Available via license: CC BY-NC 4.0
Content may be subject to copyright.
In Vitro
Pancreatic Lipase Inhibition by Marine Fungi
Purpureocillium
lilacinum
Associated with
Stylissa
sp. Sponge as Anti-obesity Agent
Wendi Nurul Fadillah1, Nampiah Sukarno2*, Dyah Iswantini3,4, Min Rahminiwati5, Novriyandi Hanif3, Mashuri Waite6
1
Study Program of Microbiology, Department of Biology, Faculty of Mathematics and Natural Sciences, IPB University, Bogor,
Indonesia
2
Department of Biology, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, Indonesia
3
Department of Chemistry, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, Indonesia
4
Tropical Biopharmaca Research Center, IPB University, Bogor, Indonesia
5
Department of Anatomy, Physiology, and Pharmacology, Faculty of Veterinary Medicine, IPB University, Bogor, Indonesia
6
Gill Ewa Lands, Honolulu, Hawaii, USA
1. Introduction
Sponges (Porifera) are vital organisms maintaining
the ecosystem in the coral triangle, a world marine
biodiversity hotspot that includes Indonesia. Sponges
are sessile organisms that play an important role in
maintaining coral reef ecosystems and as hosts for
microbial symbionts such as fungi (Taylor et al. 2007).
The fungal symbionts in sponges produced diverse
secondary metabolites that are beneficial for their
hosts. Sponges utilize fungal secondary metabolites
for defending against predation and surviving in toxic
or extreme environmental conditions (Debbab et al.
2010; Wahl et al. 2012). The association between
sponges and marine fungi may also produce unique
and novel natural products potentially beneficial as
new drugs to overcome medical problems such as
multidrug-resistant pathogenic microbes, infectious
diseases, and metabolic diseases (Doshi et al. 2011;
Pimentel et al. 2011). In 2017, 1490 new natural
products were reported from marine organisms
and marine fungi contributed 30% of these new
natural products (Carroll et al. 2019). However, in
spite of being one of the great epicenters of marine
biodiversity, Indonesia has few reports of marine
fungal secondary metabolites (Hanif et al. 2019).
Obesity is a common disease occurring in many
developing countries, including Indonesia. Studies in
Indonesia reported that as much as 16.5% of children
and 45.7% of adults are obese (Sari and Amaliah 2014;
Rachmi et al. 2016), with obesity prevalence higher
among male children and female adults (Rachmi et
al. 2017). In general, obesity is treated by limiting
dietary intake of fat, controlling the absorption
of dietary fat, and/or controlling the storage and
metabolism of fat and glucose in the body. Several
drugs have been developed for anti-obesity, such
as sibutramine, lorcaserin, rimonabant, and orlistat.
However, adverse effects have been reported for each
of these drugs.
ABSTRACT
This study aimed to evaluate the potential of marine fungus Purpureocillium
lilacinum isolated from an Indonesian marine sponge Stylissa sp. as an anti-
obesity agent through pancreatic lipase inhibition assay. The fungus was identied
as P. lilacinum through morphological and molecular characteristics. The fungal
extract’s inhibition activity and kinetics were evaluated using spectrophotometry
and Lineweaver-Burk plots. Ethyl acetate and butanol were used for extraction.
Both extracts showed pancreatic lipase inhibition in a concentration-dependent
manner. Both crude extracts were then fractionated once. All fractionated extracts
showed inhibitory activity above 50%, with the highest activity found in fraction
5 of ethyl acetate at 93.41% inhibition. The best fractionated extract had an IC50
value of 220.60 µg.mL-1. The most active fraction of P. lilacinum had a competitive-
type inhibitor behavior as shown by the value of Vmax not signicantly changing
from 388.80 to 382.62 mM pNP.min-1, and the Michaelis-Menten constant (KM)
increased from 2.02 to 5.47 mM in the presence of 500 µg.mL-1 fractionated
extract. Metabolite identication with LC-MS/MS QTOF suggested that galangin,
kaempferol, and quercetin were responsible for the observed lipase inhibition.
ARTICLE INFO
Article history:
Received July 18, 2021
Received in revised form October 4, 2021
Accepted October 18, 2021
KEYWORDS:
competitive inhibitor,
IC50,
inhibition kinetics,
LC-MS/MS QTOF,
marine fungal extract
* Corresponding Author
E-mail Address: nampiah@apps.ipb.ac.id
Vol. 29 No. 1, January 2022 76-86
DOI:10.4308/hjb.29.1.76-86
ISSN: 1978-3019
EISSN: 2086-4094
H A Y AT IH A Y AT I
Journal of BiosciencesJournal of Biosciences
Sibutramine, a centrally acting phenethylamine
class of drug used for long-term treatment of obesity
in adults, reduces food intake by selective inhibition
of the re-uptake of noradrenaline, serotonin, and
dopamine, resulting in thermogenesis and lipolysis.
Common side effects of sibutramine are due to
activation of the sympathetic nervous system and
include dry mouth, insomnia, constipation, headache,
anorexia, hypertension, and increased pulse rate
(Elangbam 2009). Lorcaserin, a selective 5-HT2C
receptor agonist located in various parts of the brain
and hypothalamus, has serotonergic properties
which act as an anorectic resulting in weight loss
through hypophagia (Lam et al. 2008). Rimonabant,
an appetite suppressant, blocks the cannabinoid-1
(CB1) receptors, and thus decrease demand for food.
Rimonabant side effects include mood changes,
nausea and vomiting, diarrhea, headache, dizziness,
and anxiety (Kaila and Raman 2008).
In contrast, as the most accepted drug by the
FDA for obesity treatment, orlistat is a hydrogenated
derivative of lipstatin produced by Streptomyces
toxytricini and acts by diminishing the absorption
of dietary fat by inhibiting pancreatic lipase activity.
Orlistat is an irreversible inhibitor targeting the active
site of pancreatic and gastric lipase, binds covalently
to the serine residue on the active site preventing the
intraluminal hydrolysis of triglyceride and resulting
in an increase in fecal fat excretion (Heck et al. 2000).
Drugs with covalent inhibitory mechanism of action
provides many pharmacological advantages over
drugs with reversible mechanism of action; these
advantages include enhanced potency, selectivity,
and prolonged activity (Singh et al. 2011).
