Available via license: CC BY-NC-ND 4.0
Content may be subject to copyright.
SHORT COMMUNICATION
Coprinopsis austrophlyctidospora sp. nov., an agaric ammonia
fungus from Southern Hemisphere plantations and natural forests
Toshimitsu Fukiharu •Neale L. Bougher •
Peter K. Buchanan •Akira Suzuki •
Chihiro Tanaka •Naohiko Sagara
Received: 19 January 2010 / Accepted: 28 September 2010 / Published online: 19 November 2010
ÓThe Mycological Society of Japan and Springer 2010
Abstract A new ammonia fungus, Coprinopsis austro-
phlyctidospora, is described from Nothofagus and Pinus
forests in New Zealand and from Eucalyptus forest in
Australia. In ecology and macro-morphology, this species
is similar to the Northern Hemisphere species C. phlyctido-
spora, but the new species differs in morphological char-
acters of the basidiospore, i.e., in having a plage, more
minute surface warts, and the smaller size of the
basidiospore.
Keywords Agaricales Australia Coprinus
phlyctidosporus New Zealand
Application of urea to soil stimulates the occurrence of an
ecological group of fungi called ‘ammonia fungi’ (Sagara
1975,1992). In the course of our study of ammonia fungi, we
investigated the ammonia fungi of the Southern Hemisphere
during 1991–2009. From Nothofagus and Pinus forests
in New Zealand and from Eucalyptus forest in Australia,
Coprinopsis phlyctidospora (Romagn.) Redhead, Vilgalys
& Moncalvo (Syn.: Coprinus phlyctidosporus Romagn.)
sensu lato was collected in each forest following urea
treatment (Suzuki et al. 2002). Coprinopsis phlyctidospora
sensu lato from The Netherlands, Japan, New Zealand, and
Australia was segregated into two distinct taxa, the Northern
Hemisphere group and Southern Hemisphere group, based
on nucleotide sequences of their ITS regions and on the
results of mating tests (Suzuki et al. 2002), but morpho-
logical differences were not elucidated. We have therefore
examined the macro- and microscopic characters of both
taxa to determine the taxonomic position of the Southern
Hemisphere group and to describe it as a new species.
Field observations were conducted in three forests: Not-
hofagus menziesii (Hook.f.) Oerst. and Nothofagus fusca
(Hook.f.) Oerst. dominated forests in Kaimanawa State For-
est Park, Taupo, North Island, New Zealand (NZ); a Pinus
radiata D.Don planted forest inRiverhead,North Island,NZ;
and a Eucalyptus marginata Donn ex Smith and Corymbia
calophylla (Lindl.) K. D. Hill & L. A. S. Johnson dominated
forest in Dwellingup, near Perth, Western Australia, Aus-
tralia (Table 1, Suzuki et al. 2002). In the field experiments,
urea (granular fertilizer; 46% nitrogen) was applied to the
ground surface in each forest at the rate per square meter of
800 g (in Australia), 696 g (in Taupo, NZ), and 348 g (in
Riverhead, NZ), to stimulate fruiting of ammonia fungi. In
the laboratory, urea (aqueous fertilizer; 20 mg N/g dry
weight soil) was applied to soil and incubated at 20°C under a
12 h light/12 h dark lighting regime. Humidity was sufficient
T. Fukiharu (&)
Natural History Museum and Institute, Chiba,
Aoba-cho 955-2, Chiba 260-8682, Japan
