Access to this full-text is provided by Wiley.
Content available from InfoMat
This content is subject to copyright. Terms and conditions apply.
REVIEW ARTICLE
A review of rechargeable batteries for portable electronic devices
Yeru Liang
1,2
| Chen-Zi Zhao
1
| Hong Yuan
1
| Yuan Chen
3
| Weicai Zhang
2
|
Jia-Qi Huang
4
| Dingshan Yu
5
| Yingliang Liu
2
| Maria-Magdalena Titirici
6
|
Yu-Lun Chueh
7
| Haijun Yu
8
| Qiang Zhang
1
1
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing, China
2
College of Materials and Energy, South China Agricultural University, Guangzhou, China
3
School of Chemical and Biomolecular Engineering, The University of Sydney, Sydney, New South Wales, Australia
4
Advanced Research Institute of Multidisciplinary Science, Beijing Institute of Technology, Beijing, China
5
Key Laboratory for Polymeric Composite and Functional Materials, Ministry of Education, School of Chemistry, Sun Yat-sen University, Guangzhou, China
6
School of Engineering and Materials Science, Queen Mary University of London, London, United Kingdom
7
Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu, Taiwan, ROC
8
College of Materials Science and Engineering, Key Laboratory of Advanced Functional Materials, Ministry of Education, Beijing University of
Technology, Beijing, China
Correspondence
Qiang Zhang, Beijing Key Laboratory of
Green Chemical Reaction Engineering and
Technology, Department of Chemical
Engineering, Tsinghua University, Beijing
100084, China.
Email: zhang-qiang@mails.tsinghua.edu.cn
Yingliang Liu, College of Materials and
Energy, South China Agricultural
University, Guangzhou 510642, China.
Email: tliuyl@scau.edu.cn
Yuan Chen, School of Chemical and
Biomolecular Engineering, The University
of Sydney, Sydney, NSW 2006, Australia.
Email: yuan.chen@sydney.edu.au
Jia-Qi Huang, Advanced Research Institute of
Multidisciplinary Science, Beijing Institute of
Technology, Beijing 100081, China.
Email: jqhuang@bit.edu.cn
Funding information
Australian Research Council under the
Future Fellowships scheme, Grant/Award
Number: FT160100107; National Key
Research and Development Program,
Grant/Award Number: 2016YFA0202500
and 2015CB932500; National Natural
Abstract
Portable electronic devices (PEDs) are promising information-exchange platforms for
real-time responses. Their performance is becoming more and more sensitive to
energy consumption. Rechargeable batteries are the primary energy source of PEDs
and hold the key to guarantee their desired performance stability. With the remark-
able progress in battery technologies, multifunctional PEDs have constantly been
emerging to meet the requests of our daily life conveniently. The ongoing surge in
demand for high-performance PEDs inspires the relentless pursuit of even more pow-
erful rechargeable battery systems in turn. In this review, we present how battery
technologies contribute to the fast rise of PEDs in the last decades. First, a compre-
hensive overview of historical advances in PEDs is outlined. Next, four types of rep-
resentative rechargeable batteries and their impacts on the practical development of
PEDs are described comprehensively. The development trends toward a new genera-
tion of batteries and the future research focuses are also presented.
KEYWORDS
electrochemical energy storage, information material, portable electronic device, rechargeable battery
Received: 14 January 2019 Revised and accepted: 1 February 2019
DOI: 10.1002/inf2.12000
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original
work is properly cited.
© 2019 The Authors. InfoMat published by John Wiley & Sons Australia, Ltd on behalf of UESTC.
6wileyonlinelibrary.com/journal/inf2 InfoMat. 2019;1:6–32.
Science Foundation of China, Grant/Award
Numbers: 21676160, 51602107,
21776019, 21825501, 21808124,
U1801257; the China Postdoctoral Science
Foundation, Grant/Award Number:
2017M620049; Australian Research
Council, Grant/Award Number:
DP180102210; Tip-top Scientific and
Technical Innovative Youth Talents of
Guangdong Special Support Program,
Grant/Award Number: 2017TQ04C419;
Tsinghua University Initiative Scientific
Research Program
1|INTRODUCTION
Nowadays, the information-rich world is becoming more
and more portable. With the huge demands for the timely
and efficient delivery of global information, information col-
lection and transmission require a portable information-
exchange platform for real-time response. Portable electronic
devices (PEDs) including mobile phones, portable com-
puters, tablets, and wearable electronic devices are the most
promising candidates and have promoted the rapid growth
of information processing and sharing.
With the development and innovation of electronic tech-
nology, PEDs have been rapidly growing over the past
decades. The primary motivation behind this activity is that
PEDs are widely used in our daily life from personal devices
to high-technology devices applied in aerospace due to the
ability to integrate and interact with a human, which have
brought great convenience and epoch-making changes, even
becoming an indispensable part for almost every person.
In general, stable operated energy sources are mandatory in
these devices to guarantee the desired performances.
1
Besides,
it is highly required to develop energy storage sources with high
safety due to the portability of PEDs. With the growing
demands of long runtime of PEDs, the capability of energy stor-
age systems should be upgraded. Accordingly, exploring effi-
cient, long-life, safe, and large-capacity energy storage devices
is strongly requested to meet the current challenges of PEDs.
Electrochemical energy storage systems, especially recharge-
able batteries, have been widely employed as the energy sources
of PEDs for decades and promoted the thriving growth of
PEDs.
2,3
To satisfy the continually high requirements of
PEDs, significant improvements in electrochemical perfor-
mances of rechargeable batteries have been attained.
4–6
The
rechargeable batteries of PEDs have gone through the lead-
acid, nickel-cadmium (Ni-Cd), nickel-metal hydride (Ni-MH),
lithium-ion (Li-ion) batteries, and so on (Figure 1). Their spe-
cific energy and specific power are substantially improved as
time goes on.
However, the current battery technology cannot fully
catch up with the rapid growth of PEDs.
7
The state-of-the-art
technology of rechargeable batteries for PEDs has exposed
many drawbacks, that is, limited energy storage capacity, short
cycle life, and high self-discharge, which have become con-
strained bottleneck for the further development of PEDs.
8–10
For
instance, the high-power consumption of multifunctional PEDs
requires energy storage systems with higher energy, smaller vol-
ume, lighter weight, and longer operational time. However, it is
challenging for current batteries to satisfy the ever-increasing
demands of emerging electrical and electronic equipment.
Therefore, the rational design and production of novel batteries
has been a relentless-pursued goal for the future PEDs.
Tremendous efforts have been dedicated to improving
the electrochemical performances of batteries. Significant
progress has been made according to recent literatures.
11–16
There are also numerous excellent reviews that cover the pro-
gress of battery technologies.
17–31
Nonetheless, few reviews
are focusing on the overview of rechargeable batteries
designed for PEDs. Considering the critical contribution
of battery technologies to the development of PEDs, it is
of great interest to summarize the progress of rechargeable
batteries for PEDs in the past decades.
In this contribution, we aim to present and highlight how
battery technologies contribute to the fast rise of PEDs. We
start with a comprehensive overview of historical advances
in PEDs. Four types of representative rechargeable batteries
and their practical impacts on the development of different
types of PEDs are described in detail. Particular attention is
given to those traditional PEDs, such as mobile phones, lap-
tops, digital camera, as well as the newly emerging PEDs,
including wearable electronic devices and consumer drones.