Despite the great potential of covalent inhibition,
there are several potential downsides and limitations
of this drug class. The longer residence time of
these drugs can lead to toxicity which can generate
adverse clinical outcomes if these drugs are used
long-term (De Cesco et al. 2017). Orlistat is reported
to deliver adverse effects, including liquid stools,
steatorrhea, fat-soluble vitamin deficiencies, fecal
urgency, and flatus incontinence (Kaila and Raman
2008). If consumed long-term, orlistat may cause
hepatotoxicity, gallstone formation, kidney stones,
and acute pancreatitis (Katoch et al. 2017). There has
been a great interest among researchers in finding
pharmacologically active molecules with fewer side
effects.
The marine sponge Xestospongia testudinaria
from China has been found to produce the
pancreatic inhibitor xestonariena I from an unknown
biosynthetic pathway (Yang et al. 2017). Several
marine fungi from the Aspergillus group associated
with a marine sponge from the South China Sea
were also reported to be a source of pancreatic lipase
inhibitors, such as flavipesides A−C from Aspergillus
flavipes 164013 isolated from a marine sponge
Dysidea sp. and petrosamides A-C from Aspergillus
sp. 151304 associated with marine sponge Petrosia sp.
(Jiao et al. 2020; Tang et al. 2020). The biodiversity
of marine fungi and their potential in Indonesia are
high. However, research on sponge-associated marine
fungi in Indonesia and their potential as a source of
anti-obesity metabolites has not been carried out. We
began our research to fill this gap. The main objective
of this research was to investigate marine sponge-
associated fungi as secondary metabolite producers
to inhibit pancreatic lipase activity to potentially
treat obesity.
2. Materials and Methods
2.1. Marine Fungal Isolation
Purpureocillium lilacinum IPBCC.19.1498 was
isolated from a marine sponge Stylissa sp. collected
from Pramuka Island, Seribu Archipelago, Jakarta,
Indonesia. Fungal isolation was carried out as
follows: the sponges were cut and collected into a
sterile container at depths of ±10 m underwater to
prevent contact of tissue with air. Then, the samples
were stored at 4°C prior to fungal isolation at the
laboratory. The sample was surface sterilized with
sterile water prior to fungal isolation by a direct
isolation method following Zhang et al. (2009) with
slight modification. Samples were cut into large
pieces and separated based on cuts such as an outer
layer, middle layer, and inner layer of sponges. Then,
the big pieces were rinsed three times with sterile
water and cut further into small pieces of 1 cm3. The
pieces were then planted into potato dextrose agar
(PDA) with 75% seawater. The medium also contained
chloramphenicol antibiotic (500 mg.L-1) and rose
bengal fungistatic (30 mg.L-1) and then incubated at
27°C for a month. The fungal isolation was done by
planting three pieces of sample in each petri dish and
was done in three replicates. The fungi that emerge
from the samples' pieces were transferred onto new
medium to obtain pure cultures.
2.2. Morphological and Molecular Analysis of
Fungi
The morphological identification followed the
method reported by Luangsa-Ard et al. (2011).
In brief, the fungus was grown on PDA at 27°C
and incubated for seven days. The morphological
and micromorphological features such as colony
HAYATI J Biosci 77
Vol. 29 No. 1, January 2022
characteristics, conidiophores, phialides, and conidia
were measured.
The fungal genome DNA was isolated following the
method of Sambrook et al. (1989). Briefly, the fungal
isolates were cultured on cellophane membrane on
PDA at 27°C for five days prior to cell harvesting for
genome DNA extraction. The mycelium was harvested
and ground with a sterile pestle. Fungal genome DNA
was extracted using the cetyl trimethyl ammonium
bromide (CTAB) buffer lysis solution followed by
washing with phenol-chloroform-isoamyl alcohol
(PCI) mixture and precipitated with ethanol. The DNA
quality was measured with nanodrop.
The fungal genome DNA was used in PCR as a
template to amplify the fungal ITS rDNA region
using primers ITS1 and ITS 4 (White et al. 1990). The
reaction mixture contained 12.5 µl PCR master mix
(kappa fast 2G), 1.5 µl of 10 pmol primer (each), 3
µl of 100 ng DNA template and 6.5 µl NFW. PCR
conditions were as follows: initial denaturation (94°C
for 5 min); 30 cycles of denaturation (94°C for 30 s),
primer annealing (55°C for 1 min), and elongation
(72°C for 2 min), with a final elongation at 72°C for 10
min. PCR products were purified prior to sequencing
analysis. The sequencing result was then analyzed for
its homologous sequences within the GenBank and
MycoBank databases by using the program Basic Local
Alignment Search Tool (BLAST) at http://blast.ncbi.
nlm.nih.gov. Phylogenetic trees were constructed by
using MEGA 6.0 program.
2.3. Fungal Extract Production
Fungal extract and fractionated extract were
conducted following protocols by Ebadda et al. (2008)
and Kjer et al. (2010). The fungus was cultivated in PDA
with 75% seawater without antibiotic. Plugs of agar
supporting mycelium growth were transferred to 3 l
of potato dextrose broth (PDB) medium. Flasks were
incubated at 27°C in constant shaking at 150 rpm for
seven days. The broth culture was then harvested by
separating from mycelia.
The crude extract was obtained by extracting
broth culture gradually using ethyl acetate followed
by n-butanol as organic solvent and evaporated
under vacuum. The extracts were chromatographed
on Silica and XAD resins with gradient eluent to give
extract fractions. The dried crude extract and the
fractionated extract were reconstituted in DMSO for
further analysis.
2.4. Assay of Inhibitory Effect on the Pancreatic
Lipase Activity
Lipase activity was determined by measuring
p-nitrophenol (pNP) concentration released from
the activity between lipase and p-nitrophenyl
butyrate (pNPB). The activity was measured by
spectrophotometry following procedures from
Chedda et al. (2016) with slight modification for
absorbance measurements optimizations. Total assay
volume was 200 µl containing 25 µl of 500 µl.mL-1
pNPB, 50 µl of 200 µg.mL-1 porcine pancreatic lipases,
and 25 µl sample in dimethyl sulfoxide (DMSO). The
reaction was brought to 200 µl with 100 mM pH 7.2
phosphate buffer saline (PBS). The test solution was
incubated for 30 minutes at 37°C prior to measuring
pNP at 400 nm using an ELISA plate reader (Biotek).