e-mail: fukiharu@chiba-muse.or.jp
N. L. Bougher
Department of Environment and Conservation,
Western Australian Herbarium, Bentley Delivery Centre,
Perth, WA 6983, Australia
P. K. Buchanan
Landcare Research, Auckland, New Zealand
A. Suzuki
Faculty of Education, Chiba University, Chiba, Japan
C. Tanaka
Laboratory of Environmental Mycoscience,
Graduate School of Agriculture,
Kyoto University, Kyoto, Japan
N. Sagara
230-180 Nagatani-cho, Iwakura, Sakyo-ku,
Kyoto 606-0026, Japan
123
Mycoscience (2011) 52:137–142
DOI 10.1007/s10267-010-0077-0
to support fungal fruiting. Basidiomata observed in the field
and in the laboratory were collected, and cultures were iso-
lated from each basidioma. Cultures were grown at 25°C
under a 12 h light/12 h dark lighting regime on MYC med-
ium (Malt extract, Yeast extract, and Casamino acid; DIFCO)
for about 2–3 weeks. Anatomical observations and mea-
surements were made on material mounted in 25% aqueous
ammonia. For scanning electron microscope (SEM) obser-
vation of basidiospore ornamentation, a small portion of a
dried specimen was rehydrated in 25% aqueous ammonia,
fixed in osmium acid, coated with platinum-palladium in an
ion sputter-coater (Hitachi E-1030; Hitachi, Tokyo Japan),
and observed under a SEM (Hitachi S-800) operating at
15.0 kV. All descriptions of macro- and microscopic char-
acters were obtained from cultivated basidiomata, with
measurements of basidiospores from spore prints. Color
notation used in the species description is according to
Kornerup and Wanscher (1978). Specimens examined are
deposited in the New Zealand Fungal Herbarium (PDD),
Western Australian Herbarium (PERTH), and Natural
History Museum and Institute, Chiba (CBM).
Coprinopsis austrophlyctidospora Fukiharu, sp. nov.
Figs. 1–9
MycoBank no.: MB 518948
Pileo primo 5–10 mm lato 6–12 mm alto, usque ad
15–20 mm lato, ovato-campanulato, demum in margine
lacerato revolutoque, radiatim sulcato, candido vel eburneo,
deinde cinerascenti, primo squamis albis radiatim hirsuto-
fibrillosis squarrosis interdum recurvis ubique tecto, dein
fere glabro, interdum primo cortinato; carne tenuissima,
fragilissima, alba; sapore miti; odore nullo; lamellis liberis,
comfertis, angustis (1–2 mm), albis, deinde atratis, deli-
quescentibus; stipite 50–100 mm longo, 1–3 mm crasso,
aequali vel sursum leviter attenuato, basi leviter incrassa-
tulo, cavo, candido, fragilissimo, primo squamis albis fi-
brillosis squarrosis ubique tecto, dein glabro; basidiosporis
in cumulo atratis, sub microscopio atro-brunneis vel rufo-
brunneis, ovoideis vel ellipsoideis, 6.5–7.5 95.1–6.5
(frontali) 95.1–6.2 (laterali) lm, verrucosis, poro germi-
nationis centrali 0.7–0.8 lm lato, cum plagiis; basidiis
14–25 96–7.5 lm, tetrasporis; pleurocystidiis 65–80 9
40–50 lm, subellipsoideis vel obovatis, hyalinis, tenuitu-
nicatis; cheilocystidiis 35–50 925–30 lm, subellipsoideis
vel obovatis, hyalinis, tenuitunicatis; velo pilei ex hyphis
tenuitunicatis, divaricatis, hyalinis, 50–150 94–6 lm
composito; fibulis praesentibus.
Holotypus: New Zealand, leg. T. Fukiharu, in Herbario
Fungorum Novae Zelandiae conservatus (PDD 76873).