Finally, the current development trends of the battery tech-
nologies and the future opportunities are also presented.
2|PORTABLE ELECTRONIC
DEVICES
PED products have experienced dramatic growth and upgraded
at an incredible speed ever since their birth (Figure 2). In par-
ticular, the sales of so-called 3C products, that is, computer,
LIANG ET AL.7
communication, and consumer electronics are increasing each
year rapidly. For example, the mobile phone industry is cur-
rently the largest consumer electronics segment in the world.
The global shipment of mobile phones increased from 9.6
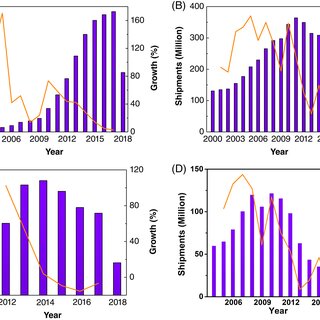
million in 2003 to 1536 million in 2017 (Figure 3A). The
rapid growth of PED products can be ascribed to the follow-
ing two reasons. First, the increasing demands by customers
for information acquisition and information processing
FIGURE 1 Schematic illustration of
representative rechargeable batteries and
their applications in traditional and
emerging portable electronic devices
FIGURE 2 Development of various
types of portable electronic devices from
1983 until today illustrated by several
representative products
8LIANG ET AL.
capabilities create a fast-growing market for PED products.
Second, the continuously reinventing of new PED products is
driven by rapid technological progress. For instance, the
mobile phones have been updated every 2 years on average
since the birth of Big Brother (ie, the first cellular phone) in
1993, where technological progress of semiconductors and
other electronic components have played a vital role. Mean-
while, this fast growth is also closely related to the introduc-
tion of new battery technologies. Since the birth of the first
commercial Li-ion batteries in 1991, PED products based on
Li-ion batteries have been springing up, ranging from mobile
phones, laptops, digital cameras, Walkman, MP3 players, and
tablets, to wearable electronic devices that have become very
popular in recent years.
Newly launched PED products usually can open new
markets with fast growth rates. With the full saturation of
market penetration, their growth would gradually slow
down. For example, the market of the traditional PED prod-
ucts, that is, laptops, mobile phones, and tablets has reached
certain penetration levels and gradually becomes saturated,
resulting in slower growth momentum in recent years. Even
though the global shipments of mobile phones increased
from 680 million in 2012 to 1536 million in 2017 and the
growth rate has dropped from 43.8% to 2.7% (Figure 3A).
The laptop market exhibited a negative growth trend since
2012, and had significant decline of 10.4% in 2015, mainly
due to the prolonged use cycle of laptops (Figure 3B). Simi-
lar negative growth phenomenon can be found in the market
of tablets and digital cameras (Figure 3C,D). The global
shipments of tablets have fallen since 2015 and decreased
15.5% year-on-year in 2016 to 175 million units. However,
due to their large outputs and extensive market penetration,
the overall number of traditional PEDs maintains a stable
growth rate.
Compared with the traditional PEDs, the emerging new
PEDs, including wearable electronic devices, consumer drones,
wireless Bluetooth speakers, and other new products, have
become an important growth point in the PED industry. For
instance, the global markets of wearable electronic devices are
growing dramatically, particularly driven by the popularity of
sports health tracking devices and smart watches. The global
shipment of wearable equipment exceeded 78.1 million in
2015, resulting in an increase of 171.6% compared to 2014. It
is estimated that the global shipments of wearable equipment
would reach 214 million with an annual growth rate of 20.3%
by 2020.
32
The market of consumer drones is another new
growth point. The shipment of consumer drones showed a
rapid growth trend from 2013 to 2015. It is estimated that the
0
400
800
1200
1600
Shipments (Million)
Year
2003 2006 2009 2012 2015 2018
(A) (B)
(C)
0
40
80
120
160
Growth (%)
0
100
200
300
400
Shipments (Million)
Yea r
2000 2003 2006 2009 2012 2015 2018
-10
0
10
20
Growth (%)
0
50
100
150
200
250
2014
Growth (%)
Year
Shipments (Million)
2012 2016 2018
0
40
80
120 (D)
FIGURE 3 Global shipments (violet) and growth rates (orange) of A, mobile phones, B, laptops, C, tablets, and D, digital cameras. Note that
data of global shipments in 2018 are taken from Jan to Jun 2018. Data of mobile phones, laptops, tablets, and digital cameras are taken from
Gartner, IDC, Wind, and Wind, respectively
LIANG ET AL.9
global shipment of consumer drones would reach 3.1 million
units by 2018.
3|RECHARGEABLE BATTERY
TECHNOLOGIES FOR PEDS
The rapid progress of PEDs is impossible without the progres-
sive improvement of rechargeable battery technologies.
33
Pri-
mary batteries have already been used as the main energy
source of PEDs for a lengthy period. However, the significant
strides of rechargeable batteries with higher energy and power
density have remarkably changed the situation since the early
21st century. Presently, rechargeable batteries have already
been applied in most PEDs.
34
PEDs evolved and incorporated several different types of
rechargeable batteries, including lead-acid, Ni-Cd, Ni-MH,
and Li-ion batteries. These rechargeable batteries often adopt
four types of shape, that is, coin, cylindrical, prismatic, and
pouch cells (Figure 4). Lead-acid and Ni-Cd batteries have
been used for a long time. The former can be dated back to
1859, while the latter was first manufactured in 1909. Ni-MH
and Li-ion batteries are relatively young. Ni-MH and Li-ion
batteries have played critical roles in realizing the widely
adaption of PEDs, especially Li-ion batteries. The key charac-
teristics of these four types of batteries are compared in
Table 1 and Figure 5. Each type of these batteries and their
practical impacts on the development of PEDs will be
described and discussed in the following sections.
3.1 |Lead-acid battery
As the first commercially successful rechargeable battery,
the lead-acid battery was invented by French physicist Gas-
ton Planté in 1859. Despite its oldest age, the lead-acid bat-
tery is continuously used widely today because of its low
cost, low self-discharge rate, high discharge currents, and
good low-temperature tolerance. These features make them
attractive for applications not only in some PEDs but also in
automobiles, golf cars, forklifts, and other vehicles.
In a standard lead-acid battery, Pb, PbO
2
, and concen-
trated H
2
SO
4
aqueous solution are used as the anode, cath-
ode, and electrolyte, respectively (Figure 6A). The reversible
electrochemistry reactions in a lead-acid battery are shown
as follows:
Cathode :PbO2+3H++ HSO−
4+2e–
Ð2H2O + PbSO4
Anode :Pb + HSO−
4ÐPbSO4+H++2e–
Overall reaction :Pb + PbO2+2H
2SO4
Ð2PbSO4+2H
2O
It is found that in the fully discharged state, both the
anode and cathode become PbSO
4
. The electrolyte loses
much of its dissolved H
2
SO
4
and becomes water primarily.
While in the fully charged state, the cathode and anode
FIGURE 4 Schematic illustration of typical rechargeable battery configurations: A, coin, B, cylindrical, C, prismatic, and D, pouch shapes
10 LIANG ET AL.
consist of PbO
2
and Pb, respectively. The electrolyte turns
back to concentrated H
2
SO
4
. Such a fully charged state
stores most of the electrochemical energy.