The sample was assayed in three replicates, and
the results were reported as a mean of replicates
measurements.
The percentage of inhibitory activity was
calculated using the following formula:
The absorbance of blank and absorbance of the
sample were corrected by measuring the activity
without using enzyme in the assay mixture. Orlistat
was used as the positive control. The test was done
in every step of bioactivity monitoring of fungal
extract. The fractionated extract with the greatest
inhibitory activity was then selected and subjected to
IC50 and inhibition kinetics assay. This selected most
active fractionated extract was diluted with DMSO
in various concentrations to prepare the required
inhibitor concentration used in the IC50 assay.
2.5. Inhibition Kinetics of Selected
Fractionated Extract
The substrate stock solution of pNPB was prepared
freshly in acetonitrile (1 µl.mL-1). The stock was then
diluted with phosphate buffer to prepare the required
concentrations between 2.3 to 6.0 mM. The inhibition
kinetics of the most active fractionated extract were
carried out by measuring assay mixtures with various
substrate concentrations and incubated at 37°C for
1 hour. The mixtures were measured for every 5
minutes of incubation to obtain the enzyme activity
rate across multiple substrate concentrations.
2.6. Metabolites Identification by LC-MS/MS
QTOF Screening
The most active fractionated extract was
then subjected to LC-MS/MS QTOF to screen the
metabolites present in the extract. The screening
criteria was selected from flavonoid, alkaloid,
tannin, steroid, saponin, and triterpenoid groups.
The screening was required to meet four criteria to
% Inhibition = Absorbance of blank
-Absorbance of
sample
Absorbance of
blank x 10 0
78 Fadillah WN
et al.
HAYATI J Biosci 79
Vol. 29 No. 1, January 2022
ensure the metabolites were present in the extract.
The criteria were as follows:
- Mass error ≤ 5 ppm
- Isotope match MZ RMS PPM ≤ 6 ppm and isotope
match MZ RMS % ≤10 %
- Intensity/Response ≥300
- Fragment match ≥1 mass fragment
3. Results
3.1. Fungal Isolation and Identification
3.1.1. Fungal Isolation and Morphological
Characteristics
The fungus was successfully isolated from the
middle layer of the Stylissa sp. sponge. The fungus
was found grown in two pieces of the sponges. The
fungus was identified as Purpureocillium lilacinum
IPBCC.19.1498 based on morphological characteristics
(Figure 1). The fungal characteristics were as follows.
Colonies at 27°C on PDA (Oxoid) attained 17.00-
18.47 mm diameters after seven days of incubation.
Colonies consisted of basal felt of numerous
conidiophores with a floccose overgrowth of aerial
mycelium, purple in color; reverse colony mostly
in shades of purple. Somatic hyphae were hyaline,
smooth-walled, 1-3 µm wide. Conidiophores were
up to 100 µm long; stalks with roughened thick walls
3–6 µm wide consisting of verticillate branches with
whorls of 2–4 phialides. Conidiophores sometimes
grew irregularly branched, septate, hyaline and
smooth-walled. Phialides were 7–15 × 2–3 µm,
having a swollen basal portion tapering into a short
distinct neck about 1 µm wide. Conidia were in long
dry chains, ellipsoidal to fusiform, 2–3 × 2–4 µm,
smooth-walled, mostly subhyaline, chlamydospores
absent.
3.1.2. Molecular Analysis of Fungi
Further identification using molecular features
showed that the identification result with BLAST
analysis is in agreement with that of the morphological
analysis. The fungus has 100% similarity with P.
lilacinum (AB_103380). The results of the phylogenetic
analysis supported that of the BLAST analysis. The
phylogenetic tree of the fungus was constructed with
the maximum likelihood method based on the Tamura
3-parameter model by using MEGA 6.0 program, and
a total of 17 OTU were used in the analysis of ITS rDNA
sequences with Paracremonium binnewijzendii CBS
143277 as an outgroup (Table 1). The dataset consists
of 665 nucleotide characters for the analyses, 479
were constant and 186 were variable.
The Maximum likelihood analysis inferred by
Tamura 3-parameter model yielded the highest
likelihood value -1689.9489 shown in Figure 2. A
discrete gamma distribution was used to model
evolutionary rate differences among sites (5 categories
(+G, parameter = 0.2150)). The tree is drawn to scale,
with branch lengths measured in the number of
substitutions per site. Bootstrap values above 50% are
indicated above branches. A bootstrap value greater
than 70 % is considered strong. The phylogenetic tree
constructed showed that P. lilacinum IPBCC.19.1498 is
in the same cluster with P. lilacinum AB_103380 with
bootstrap value 99% and sisters to P. lavendulum CBS
128677 with 99% bootstrap value.
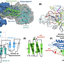
Figure 1. Colony and microscopic structures of
Purpureocillium lilacinum IPBCC.19.1498
grown on PDA at 7 days after inoculation.
(A-B) IPBCC.19.1498 sporulating colonies on
dark and light backgrounds at 27°C, (C-D)
conidiophore, (E) branched conidiophore, (F)
phialides, (G) typical fusiform conidia. Scale
bars are 1 cm (A-B) and 10 µm (C-G).
a
b
c
d
e
f
g
A
C
F
B
D E
G
Table 1. ITS rDNA of organisms used in phylogenetic tree construction
Taxa Strain/isolate ITS GenBank accession number
Purpureocillium lilacinum
P. lilacinum
P. lavendulum
Tolypocladium album
T. cylindrosporum
T. ovalisporum
Drechmeria balanoides
D. campanulata
D. zeospora
Verticillium zeosporum
V. campanulatum
Hypomyces samuelsii
H. semicircularis
Cladobotryum semicirculare
C. tchimbelense
Haptocillium glocklingiae
Paracremonium binnewijzendii (outgroup)
IPBCC.19.1498
CBS 128677
CBS 869.73
ARSEF 2920
CBS 700.92
CBS 250.82
IMI 356051
CBS 335.80
CBS 335.80
IMI 356051
CBS 127157
CBS 705.88
CBS 705.88
CBS 127166
CBS 101434
CBS 143277
AB103380
MH864976
NR_155018
MG228381
NR_155019
NR_155044
NR_155045
NR_155046
AJ292419
AJ292416
MH864448
NR_121425
FN859417
MH864455
NR_137654
NR_157491
Figure 2. Phylogenetic tree of ITS rRNA gene obtained by maximum likelihood analysis. Phylogenetic analysis was performed
on the basis of 665 unambiguously aligned positions of ITS rRNA gene with Paracremonium binnewijzendii as
outgroup. Bootstrap values are indicated at the nodes
3.2.
