Available via license: CC BY 4.0
Content may be subject to copyright.
Agriculture2021,11,1250.https://doi.org/10.3390/agriculture11121250www.mdpi.com/journal/agriculture
Article
FungalEndophytesofVitisvinifera—PlantGrowth
PromotionFactors
MarkétaKulišová
1,
*,MariaVrublevskaya
1
,PetraLovecká
2
,BlankaVrchotová
2
,MilenaStránská
3
,
MiroslavKolařík
4
andIrenaKolouchová
1
1
DepartmentofBiotechnology,UniversityofChemistryandTechnology,Technická5,16628Prague,
CzechRepublic;maria.vrublevskaya@vscht.cz(M.V.);irena.kolouchova@vscht.cz(I.K.)
2
DepartmentofBiochemistryandMicrobiology,UniversityofChemistryandTechnology,Technická5,
16628Prague,CzechRepublic;petra.lovecka@vscht.cz(P.L.);blanka.vrchotova@vscht.cz(B.V.)
3
DepartmentofFoodAnalysisandNutrition,UniversityofChemistryandTechnology,Technická5,
16628Prague,CzechRepublic;milena.stranska@vscht.cz
4
InstituteofMicrobiology,AcademyofSciencesoftheCzechRepublic,Vídeňská1083,14220Prague,
CzechRepublic;mkolarik@biomed.cas.cz
*Correspondence:marketa.kulisova@vscht.cz
Abstract:Endophytesaremicroorganismsthatliveasymptomaticallyinsideplanttissues.Theyare
beneficialtotheirhostinmanyaspects,especiallyasadefenseagainstforeignphytopathogensthrough
theproductionofavarietyofmetabolites.Thesesubstancescanserveassourcesofnewnaturalproducts
formedicinal,agricultural,andindustrialpurposes.Thisarticleisfocusedonendophyticfungifrom
Vitisvinifera.ThepurposeoftheresearchwastheirisolationandidentificationduringtheVitisvinifera
growingseason.Subsequently,theisolatesweretestedfortheproductionofbiotechnologically
interestingmetabolites(siderophores,antioxidants,andantifungalcompounds).Intotal,24endophytic
fungiwereisolated,themostrepresentedgenuswasCladosporiumsp.Theresultsofthetestfor
antioxidantandantifungalproperties,aswellassiderophoreproduction,haveshownthatthe
populationofVitisviniferaendophyticmicroscopicfungicouldserveasapromisingsourceof
metaboliteswithawiderangeofapplications.
Keywords:microscopicfungi;endophytes;Vitisvinifera;antifungalactivity;antioxidants;siderophores
1.Introduction
Thegrapevine(Vitisvinifera)isoneofthemosteconomicallyimportantcrops,beingused
mainlyforwineproduction(approximately80%ofharvestedwinegrapesisusedforthis
purpose).GrapesandotherpartsofVitisviniferacontainanumberofhealthpromoting
metabolites[1].Aswithotherplants,thetissuesofthegrapevineareinhabitedbyvarious
typesofmicroorganisms.Theseorganismscanbeepiphytic,i.e.,superficial,orcolonizing
internaltissues,i.e.,endophytic[2].Theinteractionsbetweenendophytesandtheirplant
hostsarediverse.Plantsprovideprotectionandendophyticmicroorganismsarecapableof
producingusefulmetabolitesthatincreasenutrientuptake,induceresistancetopathogens,
increasetolerancetoosmoticstress,heavymetals,xenobioticcontaminants,andotherforms
ofabioticstress[3].Mostendophytesarerepresentedbybacteria,butmicroscopicfungiand
yeastsalsoformasignificantpartoftheendophyticpopulation.Endophytesareisolatedfrom
avarietyofplantspecies,andalmostallstudiedplantspecieshavebeenfoundtohostatleast
oneendophyticmicroorganism[4].Colonizationofthehostplantwithuptoahundred
Citation:Kulišová,M.;
Vrublevskaya,M.;Lovecká,P.;
Vrchotová,B.;Stránská,M.;
Kolařík,M.;Kolouchová,I.Fungal
EndophytesofVitisvinifera—Plant
GrowthPromotionFactors.
A
griculture2021,11,1250.
https://doi.org/10.3390/
agriculture11121250
AcademicEditor:OfirDegani
Received:11November2021
Accepted:9December2021
Published:10December2021
Publisher’sNote:MDPIstays
neutralwithregardtojurisdictional
claimsinpublishedmapsand
institutionalaffiliations.
Copyright:©2021bytheauthors.
LicenseeMDPI,Basel,Switzerland.
Thisarticleisanopenaccessarticle
distributedunderthetermsand
conditionsoftheCreativeCommons
Attribution(CCBY)license
(http://creativecommons.org/licenses
/by/4.0/).
Agriculture2021,11,12502of14
differentspeciesisnoexception.Geographiclocation,season,climate,andtypeofplanttissue
areamongthefactorsthataffectspeciescompositionandfrequencyofendophyte
colonization[5,6].
Endophytesactasimportantsourcesofstructurallyuniquebioactivenaturalmetabolites
withawidebiotechnologicalpotential.Theyrepresentanattractivesourceofnatural
productsthatcanbeusedinagriculture,industry,andmedicine[7–13].Theformationof
antibacterial,antifungal,antiviral,cytotoxic,andimmunosuppressivemetabolites,aswellas
antioxidantsandsiderophores,hasbeenpreviouslyfound[14,15].Nowadays,whennew
diseasescausedbymicroorganismsareemergingandresistancetoknowndrugsisspreading,
itisthesiderophoresofarelativelypoorlystudiedendophyticpopulationthatcanbeusedto
developactivesubstancesinthepharmaceuticalindustry[16].
Severalresearchershaverecentlyinvestigatedgrapevinefungalendophytestoclarify
theirdiversityandecologicalroleinthisplant.Theuseofchemicalsasfertilizersin
agriculture,endophytesproducingantibacterialandantifungalcompoundscouldbean
interestingalternativetotheseactivesubstances.Phomaglomerata,Chaetomiumglobosum,
Aureobasidiumpullulans,Epicoccumnigrum,andAcremoniumspp.haverepeatedlyexhibited
antibacterialandantifungalpropertieseffectiveagainstanumberofplantdiseases[6,17–22].
AlternariaalternataandFusariumproliferatumhavealsobeenidentifiedaspromisingbiocontrol
agentsagainstspecificpathologicalconditionsofVitisvinifera,suchasgrapevinedowny
mildewcausedbyPlasmoparaviticola[6,22,23].
Resveratrol,asanantioxidantcompoundknowntoincreaseresistancetostressand
prolongthelifeofavarietyoforganisms,fromyeaststovertebrates,isabundantinVitis
viniferagrapes.Manyendophytesshowtheabilitytoproducethesamefunctionalcompounds
astheirhostswhilelivingasymptomaticallyinplanttissues.Fungalendophytescapableof
resveratrolproductionincludePenicillium,Aspergillus,Mucor,Alternaria,Cephalosporium,and
Geotrichum.Alternariaspeciesappeartobethebestproducersduetothestableproductionof
resveratrol[24].TheresearchworkofYangetal.[25]investigatedtheroleofendophytesin
theformationofsecondarymetabolites(totalflavonoidsandresveratrol)andtheinfluenceof
physio‐chemicaltraitsingrapesandleaves.FungalendophytesoriginallyisolatedfromVitis
viniferawerere‐inoculatedongrowinggrapevineplantsandtheireffectongrapesandleaves
wasevaluated.Thisinoculationincreasedthecontentofreducingsugar,totalflavonoids,
polyphenols,andtrans‐resveratrolinparticularpartsofVitisvinifera.Nigrosporasp.and
Fusariumsp.appearedtobethemostpromisingofthefungalgenerastudied.