Lead-acid batteries can be made in cylindrical or pris-
matic configurations (Figure 4). According to the immobili-
zation of the electrolyte and O
2
recycle in the battery, lead-
acid batteries can be divided into two types, that is, sealed
lead-acid and valve-regulated lead-acid batteries. The sealed
lead-acid battery is sealed entirely, while the valve-regulated
lead-acid battery has a valve for releasing excess internal
pressure. Moreover, the aqueous H
2
SO
4
solution can either
be soaked into an absorbent glass material or gel by addition
of fumed SiO
2
in the valve-regulated lead-acid battery. The
sealed lead-acid battery possesses the low capacity and thus
is usually used in small-sized PED like portable radios.
34
The valve-regulated lead-acid battery has greater energy
storage capacity and is commonly used as a stationary bat-
tery, for example, uninterruptible power sources, emergency
lighting, and telecom powers. Besides, the valve-regulated
batteries are also applied in high-power portable flood lights.
TABLE 1 Characteristics of four key types of rechargeable batteries used in PEDs
Characteristics Lead-acid battery Ni-Cd battery Ni-MH battery Li-ion battery
Gravimetric energy density (Wh kg
−1
) 30-50 40-60 60-120 170-250
Volumetric energy density (Wh L
−1
) 60-110 150-190 140-300 350-700
Battery voltage (V) 2.0 1.2 1.2 3.7
Cycle life (to 80% of the initial capacity) 300 1500 1000 500-2000
Self-discharge per month (%) 5 20 30 <10
Fast charging time (h) 8-16 1 1-4 1 or less
In use since Late 1800s 1950 1990 1991
Toxicity High High Low Low
Overcharge tolerance High Moderate Low Low
Operating temperature range (C) −20 to 60 −40 to 60 −20 to 60 −20 to 60
Abbreviation: PEDs, portable electronic devices.
Values are taken from References 34 and 35.
FIGURE 5 Performance comparison of A, lead-acid, B, Ni-Cd, C, Ni-MH, and D, Li-ion batteries
LIANG ET AL.11
In spite of their fascinating advantages, lead-acid batte-
ries suffer several limitations, such as short lifetime (about
300-500 cycles), toxic for humans and the environment, and
slow charge rates (Table 1). In particular, the main drawback
of lead-acid batteries is their low gravimetric energy density
of about 40 Wh kg
−1
. They have the lowest specific energy
storage capacity among Ni-Cd, Ni-MH, and Li-ion batteries,
and usually, have a large size and heavyweight. This indi-
cates that lead-acid batteries store the least amount of energy
based on the battery weight, which limits their usability in
small PEDs.
3.2 |Nickel-cadmium battery
The Ni-Cd battery was invented by Waldemar Jungner in
1899. It offers several advantages over the lead-acid battery,
such as longer lifetime, attractive low-temperature perfor-
mances, higher charge-discharge rates, and versatile in size
ranging from small sealed portable types to large vented
cells. Due to these exceptional characteristics, the Ni-Cd
battery was once the dominant battery choice for both porta-
ble and standby power sources.
A Ni-Cd battery usually consists of NiO(OH) as cathode,
Cd as anode, and KOH alkaline solution as electrolyte
(Figure 7). Its operating principle is based on the redox reac-
tions between NiO(OH) and Cd. The reversible electrochem-
istry reactions in a Ni-Cd battery are shown as follows:
Cathode :NiO OHðÞ+H
2O+e–ÐNi OHðÞ
2+OH–
Anode :Cd + 2OH –ÐCd OHðÞ
2+2e–
Overall reaction :2NiO OHðÞ+Cd+2H
2O
Ð2Ni OHðÞ
2+CdOHðÞ
2
Nickel oxide and Fe/Cd materials were used as the cath-
ode and anode in the first Ni-Cd battery built by Jungner.
Pure Cd metal and Ni(OH)
2
were used in subsequent Ni-Cd
batteries. The development of the Ni-Cd battery was slow
before 1932. In 1932, an important advancement was made
by depositing the active battery materials inside a porous
Ni-plated electrode. In 1947, the absorption of gases gener-
ated during the charge process promoted further improve-
ments, leading to the modern sealed Ni-Cd battery. The
widespread manufacture of this type of sealed Ni-Cd batteries
began in the 1950s. From then on, the Ni-Cd battery occupied
an overwhelming majority of the market as rechargeable bat-
teries in various PEDs, including mobile phones, laptop flash-
lights, video cameras, and radios up to 1990s.
Nevertheless, one major drawback of Ni-Cd batteries is
their memory effects, where their maximum energy capacity
is gradually lost when they are not fully discharged before
recharging or are not used for a while. Hence, Ni-Cd battery
was often limited to electronic devices, such as mobile
phones, which are frequently recharged after being only par-
tially discharged. Another limitation of Ni-Cd batteries is
their high self-discharge rate (Table 1). Besides, Cd is an
expensive, heavy metal with high toxicity. Considering that
a large number of PEDs were disposed every year, the
FIGURE 6 Schematic illustration of the lead-
acid battery in different operational conditions:
A, fully charged state, B, discharge process, C, fully
discharged state, and D, charge process
12 LIANG ET AL.
abandoned Ni-Cd batteries also raise significant environ-
mental concerns. Since the 1990s, Ni-Cd batteries gradually
lose their popularity due to the development of Ni-MH and
Li-ion battery technologies. Today, Ni-Cd batteries are only
used for some specialty applications.
3.3 |Nickel-metal hydride battery
First patented in 1986 and commercially available in 1989,
Ni-MH battery is an important type of rechargeable battery
used in PEDs. Its configuration is very similar to that of Ni-
Cd battery. They both use the same cathode materials and
electrolyte, but instead of Cd, a hydrogen absorbing alloy is
used as the anode in Ni-MH battery (Figure 8). As a result,
the reversible electrochemistry reactions in a Ni-MH battery
are shown as follows:
Cathode :NiO OHðÞ+H
2O+e–ÐNi OHðÞ
2+OH–
Anode :MH + OH –ÐM+H
2O+e–
Overall reaction :NiO OHðÞ+MHÐNi OHðÞ
2+M
The replacement of metal Cd makes Ni-MH batteries less
expensive and eco-friendlier when compared to Ni-Cd batte-
ries. Besides, Ni-MH batteries have several other key advan-
tages over Ni-Cd batteries, such as minimal memory effect,
superior cycle life, excellent performances over a broader
range of operating temperatures, high charge rates, and high
energy density. Their energy density is more than two times
that of lead-acid batteries, about 50% higher than that of Ni-
Cd batteries, and even able to approach that of Li-ion batteries.
The average cycle life can reach 500 cycles on a high-capacity
Ni-MH battery and almost 3000 cycles on a low-capacity one.
The Ni-MH battery also renders fast charge ability. For
instance, it can be rapidly charged within 1 hour. Because of
all these advantages, Ni-MH batteries soon replaced Ni-Cd
batteries in PEDs and became the primary power solution in
the early 1990s. Moreover, Ni-MH batteries have also been
appliedincommercialhybridelectric vehicles, such as Toyota
Prius. However, in recent years, the usage of Ni-MH batteries
has decreased significantly, mainly due to the development of
Li-ion batteries and some of their disadvantages.