In Vitro
Inhibitory Pancreatic Lipase
and Assay of
Purpureocillium lilacinum
IPBCC.19.1498 Extract
The inhibitory effect of P. lilacinum IPBCC.19.1498
crude extracts on pancreatic lipase activity is
presented in Figure 3. Both P. lilacinum IPBCC.19.1498
ethyl acetate and butanol extracts inhibited the
activity of pancreatic lipase in a dose dependent
manner. The extracts show increased inhibition
with increased extract concentration from 250 to
1,000 µg.mL-1. The inhibitory activity observed for
both extracts shows the inhibitory concentration 50
(IC50) value was between 250 to 500 µg.mL-1 of crude
extract.
Both extracts were then chromatographed in silica
and XAD resins and subjected to the gradual polarity
of eluent to obtain 12 fractionated extracts separated
based on eluent used. All fractionated extracts from
ethyl acetate and butanol showed inhibitory activity
above 50%, with the highest activity found in fraction
5 of ethyl acetate extract and fraction 12 of butanol
extract at 93.41 % and 62.76% inhibition, respectively
(Figure 4).
►
80 Fadillah WN
et al.
Fraction 5 of ethyl acetate extract was chosen
as the most active fractionated extract for IC50 and
enzyme kinetics analyses. The IC50 can be determined
by constructing a dose-response curve and examining
the effect of different concentrations of inhibitors on
enzyme activity. The IC50 value is determined by the
concentration needed to inhibit half of the maximum
enzymatic response. IC50 values can also be used to
compare the potency of inhibitors. In this study, the IC50
value was measured under different concentrations of
the best-fractionated extract and orlistat as the positive
control (data not shown). The most active fractionated
extract had efficacy of 61.75% at 500 µg.mL-1 and an IC50
of 220.60 µg.mL-1; meanwhile, orlistat as positive control
had IC50 of 30.57 µg.mL-1.
3.3. Kinetic Behavior of Best-fractionated Extract
The reaction rates of the most active fractionated
extract are shown in Figure 5. The most active fractionated
ethyl acetate extract of P. lilacinum had a competitive-type
behavior on inhibition of pancreatic lipase based on the
maximum rate (Vmax) not significantly changing, and the
enzyme constant (KM) increasing from 2.02 mM to 5.47
mM. The increased value of KM means the presence of
substrate reduces the inhibitory activity of the extract.
The kinetics parameters are presented in Table 2.
Ethyl acetate
250 500
Inhibition (%)
750 1,000
concentration of Purpureocillium lilacinum extract (µg.mL-1)
n-butanol
Figure 3. Effect of Purpureocillium lilacinum crude extracts on pancreatic lipase activity. Extract of ethyl acetate and
butanol were measure thrice. Values are inhibition (%) converted from means of pNP absorbance
Inhibition (%)
Ethyl acetate extract
fraction number
butanol extract
Figure 4. Effect of Purpureocillium lilacinum fractionated extracts of ethyl acetate and butanol on pancreatic lipase activity.
All fractionated extracts were measured thrice. Values are inhibition (%) converted from means of pNP absorbance
HAYATI J Biosci 81
Vol. 29 No. 1, January 2022
3.4. Fungal Metabolites Identification by LC-
MS/MS QTOF Screening
The metabolite screening of the most active
fractionated P. lilacinum extract by LC-MS/MS QTOF
showed 33 metabolites dominated by flavonoids,
tannins, and terpenes. Three out of the identified
metabolites are reported as lipase inhibitors,
namely: galangin, kaemferol, and quercetin. The
characteristics of the three predicted metabolites are
presented in Table 3.
Table 3. Predicted metabolites identified with LC-MS/MS QTOF from the most active fractionated extract of Purpureocillium
lilacinum as pancreatic lipase inhibitor
Compound name
Galangin
(Norizalpinin)
Kaempferol
Quercetin
ESI mode
+
-
-
IUPAC name Observed RT (min) Response Activity based on
reference
Reference
3,5,7-trihydroxy-2-
phenylchromen-
4-one
3,5,7-trihydroxy-
2-(4-hydroxy
phenyl)chromen-
4-one
2-(3,4-dihydroxy
phenyl)-3,5,7-
trihydroxy
chromen-4-one
6.55
12.60
13.23
17638
2335
1008
Not defined, IC50:
48.20 mg.mL-1
Competitive
inhibition
Mixed type
inhibition
Kumar and
Alagawadi
2013
Li et al. 2020
Martinez-
Gonzalez
et al. 2017
Table 2. Kinetic parameters of pancreatic lipase in the
presence of the most active fractionated extract
as inhibitor KM (mM) Vmax
(mM.min-1)
Type of
inhibition
2.02
5.47
Normal activity
(without inhibitor)
Most active
fractionated
Purpureocillium
lilacinum extract
as inhibitor
388.80
382.62 competitive
Figure 5. Substrate transformation in the presence and absence of the most active fractionated extract of Purpureocillium
lilacinum. Determination of the kinetic parameters reaction rate of pancreatic lipase on pNPB according to
Lineweaver-Burk Plot
R² = 0.9943
R² = 0.9696
-1500
-1000
-500
0
500
1000
1500
2000
-1 -0.8 -0.6 -0.4 -0.2 00.2 0.4 0.6 0.8
1/ velocity
[1/substrate - PNPB] (mM)
normal test
82 Fadillah WN
et al.
4. Discussion
The fungus was identified as Purpureocillium
lilacinum IPBCC.19.1498 based on morphological and
molecular characteristics, including the phylogenetic
tree. The fungus obtained in this study is in the same
clade with soil fungus P. lilacinum (AB_103380) with
a bootstrap value of 99%. The genus Purpureocillium is
proposed by Luangsa-Ard et al. (2011) for Paecilomyces
lilacinus, along with Paecilomyces nostocoides, Isaria
takamizusanensis, and Nomuraea atypicola, which
belongs to Ophiocordycipitaceae based on the
phylogenetic analysis of the 18S rRNA. The genus was
proposed because the type species of Paecilomyces, P.
variotii, is located in the family of the Trichocomaceae
(Eurotiales) near Aspergillus, Penicillium and related
species, forming a sister clade with the Onygenales
(Luangsa-Ard et al. 2004; Sung et al. 2007; Luangsa-
Ard et al. 2011).