Theaimofourstudywastoisolateandcharacterizetheendophyticfungithatoccurin
Vitisviniferaleaves,canes,andberriesgrowninvineyardswithintheCzechRepublic.Another
goalwastoinvestigatetheirpotentialtoactasplantgrowthpromotersanddiseaseprotective
agents.Thiswasdonebytestingtheirabilitytoproduceantioxidants,siderophores,and
antifungalcompounds.
2.MaterialsandMethods
2.1.Samples
SamplesofMullerThurgau,PinotGris,PinotNoirandRieslingRheinhessengrapevine
varietieswerecollectedfromtwodifferentvineyardswithintheCzechRepublic,KutnaHora
(49.9336N,15.2889E;grapevinegrownaccordingtotheprinciplesoforganicfarming)and
Prague(50.0690N,14.4454E;conventionallygrowngrapevine).Threedifferentexperimental
plantslocatedatdifferentsiteswithinthevineyardswereselectedforcontinuoussampling
duringtheentirevegetationyear.Thesamplingofleavesandcanesaslignifiedstemsofthe
plantswascarriedoutinJanuary,May,AugustandOctober2019inapproximateamounts
between3and10gofleaves,dependingonthesamplingseason(leaveswerenotsampledin
Januaryduetotheirfallduringtheautumn),andcaneswerecollectedinapproximate
Agriculture2021,11,12503of14
amountsof50g.BerriesweresampledinSeptember2019inanamountof500g,onlyfrom
thePraguevineyard(strongstormsinKutnaHoraregionruinedthecropsandsamplingwas
notpossible).Thesampleswerestoredat−80°Cbeforeprocessinginthelaboratory.
CharacterizationofthesamplesfromwhichwerefungalendophytesisprovidedinTable1.
Table1.CharacterizationofVitisviniferaleaves/canes/berriesfromwhichfungalendophyteswereisolated.
SampleCodeSamplingPeriodGrapevineVarietyPlantPartGrowingLocality(FarmingSystem)
Z‐MT‐G‐S6January2019MullerThurgaucanesPrague(conventional)
Z‐RR‐G‐SJanuary2019RieslingRheinhessencanesPrague(conventional)
Z‐MT‐KH‐S1January2019MullerThurgaucanesKutnaHora(organic)
Z‐MT‐KH‐S2January2019MullerThurgaucanesKutnaHora(organic)
Z‐RM‐KH‐S1January2019PinotNoircanesKutnaHora(organic)
Z‐RM‐KH‐S2January2019PinotNoircanesKutnaHora(organic)
J‐MT‐G‐L2May2019MullerThurgauleavesPrague(conventional)
J‐RR‐G‐S2May2019RieslingRheinhessencanesPrague(conventional)
J‐RS‐KH‐L1May2019PinotGrisleavesKutnaHora(organic)
J‐RS‐KH‐L2May2019PinotGrisleavesKutnaHora(organic)
J‐MT‐KH‐S4May2019MullerThurgaucanesKutnaHora(organic)
J‐RM‐KH‐S3May2019PinotNoircanesKutnaHora(organic)
J‐RM‐KH‐S4May2019PinotNoircanesKutnaHora(organic)
J‐RM‐KH‐S5May2019PinotNoircanesKutnaHora(organic)
J‐RR‐KH‐S2May2019RieslingRheinhessencanesKutnaHora(organic)
J‐RR‐KH‐S3May2019RieslingRheinhessencanesKutnaHora(organic)
L‐MT‐KH‐L5August2019MullerThurgauleavesKutnaHora(organic)
L‐RS‐KH‐L4August2019PinotGrisleavesKutnaHora(organic)
L‐RR‐KH‐L4August2019RieslingRheinhessenleavesKutnaHora(organic)
L‐RM‐KH‐S6August2019PinotNoircanesKutnaHora(organic)
P‐RM‐G‐L1October2019PinotNoirleavesPrague(conventional)
P‐RS‐G‐S2October2019PinotGriscanesPrague(conventional)
P‐MT‐KH‐L7October2019MullerThurgauleavesKutnaHora(organic)
P‐RM‐KH‐L7October2019PinotNoirleavesKutnaHora(organic)
MT‐M1September2019MullerThurgauberriesPrague(conventional)
MT‐M4September2019MullerThurgauberriesPrague(conventional)
RR‐M1September2019RieslingRheinhessenberriesPrague(conventional)
RR‐M2September2019RieslingRheinhessenberriesPrague(conventional)
RS‐M2September2019PinotGrisberriesPrague(conventional)
2.2.FungalEndophytesIsolationandCultivation
Theplantmaterialwassurfacesterilizedbysequentialimmersionin0.625%aqueous
sodiumhypochloritewithadropofTween80(7min),followedby70%aqueousethanol(3
min).Aftertheseprocedures,thesampleswererinsedfourtimeswithsterilizedwater(15
min).Thesurface‐sterilizedtissueswerehomogenizedandusedtoinoculateYGCmedium
(yeastextractglucosechloramphenicolagar)andincubatedat20°Cfor72hormore.
2.3.MolecularGeneticIdentificationofEndophytes
GenomicDNAwasisolatedfrompurefungusculturebyusingtheArchivePureDNA
YeastandGram‐+Kit(5PRIME,Hamburg,Germany).Subsequently,thenuclearribosomal
ITS1‐5,8S‐ITS2regionwasdeterminedforallstrainsaccordingtoKolaříketal.[26].Dueto
Agriculture2021,11,12504of14
thelowresolutionoftheITSregioninsomefungalspecies,thesequencingofothersections
wasmadetoclarifytheidentification.Elongationfactor1alpha(EF1α)wasamplifiedand
sequencedusingprimersEF‐728F/EF‐986RandEF1‐983F/EF1‐2218RaccordingtoKolaříket
al.[26].Thepartialβ‐tubulin(TUB2)genewasamplifiedusingT1/T2accordingtoPíchováet
al.[27].Thesequencesobtainedweremanuallycutfromunreadablesectionsandthehighest
probabilityoftheacquiredsequencewassearchedintheGenBankdatabase.
2.4.DeterminationofSiderophoresProductionoftheIsolates
ThemethodofMarquesetal.[28]wasfollowedtodeterminesiderophoreproduction.
FungalcultureswereinoculatedonChromeazurolS(CAS)agar,andcultivatedat28°Cfor
7days.Aftercultivation,thecolorchange(bluetoyellow)wasevaluatedandscaled(0=blue
mediumsurface,nosiderophoreproduction;1=30%yellowmediumsurface—low
siderophoreproduction;2=60%yellowmediumsurface—mediumsiderophoreproduction,
3=yellowmediumsurface,highsiderophoreproduction(Figure1.)).
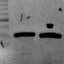
Figure1.FungalendophyteculturedonCASagarwithhighsiderophoreproductionactivity(color
changefrombluetoyellow).
2.5.DeterminationofAntioxidantActivityoftheIsolates
ThefungalendophyteisolatesweregrowninPDBmediumat30°Cfor7dayswith
constantshaking.Theantioxidantactivityofthesupernatantofthefilteredculturewas
determinedaccordingtoFidlerandKolářová[29].Theanalyseswereperformedonthe
microtiterplatesinthreeparallelsforeachsample.Analiquotof100μLofthesamplewas
pipettedtogetherwith200μLofDPPHataconcentrationof52mgL−1(inmethanol)inthe
wells.Distilledwaterwasusedasablank.Theplatewasincubatedinthedarkfor15min.