The largest drawback of Ni-MH batteries is their high
self-discharge rate, which is up to three times higher than
that of Ni-Cd batteries, and even more times higher than that
of Li-ion and lead-acid batteries. For example, Ni-MH batte-
ries would lose approximately a third of their stored charges
in a month. This situation becomes even worse with the
increasing operating temperature. Although the high self-
discharge rate might be ignored in PEDs which are charged
every day, this limitation poses severe problems for occa-
sionally used PEDs.
3.4 |Lithium-ion battery
As the most commonly used rechargeable batteries nowa-
days, Li-ion batteries bring PEDs to a new age since 1991
FIGURE 7 Schematic illustration of the Ni-Cd
battery in different operational conditions: A, fully
charged state, B, discharge process, C, fully
discharged state, and D, charge process
LIANG ET AL.13
when the Sony Corporation commercialized the first Li-ion
battery. Their advent has been very challenging to other
types of batteries, which could be ascribed to a number of
advantages, such as high specific energy (typically twice that
of standard Ni-Cd batteries), low self-discharge rate, high
voltage of about 3.6 V (three times that of typical Ni-based
battery), maintenance free, lightweight, good safety, and
excellent cycling performance. These advantageous features
make Li-ion batteries the best energy storage option for
small-sized PEDs, such as mobile phones, laptops, digital
cameras, and others, which was once dominantly by Ni-MH
and Ni-Cd batteries. Meanwhile, Li-ion batteries are also
growing in popularity for military, electric vehicle, and aero-
space applications. More detailed information about the his-
torical development of Li-ion batteries can be found in
several excellent reviews.
33,36–38
The electrochemistry reactions in Li-ion batteries are based
on the intercalation and deintercalation of Li ions, in which Li
ions move from the anode to the cathode during the discharge
process and come back during the charge process (Figure 9).
In a typical Li-ion battery, the anode is made of carbonaceous
materials, that is, graphite. Metal oxides, such as Li cobalt
oxides, Li iron phosphates, and Li manganese oxides are usu-
ally used as the main components for the cathode. Li salts,
such as Li perchlorate, Li tetrafluoroborate, or Li hexafluoro-
phosphate dissolve in organic solvents, such as diethyl car-
bonate, ethylene carbonate, or dimethyl carbonate, serve as
liquid electrolytes for conventional Li-ion batteries.
39
When
polymer electrolytes replace liquid electrolytes, the resulting
batteries are called Li-ion polymer batteries. A high-conductivity
gel containing lithium salts is often used as the polymer
electrolyte. It should be mentioned that because of their
thin and customizable shape, Li-ion polymer batteries are
very attractive for PEDs, especially for ultra-slim laptops,
mobile phones, tablets, and wearable electronic devices. To
avoid confusion, Li-ion batteries containing liquid electro-
lytes are referred to as “liquefied Li-ion batteries”in the
following discussion.
Li cobalt oxide (LiCoO
2
) and graphite (C) are used as the
representative cathode and anode materials, respectively, to
describe the basic chemistry of Li-ion batteries. The revers-
ible electrochemistry reactions are shown as follows:
Cathode :LiCoO2ÐLi1−xCoO2+xLi ++e–
Anode :C+xLi ++e–ÐLixC
Overall reaction :LiCoO2+CÐLi1−xCoO2+Li
xC
During the discharge process, Li ions move from the
anode (ie, graphite) through the electrolyte and the separator
to the cathode (ie, LiCoO
2
). At the same time, electrons
move from the graphite to the LiCoO
2
. During the charge
process, the reaction is reversed.
With the introduction of new materials and technolo-
gies, Li-ion batteries continuously improve their energy
density, power density, lifespan, and safety.
40,41
However,
Li-ion batteries are still suffering from some drawbacks. For
instance, the higher manufacturing costs result in higher prices
FIGURE 8 Schematic illustration of Ni-MH
battery in different operational conditions: A, fully
charged state, B, discharge process, C, fully
discharged state, and D, charge process
14 LIANG ET AL.
when compared with other rechargeable batteries. Although
the price is getting lower year-by-year, Li-ion batteries still cost
more than other competing batteries (Figure 5). Further, Li-ion
batteries require additional protection circuits to limit voltages
and currents to ensure safe operations. Besides, Li-ion batteries
would lose their capacity and cycle life when are stored in
temperatures over 30C for an extended period. Nowadays,
battery scientists and engineers are making significant efforts
to address the drawbacks of Li-ion batteries.
4|DEVELOPMENT OF
RECHARGEABLE BATTERIES
FOR PEDS
In this section, we briefly review the development process of
rechargeable batteries for several types of PEDs. As detailed
in this session, PED products continually being renovated at
a faster speed since their birth, and the demands for better
batteries are also explosively expanding.
4.1 |Traditional PEDs
4.1.1 |Mobile phones
The earliest mobile phone was Motorola’s DynaTac 8000X
released in 1983. It is commonly known as “Big Brother,”
and used a Ni-Cd battery with a small capacity of 500 mAh
(Table 2). Such a battery provided the DynaTac 8000X a
talk time of 20 minutes, but it took as long as 10 hours for
recharging. In spite of this limitation, the number of the
mobile phone sold each year increased rapidly. New mobile
phone products were put on the market very quickly. In 1993,
IBM launched the world’s first smartphone “Simon.”One
year later, Nokia produced its landmark smartphone “9000.”
In 1995, Motorola launched the first flip mobile phone
“8900.”Ni-Cd batteries were the primary power sources for
all these mobile phones. They accounted for almost half of
the weight and volume of these mobile phones. Furthermore,
as discussed above, Ni-Cd batteries suffered from memory
effects. Those mobile phones using Ni-Cd batteries must be
completely exhausted before recharging. Otherwise, the batte-
ries would “remember”the shortened usage time and lead to
power lost in less than 1 hour. Moreover, a long-time com-
munication using mobile phones would lead to heat genera-
tion and eventually deform Ni-Cd batteries. In addition to the
high toxicity of Cd, new rechargeable battery technology was
urgently needed at that time.
In 1997, Ni-MH batteries were introduced to Motorola
mobile phones “166C.”Compared with Ni-Cd batteries, these
Ni-MH batteries made remarkable improvements, for example,
lighter, thinner, nontoxic, lower memory effect, and higher
energy density. The energy storage capacities increased sub-
stantially from 500 to 700 mAh for Ni-Cd batteries to
1300 mAh for Ni-MH batteries (Table 2). The standby time of
the mobile phones was extended to 50 hours. Ni-MH batteries
became very popular in mobile phones. However, Ni-MH bat-
teries exhibited serious self-discharge, which hindered their
further adoptions in mobile phones.