Purpureocillium lilacinum is commonly reported
as a terrestrial fungus, commonly occurring as
a saprobic species isolated from soil, decaying
vegetation, insects and insect larvae, nematodes,
and other animals. Although widely reported as a
component of the soil mycobiota, several strains
were reported to cause keratitis typically occurring
by external invasion (Domniz et al. 2001; Yuan et al.
2009). It has also been reported that P. lilacinum has
been isolated from deep seawater samples in China
and associated with a sponge from Malaysia (Cui et al.
2013; Mosadeghzad et al. 2013). But none have been
reported from Indonesia.
Purpureocillium lilacinum is categorized as a
facultative marine fungi since the fungus has the
ability to grow on medium without sea water.
Therefore, there is the possibilty the fungus was
carried from land by streams and rivers into the sea
and filtered by sponges. The capacity of P. lilacinum
to colonize substrates under harsh conditions with
low nutrient concentrations and low oxygen levels
suggests that this species is able to survive in sea
water (Mountfort and Rhodes 1991; Vigueras et
al. 2008). Moreover, strains of Purpureocillium sp.
have been reported to survive in a wide range of
temperatures and pH, which allows them to grow in
a variety of substrates and makes them a rich source
of biologically active natural products (Mosadeghzad
et al. 2013). Purpureocillium lilacinum was frequently
found in water distribution systems, including water
tanks, sinks, showers (including drains), toilets, and
air in a three-year study by Anaissie et al. (2003). The
fungi can survive in water distribution systems and
form a biofilm with other genera such as Aspergillus,
Fusarium, and Acremonium (Anaissie et al. 2003).
Numerous kinds of marine fungi have been reported
to produce diverse secondary metabolites with potential
as medicine for many diseases. Purpureocillium
lilacinum has produced diverse metabolites with unique
properties such as α-pyrone analogue phomaligol A,
exhibiting antibacterial activity against Staphylococcus
aureus, methicillin-resistant S. aureus and multidrug-
resistant S. aureus, leucinostatin A and B as antibacterial,
antifungal, and antitumor agent, and caused uncoupling
effect on rat liver mitochondrial function. Other
metabolites such as phomapyrone C also showed anti-
bacteria activity against Acinetobacter baumannii, and
1,2-dilinolylglycero-O-4’-(N,N,N-trimethyl)homoserine,
methyl myristate and cerebroside B–D inhibited the
human cancer K562, MCF-7, HL-60, and BGC-823 cell
lines. Purpureocillium lilacinum also reported to produce a
pyridone alkaloid, paecilomide as acetylcholine esterase
inhibitor, and cerebrosides A showed nematicidal
activity against Bursaphelenchus xylophilus (Arai et al.
1973; Fukushima et al. 1983; Elbandy et al. 2009; Yang
et al. 2011; Cui et al. 2013; Teles and Takahashi 2013).
The present study is the first report of P. lilacinum
marine fungus associated sponge showing inhibitory
effects on pancreatic lipase enzyme activity.
Pancreatic, endothelial, hepatic, lipoprotein
lipases are human lipases that possesses structural
similarity. Other tissues like lungs, kidneys, skeletal
muscles, adipose tissue and placenta also secrete
lipase enzymes. Pancreatic acinar cells secrete
pancreatic lipase (triacylglycerol acyl hydrolase
EC 3.1.1.3), an essential enzyme of pancreatic juice
responsible for digestion, responsible for about 70% of
triglyceride breakdown and absorption from dietary
fat into the small intestine. Therefore, pancreatic
lipase inhibitors are used for medication to treat
obesity (Mukherjee 2003; Liu et al. 2020).
The IC50 value of pancreatic lipase inhibition with
most active fractionated extract of P. lilacinum was
220.60 µg.mL-1 while orlistat was 30.57 µg.mL-1. The
potencies of several plant crude extracts reported to
inhibit pancreatic lipase show IC50 values between
200 to 500 µg.mL-1 such as Nelumbo nucifera and
Salacia reticulata at 460 µg.mL-1 and 264 µg.mL-1,
respectively (Birari and Bhutani 2007). Several plants
extract used as slimming agents in jamu, namely
Zingiber cassumunar, Guazuma ulmifolia, Tamarindus
indica, Murraya paniculata, and Kaempferiae rotundae
had been reported to have pancreatic lipase
inhibitory activity. The activity of ethanol extract of
Z. cassumunar, G. ulmifolia, and T. indica were 29.17%
for 100 µg.mL-1, 25.13% for 60 µg.mL-1, and 49.0% for
150 µg.mL-1, respectively. The activity of water extract
of M. paniculata was 25.66% for 100 µg.mL-1 and K.
rotundae was 65.1% for 200 µg.mL-1 (Iswantini et
HAYATI J Biosci 83
Vol. 29 No. 1, January 2022
al. 2011, Pradono et al. 2011). Methanolic extract of
several species used as medicinal plants in Korea also
showed varied efficacy. Three of these plants were
reported to have the highest efficacy, namely Eriochloa
villosa (83%), Orixa japonica (81.3%), and Setaria italica
(80.3%) at 200 µg.mL-1 extract concentrations (Sharma
et al. 2005). Therefore, the extract of P. lilacinum in
this study may potentially inhibit pancreatic lipase
and could be categorized as a good source of inhibitor,
similar to plant extracts mentioned above. Although
the extract in this study had been fractionated once,
the extract showed promising result.
The value of the Vmax was not significantly changed
from 388.8 mM pNP.min-1 to 382.62 mM pNP.min-1,
and the Michaelis-Menten constant (KM) increased
from 2.02 mM to 5.47 mM in the presence of 500
µg.mL-1 fractionated extract. The Vmax value showed a
saturated state where the enzymes were fully occupied
with the substrate in the active site. In contrast, the
Michaelis-Menten constant showed a stable enzyme-
substrate complex where half of the Vmax condition
was achieved. The KM value also showed an affinity
of the enzyme for substrate. A lower KM value shows
a higher enzyme affinity to the substrate (Nelson and
Cox 2017). Therefore, the competitive type inhibition
exhibits an increasing value of KM, which means that
the substrate loses the affinity with the enzyme in
the presence of the inhibitor. Thus, the most active
fractionated extract of P. lilacinum is a competitive-
type inhibitor.