Etymology: The Latin austro- (Southern) refers to the
Southern Hemisphere origin of this species, and
Table 1 Study sites, specimens and cultures of Coprinopsis austrophlyctidospora
Locality of collection Vegetation Plot size (m) Urea application
date
Treatment
b
Isolation and
collection date
Specimen no. Isolate no.
a
Australia Dwellingup, near Perth,
Western Australia
Eucalyptus marginata,
Corymbia calophylla
dominated forest
192 8 May 1997 Field (800 g) 14 July 1997 FB-24556 (CBM) CHU3026
192 18 July 1996 Field (200–800 g) 19 September 1996 PERTH 07598351
PERTH 07599137
New Zealand Kaimanawa State Forest
Park, Taupo, North
Island
Nothofagus menziesii,
Nothofagus fusca
dominated forest
190.5 9 March 1994 Field (696 g) 20 November 1994 PDD 76873 (Holotype);
FB-24558, 30231, 30236,
30238, 30240 (CBM)
CHU3002
190.5 21 May 1993 Field (696 g) 22 March 1994 FB-24568 (CBM) CHU3009
– 19 November 1994 Laboratory 8 June 1995 FB-24562 (CBM) CHU3013
Riverhead, North Island Pinus radiata
(plantation)
190.5 6 June 1991 Field (348 g) 18 September 1991 FB-24554 (CBM) CHU3007
a
Isolates indicated by CHU numbers are stock cultures of the Faculty of Education, Chiba University, Japan
b
Weights shown in parentheses denote the amount of urea (granular form fertilizer; 46% nitrogen) spread per square meter in the field experiment. In the laboratory, aqueous urea was added to
each soil at the rate of 20 mg (nitrogen)/g dry weight soil
138 Mycoscience (2011) 52:137–142
123
-phlyctidospora reflects the morphological resemblance of
this species to C. phlyctidospora.
Pileus 5–10 mm broad, 6–12 mm high in button stage,
when young ellipsoid to ovoid, later convex to plane,
15–20 mm broad when expanded, radially sulcate, at
length the edge somewhat recurving and splitting irregu-
larly, pileipellis color at first white, soon becoming brown
(6d6), surface when young densely covered with white,
radially arranged, hairy-fibrillose scales (Figs. 1,2)or
squarrose recurved scales (Figs. 4,5), sometimes with
cortinate veil in very young stage (Fig. 4), later almost
glabrous or veil remaining only in the center (Fig. 6). Flesh
very thin, fragile, white, taste mild, odorless. Lamellae
free, crowded (number of lamellae reaching stipe =
70–85), narrow (1–2 mm wide), edge slightly pruinose, at
first white, then grayish, finally blackish, deliquescent.
Stipe up to 50–100 mm 91–3 mm, cylindrical, equal or
somewhat tapering upward, sometimes the base clavate,
not rooting, hollow, fragile, surface white, at first with
white fibrillose or squarrose scales (Figs. 3,4,5), soon
becoming smooth (Fig. 6). Basidiospores black in mass,
dark red-brown under the microscope, ovoid to ellipsoid,
6.5–7.5 (7.0 ±0.3: mean ±SD, n=40) lm long, 5.1–6.5
(5.7 ±0.4, n=20) lm broad in face view, 5.1–6.2
(5.6 ±0.3, n=20) lm in side view (dimensions includ-
ing ornamentation); 6.2–6.8 (6.6 ±0.3, n=40) lm long,
4.9–6.0 (5.4 ±0.3, n=20) lm broad in face view,
4.8–5.6 (5.3 ±0.2, n=20) lm in side view (dimensions
without ornamentation), with warty ornamentation, an
apical, central germ pore 0.7–0.8 lm wide and a clear
plage (Figs. 7a, 8,9). Basidia 14–25 96–7.5 (long type:
20–25 96–7.5, short type: 14–18 96–7) lm, 4-spored
(Fig. 7b). Pleurocystidia 65–80 940–50 lm, subellipsoid
to obovoid, thin-walled, hyaline, scattered and projecting
from the hymenium (Fig. 7c). Cheilocystidia 35–50 9
25–30 lm, subellipsoid to obovoid, thin-walled, hyaline,
numerous, but not forming sterile margin (Fig. 7d). Veil on
the pileal surface composed of thin-walled, diverticulate,
hyaline hyphae, 50–150 94–6 lm (Fig. 7e). Clamp con-
nections present on veil hyphae (Fig. 7e), on pleuro- and
cheilocystidia hyphae (Fig. 7c, d) and on pileal trama
hyphae.