The use of Li-ion batteries is a milestone in the mobile
phone battery development. Without Li-ion battery, mobile
FIGURE 9 Schematic illustration of Li-ion
battery in different operational conditions: A, fully
charged state, B, discharge process, C, fully
discharged state, and D, charge process
LIANG ET AL.15
phones could not be shrunk from huge “bricks”to the size
of pockets. Li-ion batteries are light weighted and have a
higher energy density (30% higher than that of Ni-MH batte-
ries), no annoying memory effects, and better safety. In
1998, Motorola started to produce mobile phones containing
Li-ion batteries. From 1998 to 2000 is the transition period
from Ni-MH to Li-ion batteries, when they fought nose-to-
nose to gain customers’acceptance. It was not until 2001
that Li-ion batteries replaced Ni-MH batteries, due to the
urgent needs of large energy storage capacity for multifunc-
tional mobile phones and the reduction in the cost of Li-ion
batteries. After 2002, Li-ion batteries have become the most
promising battery technology for mobile phones. Since
2007, with the launch of Apple’s iPhone, mobile phones
entered the era of new smartphones. Various types of new
mobile phones with more powerful functions were devel-
oped every year. They have increasingly become more than
just devices for voice communications as they offer a multitude
of features including connectivity, enterprise, and multimedia
capabilities. Batteries with larger capacity are now demanded
to satisfy the need of the increased safety requirement and
power consumption by these new mobile phones. For example,
the energy storage capacity of batteries used in the latest Hua-
wei mate20p (Table 2) has reached 4200 mAh. The mobile
phone industry is currently the largest consumer electronics
segment in the world. With the rapid evolution of mobile
phones, further advances in rechargeable batteries are expected
for years to come.
4.1.2 |Laptops
The earliest portable computers appeared in the early 1980s.
But their appearance is more like calculators. AA batteries or
Ni-Cd batteries powered most of these portable computers,
which can run for tens or even hundreds of hours because of
their relatively limited functions (Table 3). The first laptop in
the world is Toshiba T1100 released in 1985, which was still
a big and bulky luxury without built-in batteries due to the
limited performance of rechargeable batteries at the time.
Since 1989, Toshiba led the portable computer industry by
introducing laptops with built-in batteries. These laptops
offered more computing power with thinner and smaller pack-
ages, which also demanded batteries with better performance.
After 2 years of commercialization, Li-ion batteries were first
TABLE 2 Development of mobile phones and their batteries
Period Historical stages
Representative
product
Actual
photo
Launch
time Battery type
Voltage
(V)
Capacity
(mAh)
1983-1993 Era of cellular phone Motorola DynaTac
8000X
1983 Ni-Cd battery 7.5 500
(the world’s first mobile
phone)
1993-1995 Miniaturization and
intelligence
IBM Simon (the world’s
first smart phone)
1993 Ni-Cd battery 7.5
Motorola 8900 (the
world’s first flip
mobile phone)
1995 Ni-Cd battery 6 950
1995-1997 Era of Ni-MH
battery
Motorola 166C 1997 Ni-MH battery 6 1300
1998-2007 Era of popularization
of mobile phone
Motorola GC87C 1998 Liquefied Li-ion battery 7.2 1200
2007-present Era of new smart
mobile phone
iPhone 2007 Li-ion polymer battery 3.7 1500
Galaxy SIII 2012 Soft-pouch liquefied Li-
ion battery
3.8 2100
iPhone 6 2014 Li-ion polymer battery 3.8 1810
Huawei mate 20p 2018 Li-ion polymer battery 3.82 4200
16 LIANG ET AL.
introduced in the Toshiba laptop Portege T3400CT in 1993,
which enabled a stand-by time of 6 hours.
With the introduction of a series of high-energy-consumption
components in laptops, such as color display screens, high-
performance CPUs, and independent graphics cards, the
capacity of Li-ion batteries used in laptops increased signifi-
cantly in the subsequent 10 years. During this period, the
typical Li-ion batteries used in laptops were the cylindrical
18650 with a standard size of 18 mm in diameter and 65 mm
in length, mainly due to their cost-to-energy ratio and ultra-
thin geometry. A typical laptop battery pack comprised an
average of approximately four to six such batteries to enable
several-hour operation time.
The large-scale use of the 18650 batteries in laptops con-
tinued until Apple launched the MacBook series laptops. In
2006, Apple used soft-packed liquefied Li-ion batteries in
MacBook Pro laptops to make them thinner.
42
In 2008, Apple
switched to Li-ion polymer batteries in MacBook Air. Since
then, Li-ion polymer batteries have been widely used in ultra-
books and other laptops (Table 3).
Currently, the portable computer market is trending toward
thinner ultrabooks and tablets combined with high-end proces-
sors, which need to run complex programs while still require
high responsiveness. Lenovo Yoga-series computers are one
of the representative examples of the recent products of tech-
nological convergence. These crossover devices usually have
a touch-screen display to allow consumers to work in a tablet
mode, and then share traits of both laptops and tablets. All
such devices are often very thin and light-weight. Accord-
ingly, all of these developing trends in portable computers are
driving the demand for even lighter, larger-capacity, safer, and
longer-lifetime batteries.
4.1.3 |Digital cameras
The world’s first digital camera was a laboratory product of
Kodak in 1975 (Table 4). Later, Sony’s Mavica was produced
in 1981 as the first practical digital camera. These digital cam-
eras used cylindrical batteries. The first commercial digital
camera was Casio’s QV-10 launched in 1995 (Table 4). Two
AA batteries were used in this camera because its low-
resolution pixels of 250 000 without a built-in flash. With the
incorporation of built-in flashes and the increase in the pixel
resolution, the requirement for high-capacity batteries contin-
ued increasing.
In 1998, digital single-lens reflex cameras appeared on
the market. A considerable portion of these cameras used
Ni-Cd or Ni-MH batteries. For example, Canon D2000 used
TABLE 3 Development of laptops and their batteries
Period Historical stages Representative product
Actual
photo
Launch
time Battery type
Capacity
(Wh)
Operating
voltage (V)
1979-1984 Laptop prototype Hewlett-Packard HP-110 1984 Lead-acid battery 15 6
1985-1994 Birth of laptop Toshiba T1100 1985 Ni-Cd battery 19.2 4.8
1995-2005 Laptops entered the era
of Li-ion batteries
Toshiba Portege T3400CT 1993 Cylindrical Li-ion
battery
32.4 10.8
2006-2007 Apple laptop led the
high-performance
trend
Macbook Pro 2006 2006 Soft-pouch liquefied
Li-ion battery
60 10.8
2008-2010 Birth of ultrabook Macbook Pro 2008 2008 Li-ion polymer battery 60 10.8
2011-present Popularization of
ultrabook
Lenovo Yoga 13 2012 Li-ion polymer battery 54 14.8
Lenovo YOGA 4 Pro 2016 Li-ion polymer battery 66 7.6
Macbook Air 2018 2018 Li-ion polymer battery 49.9 11.4
LIANG ET AL.17
Ni-Cd batteries, while Nikon D1 used Ni-MH batteries.
Afterward, Li-ion batteries became widely used in digital
cameras along with the continuous improvement in their
functions and the decrease in their volume. For example,
Canon D30 appeared in 2000 used Li-ion batteries with a
capacity of 1390 mAh (Table 4). Currently, most of the digi-
tal cameras have used Li-ion batteries, while some are still
using standard AA batteries. Similar to other PEDs, digital
cameras are getting smaller over the time, which leads to the
increasing demand for batteries with lower volume and
higher energy storage capacity.
4.2 |Emerging PEDs
With the expansion of human demands and the constant tech-
nological progress, the development speed of novel PEDs is
continually accelerating. After the initial high-speed growth
stage, the traditional PEDs discussed early have entered the
phase of the stock competition. On the other hand, new PEDs,
such as wearable electronic devices and consumer drones, are
emerging. These new PED products offer multifunctionality,
lightweight, integrability, and artificial intelligence (AI), are
opening up broad market prospects, and are driving the con-
tinued growth of the whole PED industry. Correspondingly,
their demands for innovative battery technology increase sub-
stantially. For example, flexible, smart, and wearable batteries
are now desirable to power some of emerging wearable PEDs.