A competitive inhibitor binds to the enzyme's
active site and prevents the substrate from binding
to that active site. A reversible competitive inhibitor
diminishes the rate of catalysis by reducing the
proportion of enzyme molecules bound to a substrate.
At any given inhibitor concentration, competitive
inhibition can be relieved by increasing the substrate
concentration. With increased concentration, the
substrate can outcompete the inhibitor for the active
site (Berg et al. 2002). Meanwhile, with orlistat as an
irreversible inhibitor, the enzyme-inhibitor complex
is permanently unable to function as an enzyme and
only production of new enzyme will allow reaction
of the substrate. The irreversible inhibitor cannot be
overcome by increasing the substrate concentration.
The residence time of an irreversible inhibitor for
pancreatic lipase in the intestinal lumen may lead to
an unwanted effect in the body such as liquid stools,
steatorrhea, fat-soluble vitamin deficiencies, fecal
urgency, and flatus incontinence since there is a need
to produce fresh enzyme to replace the inhibited
lipase. The present study is the first step and exhibits
the results of in vitro experiments, however it shows
that P. lilacinum extract may become a potential
source of metabolites to develop an anti-obesity drug
with a competitive inhibitory mechanism.
Metabolite identification by LC-MS/MS QTOF
revealed 33 metabolites dominated by flavonoids,
tannins, and terpenes. Flavonoids and terpenes are
two molecular groups known to inhibit pancreatic
lipase (Birari and Bhutani 2007). The three identified
metabolites, galangin, kaempferol, and quercetin,
are known lipase inhibitors. Therefore, these three
metabolites could be responsible for inhibiting
pancreatic lipase in the most active fractionated
extract assessed in this study.
In conclusion, the Indonesian marine fungus
associated with Stylissa sp. sponges produced
potential secondary metabolites with pancreatic
lipase inhibition in in vitro tests and was isolated
and identified as P. lilacinum IPBCC.19.1498. The
fungal compounds were extracted from the culture
broth using ethyl acetate and butanol, and the most
active fractionated extract was obtained from ethyl
acetate. The most active fractionated extract had
an IC50 value of 220.60 µg.mL-1. The IC50 value of the
most active fraction obtained from the crude extract
was eight times higher than the orlistat as positive
control. The present study also revealed that the
most active fractionated extract of P. lilacinum had
a competitive inhibitory mechanism. Metabolite
screening of the most active fractionated extract
suggested the presence of three metabolites known
to be responsible for pancreatic lipase inhibition,
namely galangin, kaempferol, and quercetin.
Conflict of Interest
The authors declare no conflicts of interest.
All experiments were undertaken in this study in
compliance with the current laws of the country
where they were performed.
Acknowledgements
This research was funded by the Ministry
of Research, Technology and Higher Education,
Indonesia through PMDSU (agreement No 4137/IT3.
L1/PN/2018, 4282/IT3.L1/PN/2019 and 4144/IT3.
L1/PN/20200), PKPI-PMDSU (agreement No T/38/
D3.2/KP.06.02/2019) and PPKI-SAME (agreement
No T/2241/D3.2/KD.02.00/2019) Batch III. We thank
to Scientific Diving Laboratories – IPB for sampling
support and to BKSDA Jakarta for the sampling
permit.
84 Fadillah WN
et al.
References
Anaissie, E.J., Stratton, S.L., Dignani, M.C., Lee, C., Summerbell,
R.C., Rex, J.H., Monson, T.P., Walsh, T.J., 2003. Pathogenic
molds (including Aspergillus species) in hospital water
distribution systems: a 3-year prospective study and
clinical implications for patients with hematologic
malignancies. Blood. 101, 2542–2546. https://doi.
org/10.1182/blood-2002-02-0530
Arai, T., Mikami, Y., Fukushima, K., Utsumi, T., Yazawa, K.,
1973. A new antibiotic, leucinostatin, derived from
Penicillium lilacinum. Antibiot. 26, 157–161. https://
doi.org/10.7164/antibiotics.26.157
Berg, J.M., Tymoczko, J.L., Stryer, L., 2002. Enzymes Can Be
Inhibited by Specific Molecules in Biochemistry, fifth
ed. WH Freeman, New York.
Birari, R.B., Bhutani, K.K., 2007. Pancreatic lipase inhibitors
from natural sources: unexplored potential. Drug.
Discov. Today. 12, 879-889. https://doi.org/10.1016/j.
drudis.2007.07.024
Carroll, A.R., Copp, B.R., Davis, R.A., Keyzers, R.A., Prinsep,
M.R., 2019. Marine natural products. Nat. Prod. Rep.
36, 122-173. https://doi.org/10.1039/C8NP00092A
Chedda, U., Kaikini, A., Bagle, S., Seervi, M., Sathaye, S.,
2016. In vitro pancreatic lipase inhibition potential
of commonly used Indian spices. Journal of Pharmacy.
6, 10-13.
Cui, X., Li, C.W., Wu, C.J., Hua, W., Cui, C.B., Zhu, T.J., Gu, Q.Q.,
2013. Metabolites of Paecilomyces lilacinus ZBY-1
from deep-sea water and their antitumor activity.
Int. Pharm. Res. 40, 177–186.
De Cesco, S., Kurian, J., Dufresne, C., Mittermaier, A.K.,
Moitessier, N., 2017. Covalent inhibitors design and
discovery. Eur. J. Med. Chem. 138, 96-114. https://doi.
org/10.1016/j.ejmech.2017.06.019
Debbab, A., Aly, A., Lin, W., Proksch, P., 2010. Bioactive
compounds from marine bacteria and fungi. Microb.
Biotechnol. 3, 544–563. https://doi.org/10.1111/j.1751-
7915.2010.00179.x
Domniz. Y., Lawless. M., Sutton, G.L., Rogers, C.M., Meagher,
L.J., 2001. Successful treatment of Paecilomyces
lilacinus endophthalmitis after foreign body trauma
to the cornea. Cornea. 20, 109–111. https://doi.
org/10.1097/00003226-200101000-00021
Doshi, G., Aggarwal, G., Martis, E., Shanbhag, P., 2011. Novel
antibiotics from marine sources. Int. J. Pharm. Sci.
