]. However, because neither ABA (Figure 3) nor proline levels (Figure 5)
changed significantly upon N starvation, an osmotic stress response that also can induce
BCAA accumulation must be excluded. The breakdown of amino acids produces interme-
diates or precursors of the tricarboxylic acid cycle (Acetyl-CoA) and thus contributes to the
production of substrates for mitochondrial respiration. The oxidation of BCAA provides an
amount of energy for ATP synthesis that is comparable to that provided by glucose [
35
].
The same holds true for lysine, which is also enriched under N starvation. Obvious is the
tissue specificity for BCAA and lysine accumulation in the shoots rather than in the roots in
all Arabidopsis lines and that the presence of M. hyalina largely eliminated the N starvation
effect (Figure 5). However, to find out whether the N-starvation-induced increases in
BCAA have a physiological function or are merely an artefact of protein degradation, more
Int. J. Mol. Sci. 2023,24, 16128 12 of 16
research needs to be pursued. In any case, upon protein degradation, the released amino
acids are subsequently recycled and allocated for the biosynthesis of proteins required
under nutrient limitation. The exact sensing of amino acid levels seems to be a key point
for any efficient regulation of protein and amino acid metabolism. Thus, the regulation of
amino acid content, flux and transport within the plant are critical for plant adaptation to
nutrient status, as well as for development and stress responses.
As long as the molecular mechanisms underlying the coordination between plant
growth and N metabolism are still not fully understood, significant improvement in con-
trolled use of beneficial fungi is limited.
4. Materials and Methods
4.1. Plant Materials and Growth Condition
Different lines of Arabidopsis thaliana seeds were used: wild-type (ecotype Columbia-0)
and transgenic line carrying the reporter construct ProNRT2.4:GFP [
12
]. Line nrt2.4-1 was
derived from a T-DNA–mutagenized population of the Col-0 accession [
12
,
36
], and nrt2.4-2
(the SAIL line CS872100) was also derived from a T-DNA mutagenized population of the
Col-0 accession [12,37].
A. thaliana seeds were surface-sterilized using 25% (v/v) sodium hypochloride (Acros
Organics
™
, Bremen, Germany) and 0.1% of Triton X-100 (Sigma-Aldrich, Taufkirchen,
Germany) for 8 min, rinsed seven times with sterile water and grown on square plates
(120
×
120
×
16 mm) (Thermo Fisher Scientific, Dreieich, Germany) (12–15 seedlings per
plate) containing MGRL medium (Table S1). The seeds were stratified for 48 h at 4
◦
C. The
plants were incubated for 14 d in a growth chamber in vertical position under long-day
conditions (16 h light/8 h dark) and a light intensity of 100
µ
mol photons m
−2
s
−1
, at 22
◦
C.
For the different NO
3−
concentrations, the A. thaliana seedlings (6 per plate) were
transferred for 10 d onto MGRL N-free (0 mM NO
3−
), N-low (0.25 mM NO
3−
) and N-
complete (7 mM NO
3−
) media, (1% sucrose, 0.5% Gelrite, pH 5.8) supplemented with KCl
and CaCl2·2H2O in an appropriate quantity to support ion balance (Table S1).
These seedlings were further used for the different experiments without/with M. hyalina
colonization. The control/fungal plugs (0.5 cm) were placed at a 0.5 cm distance from
the plant root tips (Figure S1). Plants were harvested in threes in each vial (roots and
shoots separately) and weighed. At least 18 seedlings from each treatment were taken. The
samples were frozen immediately in liquid N, and stored at −80 ◦C for RNA preparation,
and amino acid and phytohormone analysis. Only uniformly grown seedlings were used.
4.2. Phenotypic Analysis of Arabidopsis
Different lines of A. thaliana plants, after examining their growth phenotype on a
NO
3−
-complete medium, were photographed on days 6 and 10 using a Samsung Galaxy
A52 (Samsung Electronics Co., Seoul, Republic of Korea). The images were processed using
Adobe Photoshop CS. The seedling root length (main and laterals) was measured using the
Fiji ImageJ-2.9.0 Analysis software.