Theabsorbancewasmeasuredat517nm.Theresultsoftheanalysiswereexpressedasthe
percentdecreaseinthediscolorationofthesolutionagainsttheblank.Theresultswere
expressedasanascorbicacid(AA)equivalent,whichwaschosenasananalyticalstandardin
theconcentrationrangeof2.5–25mgL−1.
2.6.DeterminationofAntifungalActivityoftheIsolates
AntifungalactivitywastestedonPDAagaraccordingtoBelletal.[30].Twowells(7.5
mmdiameter)wereexcavatedintheagaratthesamedistancefromthecenter.Oneofthe
wellswasfilledwithagarwithagrownfungalendophyteandtheotherwithagarcontaining
afungalphytopathogen.Theseplateswerecultivatedat28°Cfor7days.Testingwascarried
outwiththreefungalphytopathogens—BotrytiscinereaDBM1246,FusariumsolaniCCF2967,
andMucorplumbeusCCF2626.Thephytopathogeniccultureitselfservedasacontrolsample.
Antifungalactivitywasdisplayedbyslowingorstoppingthegrowthofafungal
phytopathogeninthevicinityofthegrowthofanendophyticfungus.Thedegreeoffungal
antagonismwasevaluatedonascaleof5–1(5—theendophytecompletelyoutgrowsthe
phytopathogen;4—theendophytecolonizes2/3ofthemediumsurface;3—theendophyteand
thephytopathogenbothcolonizehalfofthemediumsurface(Figure2);2—phytopathogen
Agriculture2021,11,12505of14
colonizes2/3ofthemediumsurface;1—thephytopathogencompletelyoutgrowsthe
endophyte).
Figure2.Fungalendophytewiththedegreeofantagonismoflevel‘3’tophytopatogen(colonizationof
halfofthemediumsurface).
2.7.StatisticalAnalysis
Dixon’sQtestwasperformedtodetectoutliersindatasetsobtainedbythedetermination
ofantioxidantactivity(thedeterminationwasperformedinfiveparallels.Thedeviationof
thefivedeterminationswaslessthan5%).Thedeterminationofabilitytoproduce
siderophoresandantifungalactivitywasperformedinthreeparallels.
3.Results
3.1.FungalEndophytesCharacterizationandMolecularGeneticIdentification
Forcanesandleaves,atotalof24endophyticmicroscopicfungibelongingto14fungal
generawereisolatedfrombothvineyards.Sixisolateswereobtainedduringthewinterfrom
bothconventionalandorganicfarminglocalities,tenisolateswereobtainedfromthespring
collection,withthemajorityofendophytesoriginatingfromorganicallygrownplants,four
fungalendophyticspecieswereisolatedfromsummersamplesfromtheorganicfarming
region,andfourisolatescamefromtheautumnsampling,frombothfarmingsystem
localities.ThegenusCladosporiumwasrepresentedbytwospecies,Cladosporiumcladosporioides
andCladosporiumherbarum.ThefurthermorerepresentedgenerawereDidymellasp.,
Aspergillussp.,Aureobasidiumsp.andAlternariasp.Allfiveisolatesobtainedfromtheberries
belongtoPenicilliumsp.,specificallytothespeciesPenicilliumcructosum(fordetails,seeTable
2).
Agriculture2021,11,12506of14
Table2.Thespeciesoffungalendophytesisolatedfromcanes,leavesandberriestaxonomyidentification,togetherwith
biologicalactivitiesofparticularisolates.
SampleCode1Sample
Matrix
EndophyteSpecies
Taxonomy
AbilitytoProduce
Siderophores2
Antioxidant
Activity
(mgAAL−1)
AntifungalActivity3to:
Botrytis
cinerea
Fusarium
solani
Mucor
plumbeus
Z‐MT‐G‐S6 canesCladosporiumcladosporioides112.4222
Z‐RR‐G‐Scanes
A
lternariaarborescens013.8323
Z‐MT‐KH‐S1canesDiatrypestigma317.5222
Z‐MT‐KH‐S2canesDidymellanegriana04.8322
Z‐RM‐KH‐S1canesAspergilluspseudodeflectus221.8332
Z‐RM‐KH‐S2canesAspergillusniger313.4244
J‐MT‐G‐L2leavesEpicoccumnigrum217.6332
J‐RR‐G‐S2canesPleurophomaossicola02.7233
J‐RS‐KH‐L1leavesSporocadusrosigena16.6332
J‐RS‐KH‐L2leavesDendrophomajuglandina20222
J‐MT‐KH‐S4canesPseudogymnoascus
pannorum 00232
J‐RM‐KH‐S3canesAureobasidiumpullulans16.4222
J‐RM‐KH‐S4canesDidymellasancta18.3222
J‐RM‐KH‐S5canesCladosporiumherbarum07.5332
J‐RR‐KH‐S2canesPhaeosphaeriaceaesp.07.8222
J‐RR‐KH‐S3canesNeosetophomashoemakeri 26.7233
L‐MT‐KH‐L5leavesAspergillusfumigatus 19.3243
L‐RS‐KH‐L4leavesLophiostomacorticola18.3411
L‐RR‐KH‐L4leavesCladosporiumherbarum08.1232
L‐RM‐KH‐S6canesAureobasidiumpullulans10332
P‐RM‐G‐L1leaves
A
lternariaastroemeriae07.5332
P‐RS‐G‐S2canesAureobasidiumpullulans17.1332
P‐MT‐KH‐L7leavesCladosporiumherbarum 00111
P‐RM‐KH‐L7leavesDidymellasancta13.4333
MT‐M1berriesPenicilliumcrustosum110.5332
MT‐M4berriesPenicilliumcrustosum39.3452
RR‐M1berriesPenicilliumcrustosum113.9232
RR‐M2berriesPenicilliumcrustosum323.9453
RS‐M2berriesPenicilliumcrustosum319.1452
Boldformattingvalues—highabilitytoproducesiderophores,orhighantioxidantactivityorhighantifungalactivity;1See
Table1.forsamplecharacterization;2‘0’—nosiderophoreproduction;‘1’—lowsiderophoreproduction;‘2’—medium
siderophoreproduction,‘3’—highsiderophoreproduction;3Degreeofantagonism:5—theendophytecompletelyoutgrows
thephytopathogen;4—theendophytecolonizes2/3ofthemediumsurface;3—endophyteandphytopathogencolonizeeach
½ofthemediumsurface;2—phytopathogencolonizes2/3ofthemediumsurface;1—thephytopathogencompletelyoutgrows
theendophyte.
3.2.ProductionofSiderophores
Siderophoreproductionwasestablishedfor83%ofisolatesfromthewinterbiomass
collection,in60%ofisolatesfromthespringsampling,in75%ofisolatesfromsummer,in
50%offungalendophytesspeciesbeingisolatedfromautumnleavesandcanesamples,and
Agriculture2021,11,12507of14
inallendophytesisolatedfromberries.Thehighestabilitytoproducesiderophores(degree
‘3’)wasdetectedforendophytesfromwintersamplingandorganicallygrowncanes,in
particularforDiatrypestigma(Z‐MT‐KH‐S1isolate)andAspergillusniger(Z‐RM‐KH‐S2
isolate),andforendophytesoriginatingfromberries,i.e.,Penicilliumcrustosum(MT‐M4
isolate,RR‐M2isolate,RS‐M2isolate).