Specimens examined: Coprinopsis austrophlyctidospora:
All specimens for descriptions were cultivated from the same
field-collected stock culture (Table 1, CHU3002: Suzuki
et al. 2002), isolated from urea-treated plots in Nothofagus
forest, Taupo, New Zealand: PDD 76873—Holotype;
FB-30231, FB-30236, FB-30238, FB-30240 (CBM);
FB-24558 (spore print, CBM). Specimens produced from
other stock cultures from Taupo: FB-24562, 24568 (CBM);
from Riverhead, New Zealand: FB-24554 (CBM); from
Dwellingup, Australia: FB-24556 (CBM). Field collected
materials were also examined; from Dwellingup, Australia,
PERTH 07598351 and PERTH 07599137 (Table 1).
Figs. 1–6 Coprinopsis austrophlyctidospora. Cultured at 25°C, 17–23 days with MYC medium. 1,2FB-30238 (CBM). 3FB-30240 (CBM).
4,6PDD76873 (holotype). 5FB-30231 (CBM). Bars 10 mm
Mycoscience (2011) 52:137–142 139
123
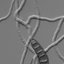
Figs. 8–11 8,9Basidiospores of Coprinopsis austrophlyctidospora. From spore print of cultured basidiomata, FB-24558 (CBM). Bars 10 lm.
10,11 Basidiospores of Coprinopsis phlyctidospora. From basidioma, Herb. Lugd. Bat. No. 989.300 068, The Netherlands. Bars 10 lm
Fig. 7 Coprinopsis
austrophlyctidospora.
aBasidiospores (face view, side
view, face view). bBasidia with
basidiospores. cPleurocystidia.
dCheilocystidia. eVeil hyphae.
afrom FB-24558 (CBM);
b–efrom FB-30240 (CBM).
Bars a5lm; b–e 50 lm
140 Mycoscience (2011) 52:137–142
123
Habit and habitat: Solitary to gregarious, appearing
2–10 months after urea treatment in Nothofagus,Pinus,
and Eucalyptus dominated forests (Table 1). This fungus
was observed at high frequency in each urea-treated plot
(Suzuki et al. 2002), indicating that it is an ammonia fun-
gus (Sagara 1975,1992).
Distribution: New Zealand (Kaimanawa State Forest
Park, Taupo, North Island; Riverhead, North Island),
Australia (Dwellingup, Western Australia) (Suzuki et al.
2002).
Other specimens examined: Coprinopsis phlyctidospora:
Herb. Lugd. Bat. No. 989.300 068, The Netherlands, No.
1026 (C.B. Ulje
´), 3 September 1989, Langeraar, Ter Aar,
prov. Zuid-Holland, The Netherlands (Ulje
´and Noordeloos
1997). Coprinopsis sp.: M-85694 (Kew), from rotted basal
stem tissue of passion vine Passiflora edulis, Lesmurdie,
near Perth in southwestern Australia.