4.3. Mortierella hyalina Cultivation
The M. hyalina (FSU-509) strains were obtained from the Jena Microbial Resource
Collection (Jena, Germany). The M. hyalina was cultured and maintained on Potato Dextrose
Agar (PDA) medium (Sigma-Aldrich, Taufkirchen, Germany), at a pH of 5.6. Fungal plugs
were transferred to the center of the PDA plates and incubated at 22–24 ◦C in the dark for
3 weeks in a growth chamber. as described by Johnson et al. (2019) [24].
To analyze whether M. hyalina can directly transfer N to the plant, it was labeled with
15
N before co-culture with Arabidopsis. A modified KM medium without N-containing
components (20 g/L dextrose, 50 mL/L macronutrients, 10 mL/L micronutrients and
1 mL/L Fe-EDTA, 1 mL/L vitamin mix, pH 6.5) was prepared and supplemented with
10 g/L ISOGRO
®
-
15
N (CortecNet, Les Ulis, France) according to the manufacturers’ proto-
col. M. hyalina plugs of 2 mm diameter were incubated (23
◦
C, 50 rpm, dark) in 2 mL of
Int. J. Mol. Sci. 2023,24, 16128 13 of 16
KM
ISOGRO
for 10 days in Greiner CELLSTAR
®®
12-well plates (Greiner Bio-One, Fricken-
hausen, Germany) sealed with 3MTM Micropore tape.
4.4. RNA Preparation and Expression Analysis
The total RNA (2.5
µ
g) was extracted using TRIzol, according to the manufacturer’s
method, followed by additional chloroform isolation and isopropanol precipitation steps,
digested to prevent DNA contamination using the TURBO DNA-free
TM
KIT (Life Tech-
nologies, Carlsbad, CA, USA) and cleaned using the RNA Clean and Concentrator
™
KIT
(
™
trademarks of Zymo Research Corporation, Irvine, CA, USA). The cDNA (20
µ
L) was
synthesized using an Thermo Fisher Scientific RevertAid First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, Dreieich, Germany), according to the manufacturers’ instructions.
The qPCR analysis was performed using a Bio-Rad CFX96
TM
Real-Time System (Bio-Rad
Laboratories Inc., Hercules, CA, USA) using the appropriate pairs for A. thaliana-specific
primers (Supplementary, Table S2). The reaction components per 20
µ
L were as follows:
6.5
µ
L H
2
O, 12.5
µ
L Brilliant II SYBR Green qPCR Master Mix (Agilent Technologies, Santa
Clara, CA, USA), 1
µ
L 10
µ
M of each primer and 1
µ
L cDNA. The thermal cycling program
was as follows: initial denaturation at 95
◦
C for 180 s, and 44 cycles at 95
◦
C for 30 s, 60
◦
C
for 30 s and 72
◦
C for 30 s. AtActin 2 (AT3G18780) was used as an internal reference gene.
The relative quantification of the gene expression was evaluated using the delta–delta
Ct method according to Pfaffl (2001) [
38
]. Three biological replicates and three technical
replicates were performed for each analysis.
4.5. Extraction and Quantification of Amino Acids Using LC–MS/MS
The plant material was homogenized in a Geno/Grinder
®®
2010 (Spex Sample Prep,
Stanmore, UK) equipped with aluminum racks. The racks were cooled in liquid nitrogen
before being used to prevent the thawing of the plant material throughout the homogeniza-
tion process. The amino acids were extracted twice with a total of 2 mL of methanol on
ice. The supernatants were combined and dried using a Concentrator plus (Eppendorf,
Hamburg, Germany) and re-suspended in 500
µ
L of methanol. The extract was diluted
1:10 (v/v) with water containing the
13
C,
15
N-labeled amino acid mix (Isotec, Miamisburg,
OH, USA) as the internal standard. The amino acids in the diluted extracts were directly
analyzed using LC–MS/MS as described in [
39
] using a QTRAP 6500 mass spectrometer
(Sciex, Darmstadt, Germany) coupled to an Agilent 1260 series HPLC system.