IsolatesJ‐MT‐G‐L2(Epicoccumnigrum),J‐RS‐KH‐L2(Dendrophomajuglandina)andJ‐RR‐
KH‐S3(Neosetophomashoemakeri)fromspringsamplingshowedthemediumabilityto
producesiderophores(degree‘2’),thehighestoneforthegivenperiod.Regardingthe
summerandautumnsampling,allisolateshadloworzerosiderophoreproductionability
(degree‘1’or‘0’),withtheexceptionofthethreeabove‐mentionedisolatesfromberries.The
detailsaresummarizedinTable2.
3.3.AntioxidantActivity
Theabilitytoproduceantioxidantsintothemediumwasidentifiedinallendophytes
isolatedfromwintercanes,in80%ofendophytesisolatedfromthespringbiomasscollection,
in75%ofisolatesoriginatingfromthesummerandautumnV.viniferabiomass,andinallof
theisolatesfromberries.Thecontentofantioxidantsexpressedasascorbicacid(AA)
equivalentwasdeterminedintherangeof4.8–21.8mgAAL−1forthewinterisolates,0–17.6
mgAAL−1forthespringisolates,0–9.3mgAAL−1forthesummerisolates,0–7.5mgAAL−1forthe
autumnendophytesfromleavesandcanesand9.3–23.9mgAAL−1fortheautumnisolatesfrom
berries.Theendophyteswiththehighestantioxidantproductionwereisolatesfromberries,
i.e.,Penicilliumcrustosum(RR‐M2isolateandRS‐M2isolate),with23.9and19.1mgAAL−1,
respectively,andAspergilluspseudodeflectus(Z‐RM‐KH‐S1isolate)fromwinterorganically
farmedcanes(21.8mgAAL−1).Fordetails,seeTable2.
3.4.AntifungalActivity
Thehighestdegreeofantagonism,level‘5’explainingthehighestantagonismwherethe
endophytecompletelyoutgrowsthephytopathogen,wasagainstF.solaniandwasobserved
forPenicilliumcrustosum(MT‐4,RR‐M2,RS‐M2)isolatedfromberries.Allthesethree
endophyticisolatesalsoshowedsignificantantifungalactivityagainstB.cinerea(level‘4’).
TheotherrelativelystrongantagonistofB.cinereaDBM4111wastheendophyte
Lophiostomacorticola(L‐RS‐KH‐L4)isolatedfromorganicallygrownleavescollectedin
summer.ForthephytopathogenF.solaniCCF2967,theotherhighlyeffectiveendophytes
wereAspergillusniger(Z‐RM‐KH‐S2)isolatedfromorganicallyfarmedwintercanesand
Aspergillusfumigatus(L‐MT‐KH‐L5)isolatedfromorganicallyfarmedsummerleaves.For
MucorplumbeusCCF2626,thehighestantagonistwasAspergillusniger(Z‐RM‐KH‐S2)isolated
fromorganicallygrownwintercanes.Fordetails,seeTable2.
4.Discussion
Recently,endophyticmicroorganismsandtheirproductshavebeenattractingthe
attentionofthescientificcommunityasarelativelypoorlyunderstoodsourceofawiderange
ofchemicallydiversenaturalsubstancespotentiallyusableinbiotechnology,pharmaceutical
andfoodindustry.Theincreasedattentionforstudyingendophyticpopulationsisbasedon
thedesiretoproducenon‐chemicalbasedsolutions.Comparingendophyticpopulationsin‐
situintermsofthepresenceofindividualmicroorganismsortheformationoftheir
metabolitesischallengingduetothemanyfactors(altitude,temperature,totalprecipitation,
rhizospherecomposition,orpesticideuse)thataffectthesepopulations.Despitethis,the
methodsfortestingtheendophyticisolatesinlaboratoryconditionsarewellestablished.
Agriculture2021,11,12508of14
4.1.FungalEndophytesCharacterizationandMolecularGeneticIdentification
Anotherdegreeofvariabilityintheendophytecompositionisthephysiologicalstateof
thehostplantitself,itsgrowthphase,andthetissuefromwhichthesampleistaken.Thus,
evenisolatesfromthesamegeographicallocationmaybediametricallydifferent[31–34].This
variabilitywasconfirmedinthiswork,whereisolatesoffungalendophytesfromtwo
vineyardsofdifferentfarmingsystemswereexamined.TheKutnaHoravineyardsgrow
grapevinesaccordingtotheprinciplesoforganicfarming,andthePraguevineyardsgrow
theirgrapevinesinaconventionalway.Thetotalnumberoffungalendophytesisolatedfrom
canesandleavesfromorganicallygrownplantswasapproximatelythreetimeshigherthan
thenumberofendophytesisolatedfromconventionalvineyards,whichisconsistentwith
previousstudies[35,36].Thisdifferencecouldberelatedtotheuseofchemicalororganic
fertilizersandherbicidesthatdirectlyaffectmicroorganismsoralterthephysiologyofthe
hostplant[37,38].Theresponseofendophyticmicrobialcommunitiestotheseexternal
productsisverybeneficialforcomparingorganicandconventionalagriculture,andfurther
researchcouldgointhisdirection.
Precipitationisoneofthemainabioticfactorsthataffectthedensityofendophytesinthe
hostplant[39–41].Theproportionofendophytesintheleavesoftreesthathavebeen
protectedfromrainislowerthanintheleavesofidenticaltrees,butunprotectedfromrainfall.
Suryanarayananetal.dealtwiththisissueintherainforestenvironmentduringtherainy
seasonanddrought.InalltestedleafsamplesofBauhiniaracemosa,Ixoranigricans,Erythroxylon
monogynumandElaeodendronglaucum,anincreasedrepresentationoftheendophytic
communitywasdetectedduringtherainyseason[40].Themoreabundantcolonizationof
plantsbyfungalmicroorganismsathigherprecipitationratesmayberelatedtothe
consequentincreasednumberofendophytesinthehostplant.Precipitationisalsooneofthe
majortypesofendophyticsporetransmission.Sofar,thereisalittleinformationonwhere
endophytesporesareproduced,wheretheycanhibernateandwhatthemodeoftransmission
is.R.parkerisporulatesprolificallyonContariniamidgegallsonDouglasfirneedles,andthere
weremeasured1200spores/mLinwaterdrippingfromaheavilygalledbranchlet.R.parkeri
anditsanamorphalsosporulateinthefallinabscisedneedles.Thesporemassesofthis
endophyteareproducedinmucilage,whichisindicativeofwatertransmission.Further,
newlyflushedneedlesinthespringdonotbecomeinfecteduntiltheyarerainedoninthe
fall.Fromthesefindingswecouldsuggestthattheassociationbetweenhigherendophyte
countsandmoistureisnotaccidental[41].Ifwefollowthenumberofcaneandleafisolates
obtainedfromindividualperiodsoftheVitisviniferagrowingyear,thepredominanceof
springsamplingisevident(Table1.).May2019(springsampling)waswellabovethelong‐
termprecipitationaverage.August2019(summersampling)wasonlyslightlybelowthis
averageandOctober2019(autumnsampling)wasquiteaverage,whichcorrespondstoa
lowerendophyticproportion(Figure3).However,heavyrainandstormscouldcompletely
destroythegrapevinecrop,whichunfortunatelyoccurredinautumn2019intheKutnaHora
vineyards.Thesenaturalphenomenamadethesamplingofberriesfromthisbiodynamic
vineyardinSeptember2019impossible.
Agriculture2021,11,12509of14
Figure3.AverageprecipitationfromFebruary2019toOctober2019comparedtothelong‐termaverage(1981–2010)(data
fromtheCzechHydrometeorologicalInstitute).