In this section, the development of the emerging PEDs and
their responding battery technologies are summarized.
4.2.1 |Wearable electronic devices
As the next hot spot in the PED industry, wearable electronic
devices have been attracting more and more attention in both
the academic research institutions and industry since Google
launched Google Glasses in 2012.
43–46
Wearable electronic
devices refer to clothing and accessories incorporating the
body’s sensing, communication, and digital entertainment
functions. Typical examples include smart watches, smart
glasses, smart clothing, heart rate monitors, fitness trackers,
and so on (Figure 10). In general, they can be directly worn
on the body or integrated into clothes or their wearable acces-
sories, aiming at liberating human hands and enabling intelli-
gent devices to meet people’s requirements automatically.
TABLE 4 Development of digital cameras and their batteries
Period Historical stages
Representative
product
Actual
photo Launch time Pixel Battery type
Capacity
(mAh)
1975-1995 Test era of digital
camera
Kodak’s laboratory
product (the world’s
first digital camera)
1975 10 000 16 series AA batteries
Mavica (the earliest
practical digital
camera)
1981 279 000 3 series AA batteries
1995-1998 Commercialization of
digital camera
Casio QV-10 (the first
commercial digital
camera)
1995 250 000 4 series AA batteries
1998-present Birth and popularization
of digital single-lens
reflex cameras
Canon EOS D2000 1998 2 000 000 Ni-Cd battery 1500
Canon EOS D30 2000 3 100 000 Li-ion battery 1390
Canon EOS5D 2005 13 300 000 Li-ion battery 1390
Canon EOS R 2018 30 300 000 Li-ion polymer battery 1800
18 LIANG ET AL.
Consequently, wearable electronic devices are expected to
improve the quality of human life further, and thus may play
a significant impact on human daily routine and lifestyle,
especially for healthcare, entertainment, and communication.
Wearable electronic devices, like other PEDs, need energy
to operate. Wearable energy systems are a pivotal integral part
of wearable electronic devices. On one hand, thin, small, and
light are basic features of wearable electronic devices, which
require their corresponding energy sources as small as possi-
ble. Conversely, the lifetime of power sources has a critical
impact on the success of wearable electronic devices, as long
endurance is the one of the first considerations for consumers
in purchasing wearable electronic devices. However, it is
challenging to meet these two requirements simultaneously
due to the contradictions between size and capacity for batte-
ries. Currently, rechargeable Li-ion batteries are the accepted
energy storage choice for wearable electronic devices due to
their advantages discussed previously.
Smart watches
A smart watch is a wearable computerized device intended to
be worn on the wrist. Most of the current smart watches have
multiple functionalities, such as long-term biomonitoring, call-
ing, messaging, and altering, which are further communicating
with mobile phone apps. Despite diverse features and designs,
usage time is a major consideration for users, and users usually
want to wear them all day long. Rechargeable Li-ion polymer
batteries power most of smart watches. For example, Apple
Watch uses built-in rechargeable Li-ion polymer batteries.
Since Apple Watch Series 1 was released in 2015, the capacity
of their batteries continuously increased from 205 mAh for
Series 1 (with a 38 mm body) to 273 mAh for Series 2, to
279 mAh for Series 3 (Table 5).
47
Other Apple Watches with
larger sizes offer slightly larger screens as well as batteries with
larger capacity. It was reported that Apple designed these batte-
ries for 18 hours of mixed usage.
48
When the battery capacity
depletes to less than 10%, the watches convert to a power-
saving mode, which enables consumers to read the time for an
additional 3 days continuously. Despite these advances, the
current battery life of smart watches is still falling short of the
demands by consumers.
Smart glasses
Smart glasses, also known as smart eye wears, are ergonomi-
cally designed wearable computers that provide, collect, and
process information alongside with what regular glasses
do. Aiming at delivering a “hands free”digital world for
wearers, smart glasses may be used in various modes, includ-
ing optical head-mounted displays, heads-up displays, virtual
reality, augmented reality, mixed reality, and smart contact
lenses.
49
Wearers may also get access to Internet via voice
commands, or take photos and record video by using a built-
in high-definition camera.
Advanced power sources that can enable smart glasses
to work for all day are essential for wearers’experiences.
Rechargeable Li-ion polymer batteries power most of exist-
ing smart glasses due to their high gravimetric and volumet-
ric energy density. Google Glass released in 2012 was
powered by an internal Li-ion polymer battery with a capac-
ity of 570 mAh, which can support the smart glasses for
about 5 hours after a full charge.
50,51
This usage time may
significantly reduce, depending on uses, configuration,
mobile network, signal strength, and many other factors. The
relatively small capacity battery was one of the major com-
plaints of the original Google Glass. Recently, Google devel-
oped a Li-ion polymer battery with a higher capacity of
780 mAh for Google Glass Enterprise Edition to extend its
run time.
52
Besides improving the capacity of incorporated
batteries, the device usage time can be extended by external
battery packs. For example, Vuzix M100 Smart Glass con-
tains a 550 mAh internal rechargeable battery and a
3800 mAh external rechargeable battery. The internal bat-
tery delivers 2-hour run time, while the external battery
increases the run time up to 6.5 times.
53
Smart clothing
Smart clothing is gaining increasing popularity because they
can effectively get targeted information by analyzing various
wearers’biometrics and provide comprehensive feedbacks
during daily life. Smart clothing is typically in the form of
FIGURE 10 Schematic illustration of representative examples of
wearable electronic devices
LIANG ET AL.19
TABLE 5 Development of wearable electronic devices and their batteries
Product
type
Representative
product
Actual
photo
Launch
time Battery type
Capacity
(mAh) Battery life
Smart watch Apple Watch
Series 1
2015 Li-ion polymer
battery
205 18 h with 90 time checks,
90 notifications, 45 min of app use,
and a 30-min workout
Apple Watch
Series 2
2016 Li-ion polymer
battery
273 Similar to Apple Watch Series 1
Apple Watch
Series 3
2017 Li-ion polymer
battery
279 10 h of indoor workout
4 h of outdoor workout with GPS and
4G LTE
Apple Watch
Series 4
2018 Li-ion polymer
battery
292 10 h of indoor workout, or 4 h of
outdoor workout with streaming
audio, GPS, and 4G
Smart glass Google Glass 2012 Li-ion polymer
battery
570 Less than 5 h
Google Glass
Enterprise Edition
2017 Li-ion polymer
battery
780 About 8 h
Vuzix M100 2014 Li-ion battery 550 (internal) and
3800 (external)
6 h of hands free, or 1 h of hands free,
display, camera, and high CPU
loading (for internal battery)
DPVR P1 2018 Li-ion polymer
battery
3000 About 4 h
HiAR G100 2017 Li-ion polymer
battery
2700 4 h of AR experience
Smart clothing OMsignal smart
T-shirt
2014 16-30 h of intensive workout time, or
3-4 d of continuous wear
Nike Mag shoes 2011 3000 h
Xiaomi Mi
smart shoes
2017 Li-ion battery 210 60 d
Digitsole
Smartshoe
2016 Li-ion polymer
battery
A couple weeks with heat off, or 5-8 h
with heat on
Samsung
WELT belt
2016 Li-ion polymer
battery
90 20 d
Abbreviation: AR, augmented reality.