Nanotechnol. 4, 1446–1461. https://doi.org/10.37285/
ijpsn.2011.4.3.2
Ebada. S., Edrada, R., Lin, W., Proksch, P., 2008. Methods
for isolation, purification and structural elucidation
of bioactive secondary metabolites from marine
invertebrates. Nat. Prot. 3, 1820–1831. https://doi.
org/10.1038/nprot.2008.182
Elangbam, C.S., 2009. Current strategies in the development
of anti-obesity drugs and their safety concerns. Vet.
Pathol. 46, 10-24. https://doi.org/10.1354/vp.46-1-10
Elbandy, M., Shinde, P.B., Hong, J., Bae, K.S., Kim, M.A., Lee,
S.M., Jung, J.H., 2009. α-Pyrones and yellow pigments
from the sponge-derived fungus Paecilomyces
lilacinus. Bull. Korean. Chem. Soc. 30, 188–192. https://
doi.org/10.5012/bkcs.2009.30.1.188
Fukushima, K., Arai, T., Mori, Y.J., Tsuboi, M., Suzuki, M.,
1983. Studies on peptide antibiotics, leucinostatins.
I: separation, physico-chemical properties and
biological activities of leucinostatins A and B.
Antibiot. 36, 1606–1612. https://doi.org/10.7164/
antibiotics.36.1606
Hanif, N., Murni, A., Tanaka, C., Tanaka, J., 2019. Marine
natural products from Indonesian waters. Mar. Drugs.
17, 364. https://doi.org/10.3390/md17060364
Heck, A.M., Yanovski, J.A., Calis, K.A., 2000. Orlistat,
a new lipase inhibitor for the management of
obesity. Pharmacotherapy. 20, 270-279. https://doi.
org/10.1592/phco.20.4.270.34882
Iswantini, D., Silitonga, R.F., Martatilofa, E., Darusman, L.K.,
2011. Zingiber cassumunar, Guazuma ulmifolia, and
Murraya paniculata extracts as antiobesity: in vitro
inhibitory effect on pancreatic lipase activity. Hayati
J. Biosci. 18, 6-10. https://doi.org/10.4308/hjb.18.1.6
Jiao, W.H., Xu, Q.H., Ge, G.B., Shang, R.Y., Zhu, H.R., Liu, H.Y.,
Cui, J., Sun, F., Lin, H.W., 2020. Flavipesides A–C,
PKS-NRPS hybrids as pancreatic lipase inhibitors
from a marine sponge symbiotic fungus Aspergillus
flavipes 164013. Org. Lett. 22, 1825-1829. https://doi.
org/10.1021/acs.orglett.0c00150
Kaila, B., Raman, M., 2008. Obesity: a review of pathogenesis
and management strategies. Can. J. Gastroenterol. 22,
61-68. https://doi.org/10.1155/2008/609039
Katoch, M., Paul, A., Singh, G., Sridhar, S., 2017. Fungal
endophytes associated with Viola odorata Linn. as
bioresource for pancreatic lipase inhibitors. BMC.
Complement. Altern. Med. 17, 385. https://doi.
org/10.1186/s12906-017-1893-y
Kjer, J., Debbab, A., Aly, A.H., Proksch, P., 2010. Methods for
isolation of marine-derived endophytic fungi and
their bioactive secondary products. nat. prot. 5, 479-
490. https://doi.org/10.1038/nprot.2009.233
Kumar, S., Alagawadi, K.R., 2013. Anti-obesity effects of
galangin, a pancreatic lipase inhibitor in cafeteria diet
fed female rats. Pharm. Biol. 51, 607-613. https://doi.
org/10.3109/13880209.2012.757327
Lam, D.D., Przydzial, M.J., Ridley, S.H., Yeo, G.S., Rochford,
J.J., O'Rahilly, S., Heisler, L.K., 2008. Serotonin
5-HT2C receptor agonist promotes hypophagia
via downstream activation of melanocortin 4
receptors. Endocrinology. 149, 1323-1328. https://
doi.org/10.1210/en.2007-1321
Li, S., Pan, J., Hu, X., Zhang, Y., Gong, D., Zhang, D., 2020.
Kaempferol inhibits the activity of pancreatic lipase
and its synergistic effect with orlistat. J. Function. Food.
72, 104041. https://doi.org/10.1016/j.jff.2020.104041
Liu, T.T., Liu, X.T., Chen, Q.X., Shi, Y., 2020. Lipase inhibitors for
obesity: a review. Biomed. Pharma.cother. 128, 110314.
https://doi.org/10.1016/j.biopha.2020.110314
Luangsa-Ard, J., Houbraken, J., van Doorn, T., Hong, S.B.,
Borman, A.M., Hywel-Jones, N.L., Samson, R.A.,
2011. Purpureocillium, a new genus for the medically
important Paecilomyces lilacinus. FEMS. Microbiol.
Lett. 321, 141-149. https://doi.org/10.1111/j.1574-
6968.2011.02322.x
Luangsa-Ard, J.J., Hywel-Jones, N.L., Samson, R.A., 2004.
The polyphyletic nature of Paecilomyces Sensu lato
based on 18S-generated rDNA phylogeny. Mycologia.
96, 773-780. https://doi.org/10.1080/15572536.2005
.11832925
Martinez-Gonzalez, A.I., Alvarez-Parrilla, E., Díaz-Sánchez,
Á.G., de la Rosa, L.A., Núñez-Gastélum, J.A, Vazquez-
Flores, A.A., Gonzalez-Aguilar, G.A., 2017. In vitro
inhibition of pancreatic lipase by polyphenols: a
kinetic, fluorescence spectroscopy and molecular
docking study. Food. Technol. Biotechnol. 55, 519-530.
Mosadeghzad, Z., Zakaria, Z., Ahmad, A., Gires, U.,
Wickneswari, R., Pittayakhajonwut, P., Farahani,
G.H.N., 2013. Chemical components and bioactivity of
the marine-derived fungus Paecilomyces sp. collected
from Tinggi Island, Malaysia. Chem. Nat. Comp. 62113,
9-31304904. https://doi.org/10.1007/s10600-013-
0693-y
HAYATI J Biosci 85
Vol. 29 No. 1, January 2022
Mountfort, D.O., Rhodes, LL., 1991. Anaerobic growth and
fermentation characteristics of Paecilomyces lilacinus
isolated from mullet gut. Appl. Environ. Microb. 57,
1963–1968. https://doi.org/10.1128/aem.57.7.1963-
1968.1991
Mukherjee, M., 2003. Human digestive and metabolic
lipases—a brief review. Journal. of Molecular. Catalysis.