Notes. According to traditional Coprinus Pers. taxonomy,
this species belongs to genus Coprinus, Section Coprinus
Singer, subsect. Alachuani Singer (Singer 1986) because
of its diverticulate veil elements, i.e., the veil is composed
of filamentous hyphae with small side branches and
branchlets. In this subsection there are three species
reported to have warty ornamented basidiospores, i.e.,
Coprinopsis echinospora (Buller) Redhead, Vilgalys &
Moncalvo (Syn.: Coprinus echinosporus Buller), Coprin-
opsis phlyctidospora (Syn.: Coprinus phlyctidosporus), and
Coprinopsis rugosobispora (J. Geesink & Imler) Redhead,
Vilgalys & Moncalvo (Syn.: Coprinus rugosobisporus
J.Geesink & Imler) (Orton and Watling 1979; Moser 1983;
Ulje
´and Noordeloos 1997; Ulje
´2005). Coprinopsis
rugosobispora is distinguished from C. austrophlyctido-
spora in having two-spored basidia. Coprinopsis echino-
spora differs in having amygdaliform basidiospores and
two types of hyphae in the pileal veil. Morphologically,
C. austrophlyctidospora is very similar to C. phlyctido-
spora, but the latter species has larger basidiospores (range:
7.5–11.0 95.5–8.0 lm, average: 8.4–10.6 96.0–7.6 lm,
Ulje
´and Noordeloos 1997; Ulje
´2005) that are more
oblong shaped and have a more coarsely warted orna-
mentation than those of C. austrophlyctidospora (Figs. 10,
11). Coprinopsis phlyctidospora has more distinct warts
surrounding the germ pore. The plage of C. phlyctidospora
is sometimes not as distinct as that of C. austrophlyctido-
spora (Figs. 8,9). The basidiospore color of C. phlyctido-
spora under the microscope is darker brown. These
morphological differences between the two species support
segregation of C. phlyctidospora sensu lato into two dis-
tinct taxa, one northern and the other southern, as first
indicated from rDNA ITS nucleotide sequences and results
of mating tests (Suzuki et al. 2002). Orton and Watling
(1979) reported that clamp connections were not observed
in C. phlyctidospora. In the present study, clamp connec-
tions were observed for both species on hyphae of the
pileal veil of cultivated basidiomata. Suzuki et al. (2002)
prepared mating tests between these two species with
matings indicated by clamp connections. Thus, clamp
connections are known to exist in cultivated mycelium and
now also in basidiomata of both species. The description
above is based on cultivated material from New Zealand.
Field collected specimens (PERTH 07598351 and PERTH
07599137, Australia, Table 1) have similar morphological
characters as cultivated specimens, both macro- and
microscopically. Basidiospores of the Australian speci-
mens (not from spore print but from lamellae of mature
basidiomata) are: 6.3-7.4 (6.9 ±0.4: mean ±SD, n=40)
lm long, 5.1–6.4 (5.7 ±0.3, n=20) lm broad in the face
view, and 5.1–6.3 (5.6 ±0.4, n=20) lm in the side view
(including ornamentation). From Australia, ‘‘Coprinus
phlyctidosporus’’ auct. non Romagn. was previously
Figs. 12–14 Coprinopsis sp.
The specimen collected from
near Perth in southwestern
Australia as Coprinus
phlyctidosporus [Doepel 1968,
M-85694 (Kew)]. Spore size
is larger than in
C. austrophlyctidospora and
C. phlyctidospora.Bars 12 10
mm; 13,14 10 lm
Mycoscience (2011) 52:137–142 141
123
recorded only once, from rotted basal stem tissue of pas-
sion vine Passiflora edulis Sims, near Perth in southwest-
ern Australia (Doepel 1968; Hilton 1982). The specimen
reported by Doepel [M-85694 (Kew), Figs. 12,13,14]is
similar to both C. austrophlyctidospora and C. phlyctido-
spora in having diverticulate pileal veil hyphae and warty
ornamented basidiospores. But the basidiospores of
Doepel’s specimen are large, i.e., 11.0–14.5 (13.0 ±0.9:
mean ±SD, n=40) lm long, 8.5–11.6 (10.0 ±0.7,
n=20) lm in the face view, and 9.2–11.0 (10.0 ±0.8,
n=20) lm in the side view (Figs. 13,14), and basidio-
mata are also more stout and large (pileus up to 14 mm
high, Fig. 12). This specimen seems to be a different and
undetermined species of Coprinopsis. Another Coprinopsis
species with warted basidiospore ornamentation reported
from South Australia is Coprinopsis karwinicola (Grgur.)