4.6. Extraction and Quantification of Phytohormones Using LC–MS/MS
The extraction procedure and phytohormone determination was carried out according
to Müller et al. (2022) [
39
]. The tissue was extracted and homogenized in 1.5 mL methanol
containing 60 ng D4-SA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 60 ng D6-JA
(HPC Standards GmbH, Borsdorf, Germany), 60 ng D6-ABA (Toronto Research Chemicals,
Toronto, ON, Canada) and 12 ng D6-JA-Ile (HPC Standards GmbH, Borsdorf, Germany) as
the internal standards. Phytohormone analysis was performed using LC–MS/MS on an
Agilent 1260 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled to
a tandem mass spectrometer QTRAP 6500 (SCIEX, Darmstadt, Germany).
4.7. Analysis of Gene Expression in GFP Reporter Lines
Fluorescence microscopy of the GFP (green fluorescent protein) signals was optimized
for live cells and detected in the roots at 10 d after transfer on a N-depleted medium after 24,
48 h and further on every 48 h. For the visualization of the GFP, images were acquired using
a Zeiss AXIO Zoom.V16 (ZEISS, Oberkochen, Germany) equipped with a 0.5
×
PlanApoZ
Objective (ZEISS, Oberkochen, Germany), an HXP120 mercury vapor lamp and a filter
set 38 HE (excitation filter BP 450–490 nm, FT 495 nm, emission filter BP 500–550 nm).
The signal intensities after treatment were measured using the Fiji ImageJ-2.9.0 Analysis
software. The images were converted into 8-bit format and processed using Fiji’s “Analyze
Particles” plugin. The average fluorescence intensity was measured in the cells of the apical
Int. J. Mol. Sci. 2023,24, 16128 14 of 16
lateral roots. For the measurement, 10 randomly selected fluorescent points in the form of
a square of four pixels for each plant were used. Confocal images were captured using a
STEDYCON imaging system (Abberior Instruments, Göttingen, Germany) on the 2nd d of
N starvation. Excitation was evoked using a 488 nm laser diode and the detection range
was around 526 nm. The final pixel size was 100 nm.
4.8. 15N Labeling Experiment
Labeled with
15
N, after 10 d of growth, the fungal tissue was separated from the
remaining medium and carefully washed 3 times with a N-free liquid MGRL medium to
remove the
15
N bound to the hyphal surface. The fungus was cut into 0.5 cm plugs and
transferred into the MGRL (N-free, N-low and N-complete) plates for co-cultivation. To
minimize the
15
N uptake by the plant from the dead fungal material due to the washing
and handling, the fungal plugs were placed at a minimum of 1 cm distance from the roots.
Under these conditions, contact between the two organisms required the growth of hyphae
toward the roots. Co-cultivation was performed for 10 d.
4.9. Isotope Analysis
Homogenous dry leaf powder (2–3 mg) was weighted in a tin capsule.
δ15
N isotope
analyses were conducted using an elemental analyzer (NA1110, CE Instruments, Milan,
Italy) coupled to a Delta+XL isotope ratio mass spectrometer (Thermo Finnigan, Bremen,
Germany) via a ConFlo III. The sample element amounts were scaled against an in-house
standard “Ali-j3” (Acetanilide) with
δ15
N values of
−
1.51
±
0.1
‰
on the
δ15
N AIR-N2
scales. “caf-j3” A (caffeine) sample was analyzed as a quality control with values of
−
15.46
±
0.1
‰
on the
δ15
N AIR-N2 scales [
33
]. Linearity, blank and drift corrections were
undertaken for each sequence according to Werner and Brand (2001) [40].
4.10. Statistical Analysis
All the experiments were performed in accordance with the relevant guidelines and
regulations. Independent experiments were treated as a completely randomized design.