B: Enzymatic. 22, 369–376. https://doi.org/10.1016/
S1381-1177(03)00052-3
Nelson, D.L., Cox, M.M., 2017. Lehninger Principles of
Biochemistrym, seventh ed. WH Freeman, New York.
Pimentel, M.R., Molina. G,. Dionísio, A.P., Roberto, M., Junior,
M., Pastore, G.M., 2011. The use of endophytes to
obtain bioactive compounds and their application in
biotransformation process. Biotechnol. Res. Int. 2011,
1-11. https://doi.org/10.4061/2011/576286
Pradono, D.I., Darusman, L.K., Susanti, A., 2011. Inhibition
of in vitro pancreatic lipase by water and ethanol
extracts of the leaves of tamarind (Tamarindus indica)
and rhizome of Kunci pepet (Kaempferia rotunda). J.
Nat. Indonesia. 13, 146-154. https://doi.org/10.31258/
jnat.13.2.146-154
Rachmi, C.N., Agho, K.E., Li, M., Baur, L.A., 2016. Stunting,
underweight and overweight in children aged 2.0–4.9
years in Indonesia: prevalence trends and associated
risk factors. PLoS ONE. 11, e0154756. https://doi.
org/10.1371/journal.pone.0154756
Rachmi, C.N., Li, M., Baur, L.A., 2017. Overweight and
obesity in Indonesia: prevalence and risk factors-a
literature review. Public. health. 147, 20-29. https://
doi.org/10.1016/j.puhe.2017.02.002
Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular
Cloning: a Laboratory Manual, second ed, Cold Spring
Harbor Laboratory Press, New York.
Sari, K., Amaliah, N., 2014. Sociodemographic factors and
obesity on adults in Indonesia, year 2007 and 2010
(data analysis of basic health survey 2007 and 2010).
Ekol. Kesehatan. 13, 328-339.
Sharma, N., Sharma, V.K., Seo, S.Y., 2005. Screening of some
medicinal plants for anti-lipase activity. J. Ethnopharm.
97, 453-456. https://doi.org/10.1016/j.jep.200 4.11.009
Singh, J., Petter, R.C., Baillie, T.A., Whitty, A., 2011. The
resurgence of covalent drugs. Nat. Rev. Drug. Discov.
10, 307–317. https://doi.org/10.1038/nrd3410
Sung, G.H., Hywel-Jones, N.L., Sung, J.M., Luangsa-ard, J.J.,
Shrestha, B., Spatafora, J.W., 2007. Phylogenetic
classification of Cordyceps and the clavicipitaceous
fungi. Stud. Mycol. 57, 5–59. https://doi.org/10.3114/
sim.2007.57.01
Tang, W.Z., Liu, J.T., Hu, Q., He, R.J., Guan, X.Q., Ge, G.B., Han, H.,
Yang, F., Lin, H.W., 2020. Pancreatic lipase Inhibitory
cyclohexapeptides from the marine sponge-derived
fungus Aspergillus sp. 151304. Nat. Prod. Rep. 83, 2287-
2293. https://doi.org/10.1021/acs.jnatprod.0c00549
Taylor, M.W., Radax, R., Steger, D., Wagner, M., 2007. Sponge
associated mircroorganisms: evolution, ecology,
and biotechnological potential. Microbiology. and
Molecular. Biology. Reviews. 71, 295-347. https://doi.
org/10.1128/MMBR.00040-06
Teles, A.P.C., Takahashi, J.A., 2013. Paecilomide, a new
acetylcholinesterase inhibitor from Paecilomyces
lilacinus. Microbiol. Res. 168, 204–210. https://doi.
org/10.1016/j.micres.2012.11.007
Vigueras, G., Shirai, K., Martins, D., Franco, T.T., Fleuri, L.F.,
Revah, S., 2008. Toluene gas phase biofiltration by
Paecilomyces lilacinus and isolation and identification
of a hydrophobin protein produced thereof. Appl.
Microbiol. Biot. 80, 147–154. https://doi.org/10.1007/
s00253-008-1490-6
Wahl, M., Goecke, F., Labes, A., Dobretsov, S., Weinberger,
F., 2012. The second skin: ecological role of epibiotic
biofilms on marine organisms. Front. Microbiol. 3, 292.
https://doi.org/10.3389/fmicb.2012.00292
White, T.J., Bruns, T., Lee, S., Taylor, J., 1990. PCR Protocols:
A Guide to Methods and Applications-A Laboratory
Manual. Academic press, New York.
Yang, G.H., Sandjo, L., Yun, K., Leutou, A.S., Kim, G.D., Hong,
D.C., Kang, J.S., Hong, J., Son, B.W., 2011. Flavusides A
and B, antibacterial cerebrosides from the marine-
derived fungus Aspergillus flavus. Chem. Pharm. Bull.
59, 1174–1177. https://doi.org/10.1248/cpb.59.1174
Yang, M., Liang, L.F., Wang, T., Wang, H.Y., Liu, H.L., Guo,
Y.W., 2017. Further brominated polyacetylenes with
pancreatic lipase inhibitory activity from Chinese
marine sponge Xestospongia testudinaria. J. Asian. Nat.
Prod. Res. 19,732-737. https://doi.org/10.1080/10286
020.2016.1274308
Yuan, X., Wilhelmus, K.R., Matoba, A.Y, Alexandrakis, G., Miller,
D., Huang, A.J., 2009. Pathogenesis and outcome of
Paecilomyces keratitis. Am. J. Ophthalmol. 147, 691–
696. https://doi.org/10.1016/j.ajo.2008.11.016
Zhang, Y., Mu, J., Feng, Y., Kang, Y., Zhang, J., Cu, P.J., Wang, Y.,
Ma, L.F., Zhu, Y.H., 2009. Broad-spectrum antimicrobial
epihitic and endophytic fungi from marine organism:
isolation, Bioassay, and Taxonomy. Mar. Drugs. 7, 97-
122. https://doi.org/10.3390/md7020097
86 Fadillah WN
et al.