20 LIANG ET AL.
wearable shirts, socks, windbreakers, sportswear, shoes,
belts, and other textiles. Similar to other types of wearable
electronic devices, smart clothing incorporates digital com-
ponents (eg, sensors, small computers, and other electronics)
to provide added functions, such as monitoring wearers’
physiological and behavioral data. To power smart clothing,
built-in batteries are needed. High safety and reliability
have made Li-ion polymer batteries as a reasonable energy
storage solution for clothing. For example, OMsignal smart
T-shirt’s built-in battery can operate for 16 to 30 hours of
intensive workout time, or 3 to 4 days of continuous wear
without recharging.
54
Nike Mag shoes were released in
2011 as the first rechargeable footwear featured by the
electroluminescent out-sole and space-age materials, which
consists of a rechargeable internal battery for a total usage
time of 3000 hours.
55
4.2.2 |Consumer drones
As unmanned aerial vehicles, consumer drones are aircrafts
without a human pilot aboard for civilian usages. In recent
years, the general public has developed a strong interest in
consumer drones, consequently creating an unprecedented
boom in the new consumer drone industry. It is estimated
that the global shipment of consumer drones would reach
8.34 million by 2020. The potentials of consumer drones
are endless. Currently, they are widely used in aerial pho-
tography. With the continuous progress in technology and
decrease in cost, consumer drones are increasing being
applied to power patrol, movie-video shooting, mobile
communication, meteorology monitoring, and express
delivery. DJI is the worldwide leader in the consumer
drone industry, which accounts for more than 70% of the
world market.
56
In addition to DJI, EHANG, Parrot, and
3D Robotics are also known for launching powerful con-
sumer drones (Table 6).
Unlike other unmanned aerial vehicles used in military
operations that often use combustion engines or solar cells,
consumer drones typically run on electricity. Consumer drones
rely on a reliable power source to achieve an ideal balance
between performance and flight time. The most commonly
used power sources are Li-ion polymer batteries (Table 6)
because of their high specific volumetric energy storage den-
sity, high power density, and long life in comparison with
other rechargeable batteries.
The voltage and capacity of used batteries play signifi-
cant roles in the flight performance of consumer drones. The
battery voltage has an important impact on the maximum
motor speed. A higher voltage provides greater motor spin-
ning speed. The voltage of single standard full-charged Li-
ion polymer batteries is 3.7 V. Multiple Li-ion polymer bat-
teries are usually connected in series as battery packs to
increase the voltage outputs. The standard battery packs are
in 1S, 2S, 3S, 4S, 5S, or 6S configurations, where “S”refers
to connected in series. A 2S battery pack can deliver 7.4 V.
Because of the optimized balance between motor speed and
battery weight, battery packs in the 4S configuration is the
most commonly used for consumer drones.
The battery capacity is often used to reflect how long a
battery can supply energy at a particular current. For exam-
ple, a battery with a capacity of 3000 mAh may supply a
3 A current for 1 hour, or a 6 A current for half an hour,
and so on. Batteries with higher capacity may provide longer
flight time, but their weight also becomes more burdensome.
The increase in weight restricts the response performance of
drones. Therefore, a balance is needed between the battery
capacity and the weight. In typical consumer drones, the
capacity of batteries with a mass of about 200 g ranges from
3000 to 4000 mAh. Such consumer drones can fly about
15 to 25 minutes under normal conditions, that is, without
heavy wind or cold weather. But the flight time is substan-
tially reduced with fast responses and high mobility. For
example, a racing drone with a 1300 mAh battery can fly
only about 3 minutes. Larger batteries with a capacity up to
2200 mAh would be required to achieve a longer flight time
of 5 to 8 minutes.
5|DEVELOPMENT TRENDS OF
BATTERY TECHNOLOGIES
FOR PEDS
Although rechargeable batteries have transformed PEDs
over the past decades, insufficient battery performance is
still the bottleneck of emerging PEDs. Comparing the rapid
process in electronics, the improvement in batteries is much
slower and shows a sign of reaching a performance plateau
in recent years. Thus, developing new high-performance bat-
teries to meet the demands of emerging PEDs remains a crit-
ical issue. In recent years, considerable research efforts have
been devoted to improving existing rechargeable batteries
and developing new batteries. Significant advances have
been attained in increasing energy density, improving safety,
lowering cost, and enabling mechanical flexibility. The
development trends in these aspects are discussed in the fol-
lowing sections.
5.1 |Increasing energy density
The task of increasing battery energy density has driven the
entire battery technology progress over the past two decades.
Up to date, battery energy density remains the primary criteria
in selecting a battery system for PEDs, which is especially cru-
cial for PEDs because of the limited space and weight allocated
for batteries in PEDs. However, the advances in increasing
LIANG ET AL.21
battery energy density fail to keep up the pace of growing
demands by PEDs. Although Li-ion batteries exhibit the high-
est energy density among various rechargeable batteries, their
energy density, ranging from 170 to 250 Wh kg
−1
or 350 to
700 Wh L
−1
, is still not able to cope with the increasing energy
storage requirements by emerging PEDs (Figure 11).
2,58
There-
fore, it is a worldwide and urgent desire to further increasing
the energy density of rechargeable batteries.
Since batteries’specific capacities and operation voltages
determine their energy density, increasing these two parame-
ters has been the primary research targets.
35,59,60
Currently,
the research efforts for improving the energy density of
rechargeable batteries can be classified into two categories.
Methods in the first category focus on optimizing existing
rechargeable batteries, including their electrode materials,
electrolytes, separators, binders, current collectors, and battery
TABLE 6 Development of consumer drones and their batteries
Company Representative product
Actual
photo
Launch
time Battery type
Capacity
(mAh)
Operating
voltage (V)
Battery
weight (g)
DJI Mavic Pro 2016 3S Li-ion polymer battery 3830 11.4 240
Mavic Air 2018 3S Li-ion polymer battery 2375 11.55 140
Mavic 2 2018 4S Li-ion polymer battery 3850 15.4 297
Spark 2017 3S Li-ion polymer battery 1480 11.4 95
Phantom 4 Pro 2016 4S Li-ion polymer battery 5870 15.2 468
Phantom 3 SE 2017 4S Li-ion polymer battery 4480 15.2 365
Phantom 4 Advanced 2017 4S Li-ion polymer battery 5870 15.2 468
ZEROTECH DOBBY 2016 2S Li-ion polymer battery 970 7.6 65
FLYPRO XEagle 2016 3S Li-ion polymer battery 5200 11.1 370
EHANG GHOSTDRONE 2.0 2017 4S Li-ion polymer battery 4500 14.8 400
22 LIANG ET AL.
manufacturing techniques (eg, increasing the packing densi-
ties and the mass ratios of active electrode materials in assem-
bled batteries). For instance, graphite is the common anode
material for commercial Li-ion batteries due to its good stabil-
ity, excellent conductivity, and high Coulombic efficiency.
However, the theoretical Li storage capacity of graphite
anodes is only 372 mAh g
−1
.