Figures were plotted using GraphPad Prism software version 9.0. The datasets of amino
acids and phytohormones analyses were subjected to analysis using RStudio version
1.1.463 with R version 3.4.4. (R Development CoreTeam, 2018). Statistically significant
differences were calculated using one- and two-way analysis of variance, with Dunnett’s
multiple comparison test and Tukey’s post hoc test and a mixed-effects model (REML) with
Dunnett’s multiple comparison test, with p< 0.05 as the threshold for significance.
5. Conclusions
Beneficial fungi can often mitigate abiotic-stress-induced physiological responses in
their host plants. However, the underlying molecular mechanisms are largely unknown. In
the present study, the role of the beneficial fungus Mortierella hyalina on Arabidopsis thaliana
plants exposed to N starvation stress was investigated. One focus was on the hypothesis
that fungal infection could alleviate N starvation stress by affecting the inducible high-
affinity nitrogen transporter NRT2.4. This hypothesis could not be confirmed. Nevertheless,
the results obtained show that the fungus has a positive effect on the plants. On the one
hand, it is not recognized as a pathogen and the plant does not have to invest in the
appropriate defense mechanisms but can continue to manage the nitrogen deficiency. On
the other hand, it becomes clear that the fungus influences the amino acid metabolism and
can restore the amino acid homeostasis disturbed by N starvation stress. This exemplifies
how a beneficial fungus can support a plant under stress conditions and benefit from the
symbiosis itself in the longer term. It also shows the potential of such interactions and
possible mechanisms of how beneficial fungi can alter the metabolism of their host plants
to mitigate stress symptoms and keep the plants alive.
Int. J. Mol. Sci. 2023,24, 16128 15 of 16
Supplementary Materials:
The following supporting information can be downloaded at https://www.
mdpi.com/article/10.3390/ijms242216128/s1.
Author Contributions: Conceptualization, N.S. and A.M. (Axel Mithöfer); methodology, N.S., S.S.S.
and A.M. (Axel Mithöfer); investigation, N.S., A.M. (Anindya Majumder) and M.R.; data curation
and statistical analysis, A.M. (Axel Mithöfer), L.Z. and V.G.; writing—original draft preparation, A.M.
(Axel Mithöfer) and N.S.; writing—review and editing, A.M. (Axel Mithöfer); visualization, N.S. and
L.Z.; supervision, A.M. (Axel Mithöfer), R.O. and A.K.; project administration, A.M. (Axel Mithöfer);
funding acquisition, A.M. (Axel Mithöfer). All authors have read and agreed to the published version
of the manuscript.
Funding:
This work was supported by the VolkswagenStiftung (Funding for Refugee Scholars and
Scientists from Ukraine (Gastforschungsprogramm für geflohene ukrainische Wissenschaftlerinnen
und Wissenschaftler; Förderantrag: A139316)).
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement:
The data supporting the findings of this study are available on request
from the corresponding author.
Acknowledgments:
We thank Heiko Moossen (MPI for Biogeochemistry, Jena) for the
15
N level
measurement, Andrea Lehr (MPI for Chemical Ecology, Jena) for technical support and the MPI
greenhouse team for growing the plants. We moreover thank the VolkswagenStiftung for supporting
N. Svietlova with a fellowship and the International Max Planck Research School (IMPRS) for
supporting Y. Zhyr. Open Access funding provided by the Max Planck Society.
Conflicts of Interest: The authors declare no conflict of interest.
References
1.
Krouk, G.; Crawford, N.M.; Coruzzi, G.M.; Tsay, Y.F. Nitrate signaling: Adaptation to fluctuating environments. Curr. Opin. Plant
Biol. 2010,13, 265–272. [CrossRef] [PubMed]
2.
Vidal, E.A.; Moyano, T.C.; Canales, J.; Gutiérrez, R.A. Nitrogen control of developmental phase transitions in Arabidopsis thaliana.
J. Exp. Bot. 2014,65, 5611–5618. [CrossRef] [PubMed]
3.
Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot.
2007
,58, 2297–2306. [CrossRef]
4.