TheamplificationofITSrDNAandsubsequentcomparisonoftheobtainedsequence
withthedatabaseiscurrentlythemostwidelyusedmethodfortheidentificationoffungal
endophytes[42,43].All29isolatesbelongingto15generaweresuccessfullyidentified,andall
ofthembelongedtotheAscomycotaphylum.Thisphylumsignificantlypredominatesinthe
proportionoffungalendophytesinVitisvinifera,regardlessofthegeographicallocationofthe
hostplant[34,44].ThemostabundantgenerawerePenicilliumsp.,Cladosporiumsp.,Didymella
sp.,Aspergillussp.,Aureobasidiumsp.,andAlternariasp.Alternariasp.andCladosporiumsp.are
oneofthemostabundantendophytesofVitisvinifera[34,43],whichisinaccordancewithour
results.
4.2.ProductionofSiderophores
Siderophoreshavereceivedgreatattentioninmedicine,biotechnology,and
environmentalresearchduetotheirhighaffinityandspecificityforFe3+.Theonlyfungal
endophyteisolatesfromcanesandleaveswithahighabilitytoproducesiderophorescame
fromthewinterperiodof2019.Theabilitytoformthesecompoundshasbeendecliningsince
winter,withonlyloworzeroproductionactivityinsummerandautumn.Thehighestresult
ofsiderophoreproductioninwintercanbeexplainedbythereducedmovementofnutrients
inthesoilduetolowtemperaturesandthereforeirondeficiencyinboththeendophyteand
thehostplantandtheincreasedneedforuptakeofthesenutrientsbyothermechanisms[45].
DiatrypestigmaandAspergillusnigerwereisolateswiththehighestdetectedsiderophore
productionactivity.Inthispaper,theproductionofsiderophoresbythegenusDiatrypewas
provedforthefirsttime.Withregardtothehighactivityofproductionofthesecompounds
identified,itwouldbeinterestingtopayfurtherattentiontothespeciesofthismicrobial
genusintheresearch.Aspergillusspeciesarewell‐researchedproducersofsiderophores,
servingasamodelorganismtoelucidatethebiosynthesis,absorption,anddegradationof
thesesecondarymetabolites[46].Threeberryisolatesalsoshowedahighabilitytoproduce
siderophores.AlloftheseisolatesbelongtoPenicilliumcrustosum,whichisinagreementwith
thefindingsintheliteraturethatthisgenusiscapableofsiderophoreproduction[47].Ina
studyonthecharacterizationofsiderophoresproducedbyendophytesfromCymbidium
aloifolium,thegenusPenicilliumwasfoundtobethebestproducerofthesecompounds[48].
0
20
40
60
80
100
precipitation[mm]
averagemonthlyprecipitation(2019)
long‐termaveragemonthlyprecipitation(1981‐2010)
Agriculture2021,11,125010of14
4.3.AntioxidantActivity
Thereisgrowingevidenceofoxidativedamagetobiomoleculesbyfreeradicals.These
injuriescouldcausemuchtissueharm.Antioxidantsareconsideredhighlyeffectivein
defendingtissueagainstdamagecausedbyreactiveoxygenspecies[49].Fungalendophytes
canbeapotentiallyverygoodsourceofantioxidants[50]whichhasbeenconfirmedinisolates
inthisstudy.Theisolatewiththehighestantioxidantactivity(21.7mgAAL−1)ofcanesand
leaveswasAspergilluspseudodeflectus.AroraandChandra[51]investigatedtheantioxidant
activityofthegenusAspergillus,specificallyAspergillusfumigatus.Thedatahaveshownthat
thismicroorganismcanserveasapromisingsourceofantioxidantcompounds.Ourresults
showanevenhigherantioxidantactivityforthefungalendophyteAspergilluspseudodeflectus
thanwasmentionedinthearticle.Otherstudiesalsomentionthehighantioxidantactivityof
endophytesofAspergillussp.andthepossibilitiesoffurtheruseoftheseproperties[52,53].
Therefore,thisfungalgenuscouldservetomoreeasilyadjusttheproductionandpurification
ofnaturalantioxidants.
Penicilliumisanotherfungalendophyticgenusstudiedinmoredetailwithhigh
antioxidantactivity[7,54].Inberries,twoofthePenicilliumcrustosumisolatesshowedhigh
antioxidantactivity(23.9and19.1mgAAL−1)whichisinconnectionwithpreviousstudies
[7,55,56].OtherfungalendophyteswithhighantioxidantactivityareFusariumsp.[57,58]and
Burkholderiaphytofirmans[59].Inotherstudy,fungalendophytesDiaporthesp.,Colletotrichum
sp.,andArthiniumsptendtogenerateawidearrayofbioactivecompounds(β‐dihydro
agarofuran,α‐agarofuran,δ‐eudesmol,β‐agarofuran,andoxo‐agarospirol)withstrong
antioxidantactivity[60].AccordingtoHamiltonandBauerle,theantioxidantactivityin
plantswithendophytesunderabioticstressishigherthaninplantswithoutthese
microorganisms[61].
Ingeneral,theproportionofgenerawithantioxidantactivityinVitisviniferaisrelatively
high,whichcorrelateswiththeassumptionoftheformationofsimilarsecondarymetabolites
betweenthehostplantanditsendophytes.Grapevineitselfisanimportantsourceof
antioxidants,especiallyphenolicsubstances.
4.4.AntifungalActivity
ThefungalendophytesofVitisviniferacouldhaveanantagonisticeffectonsome
importantphytopathogens.Studiesmappingthisantifungalabilityofendophytic
communitiesareessentialtoshapepestcontrolstrategiesbutalsotopotentialproductionof
high‐qualityagriculturalproducts.Inthecaseofgrapevine,oneofitsmostcommon
pathogensisthefungusBotrytiscinerea,whichcausesBotrytisbunchrot.Themosteffective
antifungalagentsagainstthisphytopathogenaretheendophytesAlternariasp.andEpicoccum
sp.BotharealsopromisingbiocontrolagentsagainstPlasmoparaviticola,anotherimportant
sourceofVitisviniferadiseases[34].TheantifungalabilityfoundagainstBotrytiscinereain
AlternariaandEpicoccumisolatesinthisresearchworkwasexpressedbythedegreeof
antagonismatlevel‘3’,whichcouldbeexpressedas50%.Thementionedgeneradidnotshow
aboveaverageactivityevenagainsttheothertwotestedphytopathogensFusariumsolaniand
Mucorplumbeus.Fusariumsolaniisanimportantplantpathogenthatmostoftencausesrotin
therootsystem.Ithastheabilitytopenetratecellwallsandthereforecauseplanttissuetorot
[62].Mucorplumbeusisassociatedwiththegrowthoffungiincereals,rice,soybeans,nuts,
fruits,herbs,andothers[63].