J.A. Simpson & Grgur. (Syn.: Coprinus karwinicola
Grgur.) (Grgurinovic 1997). This species is distinguished
from Coprinopsis austrophlyctidospora in having larger
basidiomata (pileus up to 62 mm wide), larger basidio-
spores (10.4–13.4 97.2–8.6 lm) and in lacking cheilocy-
stidia. Ecologically, C. karwinicola is not an ammonia
fungus, having been collected on dead tissue of the grass
tree Xanthorrhoea sp. (Grgurinovic 1997).
Acknowledgments We are grateful to the late Mr. C. B. Ulje
´(The
Netherlands) and the Royal Botanic Garden, Kew, for the loan of
specimens and to Dr. K. Katumoto for critical reading of the Latin
description. This work was financially supported in part by a Grant-
in-Aid for Scientific Research (Monbusho International Scientific
Research Program: Field research) (nos. 03041047 and 05041093)
from the Ministry of Education, Culture, Sports, Science and Tech-
nology, Japan, the Japan Society for the Promotion of Science (JSPS,
nos. 13660153 and 17405030), Hokuto Foundation for Bioscience
(fiscal year: 2000, 2007), Bilateral Exchanging Program (Australia)
(fiscal year: 1996), Australian Academy of Science (AAS), and the
New Zealand Foundation for Research, Science, and Technology. We
would like to thank Mr. Lindsay Cannon, Carter Holt Harvey Forests,
New Zealand, for providing access to Riverhead Forest, and the New
Zealand Department of Conservation for access to Kaimanawa State
Forest Park. We are thankful to Alcoa World Alumina Australia for
their support and making the experimental sites available. We also
acknowledge the technical assistance of Mses. Janine M. Catchpole
and Susan Q. Bolsenbroek at CSIRO, Perth.
References
Doepel RF (1968) Base rot of passion vine. Aus Plant Dis Rec 20:5
Grgurinovic CA (1997) Larger fungi of South Australia. The botanic
gardens of Adelaide and State herbarium, Adelaide
Hilton RN (1982) A census of the larger fungi of Western Australia.
J R Soc West Aust 65:1–15
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd
edn. Sankt Jorgen Tryk Ltd, Copenhagen
Moser M (1983) Key to Agarics and Boleti (Polyporales, Boletales,
Agaricales, Russulales), 4th edn. The Whitefriars Press Ltd,
Tongridge
Orton PD, Watling R (1979) Coprinaceae part 1: Coprinus. In:
Henderson DM, Orton PD, Watling R (eds) British fungus flora
2: Agarics and Boleti. Royal Botanic Garden, Edinburgh
Sagara N (1975) Ammonia fungi—a chemoecological grouping of
terrestrial fungi. Contrib Biol Lab Kyoto Univ 24:205–276
(7 pls)
Sagara N (1992) Experimental disturbances and epigeous fungi. In:
Carroll GC, Wicklow DT (eds) The fungal community: its
organization and role in the ecosystem, 2nd edn. Marcel Dekker,
Inc, New York, pp 427–454
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz
Scientific Books, Koenigstein
Suzuki A, Tanaka C, Bougher NL, Tommerup IC, Buchanan PK,
Fukiharu T, Tsuchida S, Tsuda M, Oda T, Fukada J, Sagara N
(2002) ITS rDNA variation of the Coprinopsis phlyctidospora
(syn.: Coprinus phlyctidosporus) complex in the Northern and
the Southern Hemispheres. Mycoscience 43:229–238
Ulje
´CB (2005) 1. Coprinus Pers. In: Noordeloos ME, Kuyper THW,
Vellinga EC (eds) Flora Agaricina Neerlandica. Taylor &
Franics, Boca Raton, pp 22–109
Ulje
´CB, Noordeloos ME (1997) Studies in Coprinus IV. Coprinus
section Coprinus: subdivision and revision of subsection
Alachuani. Persoonia 16:265–333
142 Mycoscience (2011) 52:137–142
123