Dechorgnat, J.; Nguyen, C.T.; Armengaud, P.; Jossier, M.; Diatloff, E.; Filleur, S.; Daniel-Vedele, F. From the soil to the seeds: The
long journey of nitrate in plants. J. Exp. Bot. 2011,62, 1349–1359. [CrossRef]
5.
Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci.
2012
,17, 458–467. [CrossRef]
[PubMed]
6.
Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: Responses to nitrate
provision. Plant Cell Physiol. 2003,44, 304–317. [CrossRef]
7.
Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl.
Biol. 2013,163, 174–199. [CrossRef]
8.
Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot.
2017,68, 2463–2475. [CrossRef]
9.
O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutierrez, R.A. Nitrate transport, sensing, and responses
in plants. Mol. Plant 2016,9, 837–856. [CrossRef]
10. Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino acid transporters in plants: Identification and function. Plants 2020,9, 972. [CrossRef]
11.
Liu, H.; Liu, Q.; Gao, X.; Fu, X. Role of nitrogen sensing and its interactive signaling pathways in shaping root system architecture.
Front. Agric. Sci. Eng. 2022,9, 316–332. [CrossRef]
12.
Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Bréhaut, V.; Miller, A.; Daniel-Vedele, F.;
Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2. 4 plays a double role in roots and shoots of nitrogen-starved
plants. Plant Cell 2012,24, 245–258. [CrossRef] [PubMed]
13.
Xuan, W.; Beeckman, T.; Xu, G. Plant nitrogen nutrition: Sensing and signaling. Curr. Opin. Plant Biol.
2017
,39, 57–65. [CrossRef]
[PubMed]
14. Gent, L.; Forde, B.G. How do plants sense their nitrogen status? J. Exp. Bot. 2017,68, 2531–2539. [CrossRef]
15.
Chellamuthu, V.R.; Ermilova, E.; Lapina, T.; Lüddecke, J.; Minaeva, E.; Herrmann, C.; Hartmann, M.D.; Forchhammer, K. A
widespread glutamine-sensing mechanism in the plant kingdom. Cell 2014,159, 1188–1199. [CrossRef] [PubMed]
16.
Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary
metabolites. Mycol. Res. 2002,106, 996–1004. [CrossRef]
17. Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002,154, 275–304. [CrossRef]
Int. J. Mol. Sci. 2023,24, 16128 16 of 16
18.
Furbino, L.E.; Godinho, V.M.; Santiago, I.F.; Pellizari, F.M.; Alves, T.M.; Zani, C.L.; Junior, P.A.; Romanha, A.J.; Carvalho, A.G.; Gil,
L.H.; et al. Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae
across the Antarctic Peninsula. Microb. Ecol. 2014,67, 775–787. [CrossRef] [PubMed]
19.
Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic fungi promote plant growth and
mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus.J. Plant Interact.
2015
,10,
280–287. [CrossRef]
20.
Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë fungal endophytes and plant defenses: Not just
alkaloids. Trends Plant Sci. 2017,22, 939–948. [CrossRef]
21.
Jaber, L.R. Seed inoculation with endophytic fungal entomopathogens promotes plant growth and reduces crown and root rot
(CRR) caused by Fusarium culmorum in wheat. Planta 2018,248, 1525–1535. [CrossRef] [PubMed]
22.
Zhang, K.; Bonito, G.; Hsu, C.M.; Hameed, K.; Vilgalys, R.; Liao, H.L. Mortierella elongata increases plant biomass among
non-leguminous crop species. Agronomy 2020,10, 754. [CrossRef]
23.
Ozimek, E.; Hanaka, A. Mortierella species as the Plant Growth-Promoting Fungi present in the agricultural soils. Agriculture
2021
,
11, 7. [CrossRef]
24.
Johnson, J.M.; Ludwig, A.; Furch, A.; Mithöfer, A.; Scholz, S.S.; Reichelt, M.; Oelmüller, R. The beneficial root-colonizing fungus
Mortierella hyalina promotes the aerial growth of Arabidopsis and activates calcium-dependent responses that restrict Alternaria
brassicae-induced disease development in roots. Mol. Plant-Microbe Interact. 2019,32, 351–363. [CrossRef] [PubMed]
25.