Thehighestdegreeofantagonism(level‘4’)againstBotrytiscinereawasdetectedin
isolatesfromcanesandleavesinonlyoneendophyte,Lophiostomacorticola.Thisisthefirst
papertoshowtheabilityofthisfungustoproduceantifungalcompounds.Theabilityofthe
Lophiostomagenushasbeenshowntoproducemetabolitesthatareeffectiveonlyagainst
pathogenicbacteria[64,65].However,Lophiostomacorticolahadadegreeofantagonismof
Agriculture2021,11,125011of14
level‘1’againstFusariumsolaniandMucorplumbeusandthereforezeroantifungalactivity.For
suchaquestionableresult,itwouldbeidealtoperformantifungaltestswithother
phytopathogenstodetecttheantifungalactivityofthisgenus.Whenwecontinuewiththe
antifungalresultsfromisolatesconnectedwithcanesandleaves,thehighestactivityagainst
FusariumsolaniwasdetectedintwoisolatesofthegenusAspergillus,Aspergillusnigerand
Aspergillusfumigatus.Aspergillusnigerwastheonlyspeciestoshowthehighestactivityalso
againstMucorplumbeus.AntifungalactivityagainstthephytopathogensGiberellazeae,
Thanatephoruscucumerisandsixothernonpathogenicmicroscopicfungiwasdetectedinthe
endophyteAspergillusfumigatusisolatedfromHyoscyamusmuticus[66].Aspergillusflavus,an
endophyteofLanneacoromandelica,showedhighantifungalactivityagainstCandidaalbicans
andMalasseziapachydermis.Aspergillusnigerisolatedfromthesamehostplantshoweda
moderateabilitytoformantifungalmetabolitesagainstthementionedphytopathogens[67].
Fromthementionedstudies,itcanbeconcludedthattheantifungalactivityofthegenus
Aspergillusishigh,whichisinaccordancewithourresults.ThegenusPenicilliumisknown
foritsantifungaleffectonBotrytiscinerea[7,68,69]andalsoshowsthiseffectonFusariumsp.
[70,71].Penicilliumcrustosumisolatesfromberriesconfirmedthesefindings,astheyexhibited
highantifungalactivityagainstbothBotrytiscinereaandFusariumsolani.
5.Conclusions
ThepopulationofendophyticfungiofVitisviniferahasproventobeapromisingsource
ofgrowth‐promotingandprotectivepropertiesusefulfortheplant.Furtherstudiesare
neededtoinvestigateendophyticfungiindetailasapotentialsourceofsecondary
metabolites.Asitisaverypoorlyresearchedsourceofmetabolites,itwouldbeinterestingto
usethefindingsofthisresearchwork,choosethemostproductiveendophyticspecies,and
conductdetailedresearch.Acloserfocusontheformationofsiderophorescouldbevery
usefulinconjunctionwithenhancementofplantgrowthandbiocontrolagainst
phytopathogens.Thestudyofantifungalmetabolitescouldbeusedtodevelopeffective
biopesticidesthatcouldbeamoreenvironmentallyfriendlyoptionforboththeplantandthe
environment.
AuthorContributions:Conceptualization,I.K.,P.L.andM.K.(MarkétaKulišová);methodology,P.L.,
I.K.,M.K.(MiroslavKolařík)andB.V.;formalanalysis,M.K.(MarkétaKulišová)andI.K.;investigation,
M.K.(MarkétaKulišová),B.V.,M.V.andM.K.(MiroslavKolařík);resources,P.L.,M.S.andI.K.;data
curation,M.K.(MarkétaKulišová),M.V.,P.L.andI.K.;writing—originaldraftpreparation,M.K.
(MarkétaKulišová)andM.S.;writing—reviewandediting,M.K.(MarkétaKulišová);visualization,
M.K.(MarkétaKulišová)andM.V.;supervision,P.L.andI.K.;projectadministration,I.K.andM.S.All
authorshavereadandagreedtothepublishedversionofthemanuscript.
Funding:ThisresearchwasfundedbyTheCzechScienceFoundation(GACR),grantnumber18‐26463S.
InstitutionalReviewBoardStatement:Notapplicable.
InformedConsentStatement:Notapplicable.
Acknowledgments:WeacknowledgePavelBulánekforthepossibilityofcollectingVitisvinifera
samplesonvineyardsinPragueandStanislavRudolfskýonvineyardsinKutnaHora.
ConflictsofInterest:Theauthorsdeclarenoconflictofinterest.
References
1. Kolouchová,I.;Melzoch,K.;Šmidrkal,J.;Filip,V.Thecontentofresveratrolinvegetablesandfruit.Chem.Listy2005,99,492–495.
2. Schulz,B.Mutualisticinteractionswithfungalrootendophytes.InMicrobialRootEndophytes;Springer:Berlin/Heidelberg,Germany,
2006;pp.261–279.
3. Maheshwari,D.K.;Maheshwari,R.;Rinaudo.Endophytes:BiologyandBiotechnology;Springer:Berlin/Heidelberg,Germany,2017.
Agriculture2021,11,125012of14
4. Kusari,S.;Hertweck,C.;Spiteller,M.Chemicalecologyofendophyticfungi:Originsofsecondarymetabolites.Chem.Biol.2012,19,
792–798.
5. Yadav,A.N.;Singh,S.;Mishra,S.;Gupta,A.RecentAdvancementinWhiteBiotechnologythroughFungi;Springer:Berlin/Heidelberg,
Germany,2019.
6. González,V.;Tello,M.L.TheendophyticmycotaassociatedwithVitisviniferaincentralSpain.FungalDivers.2011,47,29–42.
7. Toghueo,R.M.K.;Boyom,F.F.EndophyticPenicilliumspeciesandtheiragricultural,biotechnological,andpharmaceutical
applications.3Biotech2020,10,107.
8. Gupta,S.;Chaturvedi,P.;Kulkarni,M.G.;VanStaden,J.Acriticalreviewonexploitingthepharmaceuticalpotentialofplant
endophyticfungi.Biotechnol.Adv.2020,39,107462.
9. Dina,B.;Mamtaj,S.Endophyticmicroorganisms:Colonization,plant‐microbeinteraction,diversityandtheirBioprospecting.Res.
J.Biotechnol.2020,15,151–179.
10. Zimowska,B.;Bielecka,M.;Abramczyk,B.;Nicoletti,R.Bioactiveproductsfromendophyticfungiofsages(Salviaspp.).Agriculture
2020,10,543.
11. Yadav,G.;Meena,M.BioprospectingofendophytesinmedicinalplantsofTharDesert:Anattractiveresourcefor
biopharmaceuticals.Biotechnol.Rep.2021,30,e00629.
12. Choudhary,M.;Gupta,S.;Dhar,M.K.;Kaul,S.EndophyticFungi‐MediatedBiocatalysisandBiotransformationsPavingtheWay
TowardGreenChemistry.Front.Bioeng.Biotechnol.2021,9,419.
13. Ntemafack,A.;Kapoor,N.;Ali,S.;Jamwal,V.L.;Hassan,Q.P.;Gandhi,S.G.ComprehensiveReviewofEndophyticFlorafrom
AfricanMedicinalPlants.Curr.Microbiol.2021,78,2860–2898.
14. Ancheeva,E.;Daletos,G.;Proksch,P.Bioactivesecondarymetabolitesfromendophyticfungi.Curr.Med.Chem.2020,27,1836–1854.
15. Tan,R.X.;Zou,W.X.Endophytes:Arichsourceoffunctionalmetabolites.Nat.Prod.Rep.2001,18,448–459.
16. Górska,A.;Sloderbach,A.;Marszałł,M.P.Siderophore–drugcomplexes:Potentialmedicinalapplicationsofthe‘Trojan
horse’strategy.TrendsPharmacol.Sci.2014,35,442–449.
17. El‐Tarabily,K.A.;Sivasithamparam,K.Potentialofyeastsasbiocontrolagentsofsoil‐bornefungalplantpathogensandasplant
growthpromoters.Mycoscience2006,47,25–35.
18. Schena,L.;Ippolito,A.;Zahavi,T.;Cohen,L.;Nigro,F.;Droby,S.GeneticdiversityandbiocontrolactivityofAureobasidium
pullulansisolatesagainstpostharvestrots.PostharvestBiol.Technol.1999,17,189–199.
19. Schena,L.;Nigro,F.;Pentimone,I.;Ligorio,A.;Ippolito,A.Controlofpostharvestrotsofsweetcherriesandtablegrapeswith
endophyticisolatesofAureobasidiumpullulans.PostharvestBiol.Technol.2003,30,209–220.
20. Sullivan,R.F.;White,J.F.,Jr.Phomaglomerataasamycoparasiteofpowderymildew.Appl.Environ.Microbiol.2000,66,425–427.
21. Zhao,X.;Hu,Z.;Hou,D.;Xu,H.;Song,P.BiodiversityandantifungalpotentialofendophyticfungifromthemedicinalplantCornus
officinalis.Symbiosis2020,81,223–233.
22. Aleynova,O.A.;Suprun,A.R.;Nityagovsky,N.N.;Dubrovina,A.S.;Kiselev,K.V.TheInfluenceoftheGrapevineBacterialand
FungalEndophytesonBiomassAccumulationandStilbeneProductionbytheInVitroCultivatedCellsofVitisamurensisRupr.
Plants2021,10,1276.
23. Mondello,V.;Spagnolo,A.;Larignon,P.;Clement,C.;Fontaine,F.PhytoprotectionpotentialofFusariumproliferatumforcontrol
ofBotryosphaeriadiebackpathogensingrapevine.Phytopathol.Mediterr.2019,58,293–306.
24. Shi,J.;Zeng,Q.;Liu,Y.;Pan,Z.Alternariasp.MG1,aresveratrol‐producingfungus:Isolation,identification,andoptimalcultivation
conditionsforresveratrolproduction.Appl.Microbiol.Biotechnol.2012,95,369–379.
25. Yang,M.‐Z.;Ma,M.‐D.;Yuan,M.‐Q.;Huang,Z.‐Y.;Yang,W.‐X.;Zhang,H.‐B.;Huang,L.‐H.;Ren,A.‐Y.;Shan,H.Fungal
EndophytesasaMetabolicFine‐TuningRegulatorforWineGrape.PLoSONE2016,11,e0163186.
26. Kolařík,M.;Spakowicz,D.J.;Gazis,R.;Shaw,J.;Kubátová,A.;Nováková,A.;Chudíčková,M.;Forcina,G.C.;Kang,K.W.;Kelnarová,
I.Biatriospora(Ascomycota:Pleosporales)isanecologicallydiversegenusincludingfacultativemarinefungiandendophyteswith
biotechnologicalpotential.PlantSyst.Evol.2017,303,35–50.
27. Píchová,K.;Pažoutová,S.;Kostovčík,M.;Chudíčková,M.;Stodůlková,E.;Novák,P.;Flieger,M.;vanderLinde,E.;Kolařík,M.
Evolutionaryhistoryofergotwithanewinfragenericclassification(Hypocreales:Clavicipitaceae:Claviceps).Mol.Phylogenetics
Evol.2018,123,73–87.
28. Marques,A.P.;Pires,C.;Moreira,H.;Rangel,A.O.;Castro,P.M.Assessmentoftheplantgrowthpromotionabilitiesofsixbacterial
isolatesusingZeamaysasindicatorplant.SoilBiol.Biochem.2010,42,1229–1235.
29. Fidler,M.;Kolářová,L.Analýzaantioxidantůvchmeluapivu.Chem.Listy2009,103,232–235.
30. Bell,D.;Wells,H.;Markham,C.InvitroantagonismofTrichodermaspeciesagainstsixfungalplantpathogens.Phytopathology1982,
72,379–382.
31. Aly,A.H.;Debbab,A.;Proksch,P.Fungalendophytes:Uniqueplantinhabitantswithgreatpromises.Appl.Microbiol.Biotechnol.
2011,90,1829–1845.
Agriculture2021,11,125013of14
32. Núñez‐Trujillo,G.;Cabrera,R.;Burgos‐Reyes,R.;Silva,E.d.;Giménez,C.;Cosoveanu,A.;Brito,N.EndophyticfungifromVitis
viniferaL.isolatedinCanaryIslandsandAzoresaspotentialbiocontrolagentsofBotrytiscinereaPers.:Fr.J.Hortic.For.Biotechnol.
2012,16,1–6.
33. Muvea,A.M.;Subramanian,S.;Maniania,N.K.;Poehling,H.‐M.;Ekesi,S.;Meyhöfer,R.Endophyticcolonizationofonionsinduces
resistanceagainstviruliferousthripsandvirusreplication.Front.PlantSci.2018,9,1785.
34. Varanda,C.M.R.;Oliveira,M.;Materatski,P.;Landum,M.;Clara,M.I.E.;doRosárioFélix,M.Fungalendophyticcommunities
associatedtothephyllosphereofgrapevinecultivarsunderdifferenttypesofmanagement.FungalBiol.2016,120,1525–1536.
35. Araújo,A.S.;Leite,L.F.;Santos,V.B.;Carneiro,R.F.Soilmicrobialactivityinconventionalandorganicagriculturalsystems.
Sustainability2009,1,268–276.
36. Xia,Y.;Sahib,M.R.;Amna,A.;Opiyo,S.O.;Zhao,Z.;Gao,Y.G.Culturableendophyticfungalcommunitiesassociatedwithplants
inorganicandconventionalfarmingsystemsandtheireffectsonplantgrowth.Sci.Rep.2019,9,1–10.
37. Geissen,V.;Silva,V.;Lwanga,E.H.;Beriot,N.;Oostindie,K.;Bin,Z.;Pyne,E.;Busink,S.;Zomer,P.;Mol,H.Cocktailsofpesticide
residuesinconventionalandorganicfarmingsystemsinEurope–Legacyofthepastandturningpointforthefuture.Environ.Pollut.
2021,278,116827.
38. Burns,K.N.;Kluepfel,D.A.;Strauss,S.L.;Bokulich,N.A.;Cantu,D.;Steenwerth,K.L.Vineyardsoilbacterialdiversityand
compositionrevealedby16SrRNAgenes:Differentiationbygeographicfeatures.SoilBiol.Biochem.2015,91,232–247.
39. Wilson,D.Endophyte:Theevolutionofaterm,andclarificationofitsuseanddefinition.Oikos1995,73,274–276.
40. Suryanarayanan,T.;Murali,T.;Venkatesan,G.Occurrenceanddistributionoffungalendophytesintropicalforestsacrossarainfall
gradient.Can.J.Bot.2002,80,818–826.
41. Bacon,C.W.;White,J.MicrobialEndophytes;CRCPress:BocaRaton,FL,USA,2000.
42. Sarsaiya,S.;Jain,A.;Jia,Q.;Fan,X.;Fuxing,S.;Chen,Z.;Zhou,Q.;Shi,J.;Chen,J.MolecularIdentificationofEndophyticFungiand
TheirPathogenicityEvaluationAgainstDendrobiumnobileandDendrobiumofficinale.Int.J.Mol.Sci.2020,21,316.
43. Wijekoon,C.;Quill,Z.Fungalendophytediversityintablegrapes.Can.J.Microbiol.2021,67,29–36.
44. Jayawardena,R.S.;Purahong,W.;Zhang,W.;Wubet,T.;Li,X.;Liu,M.;Zhao,W.;Hyde,K.D.;Liu,J.;Yan,J.Biodiversityoffungi
onVitisviniferaL.revealedbytraditionalandhigh‐resolutionculture‐independentapproaches.FungalDivers.2018,90,1–84.