Tseng, Y.H.; Bartram, S.; Reichelt, M.; Scholz, S.S.; Meents, A.K.; Ludwig, A.; Mithöfer, A.; Oelmüller, R. Tris(methylthio)methane
produced by Mortierella hyalina affects sulfur homeostasis in Arabidopsis. Sci. Rep. 2022,12, 14202. [CrossRef]
26. Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi,
A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycology
2016
,108,
1028–1046. [CrossRef]
27.
Tedersoo, L.; Mikryukov, V.; Zizka, A.; Bahram, M.; Hagh-Doust, N.; Anslan, S.; Prylutskyi, O.; Delgado-Baquerizo, M.; Maestre,
F.; Pärn, J.; et al. Global patterns in endemicity and vulnerability of soil fungi. Glob. Chang. Biol. 2022,28, 6696–6710. [CrossRef]
28.
Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Vedele, H.D.; Krapp, A.
The Arabidopsis nitrate transporter NRT 2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants.
Plant J. 2014,80, 230–241. [CrossRef]
29.
Wang, L.; Chen, W.; Feng, Y.; Ren, Y.; Gu, Z.; Chen, H.; Wang, H.; Thomas, M.J.; Zhang, B.; Berquin, I.M.; et al. Genome
characterization of the oleaginous fungus Mortierella alpina.PLoS ONE 2011,6, e28319. [CrossRef]
30. Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005,109, 661–686. [CrossRef]
31.
Yu, K.; Pieterse, C.M.; Bakker, P.A.; Berendsen, R.L. Beneficial microbes going underground of root immunity. Plant Cell Environ.
2019,42, 2860–2870. [CrossRef] [PubMed]
32.
Meents, A.K.; Furch, A.C.; Almeida-Trapp, M.; Özyürek, S.; Scholz, S.S.; Kirbis, A.; Lenser, T.; Theißen, G.; Grabe, V.; Hansson, B.;
et al. Beneficial and pathogenic Arabidopsis root-interacting fungi differently affect auxin levels and responsive genes during
early infection. Front. Microbiol. 2019,10, 380. [CrossRef]
33.
Sanow, S.; Kuang, W.; Schaaf, G.; Huesgen, P.; Schurr, U.; Roessner, U.; Watt, M.; Arsova, B. Molecular mechanismsof Pseudomonas-
assisted plant nitrogen uptake: Opportunities for modern agriculture. Mol. Plant-Microbe Interact. 2023,36, 536–548. [CrossRef]
34.
Huang, T.; Jander, G. Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branched-chain
amino acids in Arabidopsis thaliana.Planta 2017,246, 737–747. [CrossRef] [PubMed]
35.
Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant
2015
,8, 1563–1579.
[CrossRef] [PubMed]
36.
Forsbach, A.; Schubert, D.; Lechtenberg, B.; Gils, M.; Schmidt, R. A comprehensive characterization of single-copy T-DNA
insertions in the Arabidopsis thaliana genome. Plant Mol. Biol. 2003,52, 161–176. [CrossRef] [PubMed]
37.
Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.;
et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana.Science 2003,301, 653–657. [CrossRef] [PubMed]
38.
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res.
2001
,29, e45. [CrossRef]
39.
Müller, A.T.; Reichelt, M.; Cosio, E.G.; Salinas, N.; Nina, A.; Wang, D.; Moossen, H.; Geilmann, H.; Gershenzon, J.; Köllner, T.G.;
et al. Combined–omics framework reveals how ant symbionts benefit the Neotropical ant-plant Tococa quadrialata at different
levels. iScience 2022,25, 105261. [CrossRef] [PubMed]
40.
Werner, R.A.; Brand, W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom.
2001,15, 501–519. [CrossRef]
Disclaimer/Publisher’s Note:
The statements, opinions and data contained in all publications are solely those of the individual
author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to
people or property resulting from any ideas, methods, instructions or products referred to in the content.