45. Matsuoka,H.;Akiyama,M.;Kobayashi,K.;Yamaji,K.FeandPsolubilizationunderlimitingconditionsbybacteriaisolatedfrom
CarexkobomugirootsattheHasakicoast.Curr.Microbiol.2013,66,314–321.
46. Haas,H.FungalsiderophoremetabolismwithafocusonAspergillusfumigatus.Nat.Prod.Rep.2014,31,1266–1276.
47. Sharma,H.;Rai,A.K.;Chettri,R.;Nigam,P.S.BioactivitesofPenicilliumcitrinumisolatedfromamedicinalplantSwertiachirayita.
Arch.Microbiol.2021,203,5173–5182.
48. Chowdappa,S.;Jagannath,S.;Konappa,N.;Udayashankar,A.C.;Jogaiah,S.DetectionandCharacterizationofAntibacterial
SiderophoresSecretedbyEndophyticFungifromCymbidiumaloifolium.Biomolecules2020,10,1412.
49. Huang,W.‐Y.;Cai,Y.‐Z.;Xing,J.;Corke,H.;Sun,M.Apotentialantioxidantresource:Endophyticfungifrommedicinalplants.
Econ.Bot.2007,61,14–30.
50. Nischitha,R.;Shivanna,M.Metabolitefingerprinting,invitroantimicrobialandantioxidantactivitiesandin‐silicodockingin
AlloteropsiscimicinaanditsendophyticfungusPenicilliumpinophilum.Mol.Biol.Rep.2021,48,4021–4037.
51. Arora,D.S.;Chandra,P.AntioxidantactivityofAspergillusfumigatus.Int.Sch.Res.Not.2011,2011,619395.
52. Sharaf,M.H.;Abdelaziz,A.M.;Kalaba,M.H.;Radwan,A.A.;Hashem,A.H.Antimicrobial,Antioxidant,CytotoxicActivitiesand
PhytochemicalAnalysisofFungalEndophytesIsolatedfromOcimumBasilicum.Appl.Biochem.Biotechnol.2021,1–19,
https://doi.org/10.1007/s12010‐021‐03702‐w.
53. Vellingiri,M.M.;Ashwin,J.K.M.;Soundari,A.J.P.G.;Sathiskumar,S.;Priyadharshini,U.;Paramasivam,D.;Liu,W.‐C.;
Balasubramanian,B.MycofabricationofAgONPsderivedfromAspergillusterreusFC36AY1anditspotentantimicrobial,
antioxidant,andanti‐angiogenesisactivities.Mol.Biol.Rep.2021,48,7933–7946.
54. Kumari,P.;Singh,A.;Singh,D.K.;Sharma,V.K.;Kumar,J.;Gupta,V.K.;Bhattacharya,S.;Kharwar,R.Isolationandpurificationof
bioactivemetabolitesfromanendophyticfungusPenicilliumcitrinumofAzadirachtaindica.S.Afr.J.Bot.2021,139,449–457.
55. Devi,N.;Prabakaran,J.BioactivemetabolitesfromanendophyticfungusPenicilliumsp.isolatedfromCentellaasiatica.Curr.Res.
Environ.Appl.Mycol.2014,4,34–43.
56. Kaur,R.;Kaur,J.;Kaur,M.;Kalotra,V.;Chadha,P.;Kaur,A.;Kaur,A.AnendophyticPenicilliumoxalicumisolatedfromCitrus
limonpossessesantioxidantandgenoprotectivepotential.J.Appl.Microbiol.2020,128,1400–1413.
57. Nasrin,M.;Afroz,F.;Begum,M.N.;Rony,S.R.;Sharmin,S.;Moni,F.;Rana,M.S.;Sohrab,M.H.IsolationandBioactivityScreening
ofEndophyticFungifromCommelinadiffusa.IndianJ.Pharm.Educ.Res.2021,55,829–836.
58. Kumar,V.;Prasher,I.PhytochemicalanalysisandantimicrobialpotentialofNigrosporasphaerica(Berk.&Broome)Petch,afungal
endophyteisolatedfromDilleniaindicaL.Adv.Tradit.Med.2021,1–13,https://doi.org/10.1007/s13596‐021‐00619‐x.
59. Acuña‐Rodríguez,I.S.;Newsham,K.K.;Gundel,P.E.;Torres‐Díaz,C.;Molina‐Montenegro,M.A.Functionalrolesofmicrobial
symbiontsinplantcoldtolerance.Ecol.Lett.2020,23,1034–1048.
Agriculture2021,11,125014of14
60. Monggoot,S.;Popluechai,S.;Gentekaki,E.;Pripdeevech,P.Fungalendophytes:Analternativesourceforproductionofvolatile
compoundsfromagarwoodoilofAquilariasubintegra.Microb.Ecol.2017,74,54–61.
61. Hamilton,C.E.;Bauerle,T.L.Anewcurrencyformutualism?Fungalendophytesalterantioxidantactivityinhostsrespondingto
drought.FungalDivers.2012,54,39–49.
62. Gams,W.;Anderson,T.‐H.CompendiumofSoilFungi;AcademicPress:Cambridge,MA,USA,1980.
63. Pitt,J.I.;Hocking,A.D.FungiandFoodSpoilage;Springer:Berlin/Heidelberg,Germany,2009;Volume519.
64. Mao,Z.;Xue,M.;Gu,G.;Wang,W.;Li,D.;Lai,D.;Zhou,L.LophiostominA–D:New3,4‐dihydroisocoumarinderivativesfromthe
endophyticfungusLophiostomasp.Sigrf10.RSCAdv.2020,10,6985–6991.
65. Shushni,M.A.M.;Azam,F.;Lindequist,U.OxasetinfromLophiostomasp.oftheBalticSea:Identification,insilicobindingmode
predictionandantibacterialevaluationagainstfishpathogenicbacteria.Nat.Prod.Commun.2013,8,193.
66. Abdel‐Motaal,F.F.;Nassar,M.S.;El‐Zayat,S.A.;El‐Sayed,M.A.;Ito,S.I.Antifungalactivityofendophyticfungiisolatedfrom
Egyptianhenbane(HyoscyamusmuticusL.).Pak.J.Bot.2010,42,2883–2894.
67. Premjanu,N.;Jaynthy,C.;Diviya,S.Antifungalactivityofendophyticfungiisolatedfromlanneacoromandelica–aninsilico
approach.Int.J.Pharm.Pharm.Sci.2016,8,207–210.
68. Rouissi,W.;Ugolini,L.;Martini,C.;Lazzeri,L.;Mari,M.ControlofPostharvestFungalPathogensbyAntifungalCompoundsfrom
Penicilliumexpansum.J.FoodProt.2013,76,1879–1886.
69. Li,H.;Wei,J.;Pan,S.‐Y.;Gao,J.‐M.;Tian,J.‐M.Antifungal,phytotoxicandtoxicmetabolitesproducedbyPenicillium
purpurogenum.Nat.Prod.Res.2014,28,2358–2361.
70. Korejo,F.;Ali,S.A.;Shafique,H.A.;Sultana,V.;Ara,J.;Ehteshamul‐Haque,S.Antifungalandantibacterialactivityofendophytic
PenicilliumspeciesisolatedfromSalvadoraspecies.Pak.J.Bot.2014,46,2313–2318.
71. Urooj,F.;Farhat,H.;Ali,S.A.;Ahmed,M.;Sultana,V.;Shams,Z.I.;Ara,J.;Ehteshamul‐Haque,S.RoleofendophyticPenicillium
speciesinsuppressingtherootrottingfungiofsunflower.Pak.J.Bot.2018,50,1621–1628.