61–64
Many other materials with
higher Li storage capacities, such as Si (4200 mAh g
−1
),
65,66
Sn (994 mAh g
−1
),
67–69
SnO
2
(782 mAh g
−1
),
70,71
Fe
2
O
3
(1007 mAh g
−1
),
72
MnO
2
(1232 mAh g
−1
),
73
Co
3
O
4
(890 mAh g
−1
),
74
and NiO (718 mAh g
−1
),
75–77
have been
explored as new anode materials. Similarly, traditional cath-
ode materials (eg, Li cobalt oxide, Li iron phosphate, and Li
manganese oxide) can be substituted by large-capacity
materials (eg, Ni-rich layered oxides and Li-rich layered
oxides)
78–80
or high-voltage materials (eg, polyanion oxides
and spinel materials).
2,81,82
These efforts have been able to
significantly improve the energy density of Li-ion batteries at
least in many research lab studies.
Methods in the second category focus on developing new
batteries. It has been speculated that existing batteries, includ-
ing Li-ion batteries, have limited room for further improve-
ment. A breakthrough in increasing the battery energy density
requires developing new electrochemical reactions.
83–89
Along
this line, new battery systems have been intensively pursued
in recent years, including Li metal batteries,
90–96
metal-sulfur
batteries,
97–104
metal-air (or metal-oxygen) batteries,
105–109
and batteries involving monovalent (eg, Na and K)
110–115
or
multivalent (eg, Mg, Ca, Zn, and Al) elements/cations.
116
Among various new battery systems, Li-sulfur, Li metal, and
Li-oxygen batteries have gained great attraction due to their
exceptionally high energy density (Figure 11). In particular,
Li-sulfur and Li-oxygen batteries have the theoretical gravi-
metric energy density of 2600 Wh kg
−1
and 3500 Wh kg
−1
,
respectively.
117–119
Researchers hope that they can deliver a
practical battery energy density of 2 to 5 times higher than
those of current Li-ion batteries.
120–122
Nevertheless, it should be noted that these new batteries
are still far from mature. There are many technical challenges
in translating research lab findings to scalable industrial pro-
duction.
37,123,124
Significant research and development efforts
are required to make them competitive with the existing state-
of-the-art Li-ion batteries for practical PED applications.
5.2 |Improving safety
Batteries present a safety risk since they store a large amount
of chemical energy in a small space, and thus they are prone
to fire or explosions if operated improperly. Batteries used
in PEDs are particularly dangerous to human due to their fre-
quently carry-on characteristics. There have been numerous
incidents related to fires and explosions of batteries world-
wide, especially involving mobile phones, laptops, and elec-
tric vehicles.
125
For instance, the United States Federal
Aviation Administration have reported over 206 air/airport
battery fire/explosion incidents from 1991 to 2018.
126
The
battery problems of Boeing 787 Dreamliner and Samsung
Note 7 have attracted worldwide attention. It is highly desir-
able to better manage safety issues for new batteries used in
emerging PEDs.
The reasons of battery fire/explosion incidents vary,
which may include short circuits, mechanical abuses, bat-
tery overcharging, or manufacturing defects.
127
Numerous
methods have been proposed to improve battery safety.
These methods can be divided into external or internal pro-
tection approaches.
128
External protection approaches are
usually using additional external devices. For example,
using temperature sensors and pressure valves to monitor
batteries under thermal or pressure abused conditions.
126
These technologies are relatively mature, which are not
discussed in details here.
Internal protection approaches focus on introducing intrin-
sically safe components to different components of batteries.
First, chemical additives are added to electrolytes. These
chemical additives usually comprise flame-retardants,
129–133
ionic liquids,
134–136
shut-down, and redox shuttle addi-
tives.
137,138
Second, some other chemical components
areusedtoachievestableelectrode/electrolyte inter-
faces.
139,140
Third, solid-state batteries based on either poly-
mer gels or inorganic electrolytes have also been explored as
internal protection approaches.
141–152
Polymer gel electrolytes
can improve battery safety because less organic solvents are
used without leakage. Further, inorganic solid ceramic electro-
lytes (eg, Li
7
La
3
Zr
2
O
12
,Li
14
ZnGe
4
O
16
,andLi
3x
La
2/3-x
TiO
3
)
or glass-ceramic electrolytes are attractive because they are
nonflammable, low-cost, no leakages, and stable against high
0 200 400 600 800
0
200
400
600
800
1000
1200
Volumetric energy density (Wh L
-1
)
Gravimetric energy density (Wh kg
-1
)
Lead-acid
Ni-Cd
Ni-MH
Li-ion
Classical Li metal
Li-O
2
Li-S
FIGURE 11 Gravimetric energy density vs volumetric energy
density plot of various types of rechargeable batteries. Values are taken
from References 7 and 57
LIANG ET AL.23
temperatures.
153–161
Besides, the mechanical rigidity of solid
ceramic electrolytes suppresses the formation of Li dendrites,
which is a major reason for battery short circuits. Forth, the
optimization of separators,
8,162–167
current collectors,
168
anode materials,
89,169–172
and cathode materials
173–175
may
also improve the safety of batteries. Detailed discussion on
these optimizations can be found in a recent review.
128
It should
be noted that these internal protection methods are more fre-
quently used in Li-ion batteries, because of the high reactivity
of materials utilized in Li-ion batteries. In general, Li-ion batte-
ries pose higher safety risks compared to other rechargeable bat-
teries discussed in this review.
5.3 |Lowering cost
Batteries used in PEDs must meet challenging cost targets to
achieve commercial success. Li-ion batteries have success-
fully dominated today’s PED battery market. However,
because of their higher cost (normally 300 $ kWh
−1
whereas
90 $ kWh
−1
for lead-acid batteries), many efforts have been
made to reduce the cost of Li-ion batteries through material
designs and synthesis, battery manufacturing, and packag-
ing. These efforts fall into two categories.
The first category involves lowering the cost of various
battery components (ie, cathodes, anodes, electrolytes, sep-
arators, binders, and current collectors) or reducing
manufacturing costs of batteries.
24,26,176
The cost of battery
materials is closely related to their synthesis process and
the price of raw materials. Over the past decades, great
efforts have been devoted to developing new eco-friendly
TABLE 7 Main characteristics of lithium, sodium, and zinc
elements
Characteristics Lithium Sodium Zinc
Price ($ kg
−1
) ~120 ~3 ~3
Specific capacity (mAh g
−1
) 3860 1166 820
Capacity density (mAh cm
−3
) 2061 1129 5855
Voltage vs S.H.E. (V) −3.040 −2.713 −0.763
Ionic radius (Å) 0.76 1.02 0.75
Abbreviation: S.H.E., standard hydrogen electrode.
Values are partially taken from References 207 and 208.
FIGURE 12 A, Schematic illustration of a flexible Li-ion battery. Reproduced with permission.
234
Copyright 2010, American Chemical
Society. B, Schematic illustration of a flexible Li-sulfur battery. Reproduced with permission.
235
Copyright 2015, Wiley-VCH. C, Schematic
illustration of a flexible Li-oxygen battery. Reproduced with permission.
236
Copyright 2015, Nature Publishing Group. D, Schematic illustration of a
flexible Zn-ion battery. Wearable applications of flexible Zn-ion batteries in E, a smart shoe, F, a smart watch, and G, a pulse sensor. Reproduced
with permission.
223
Copyright 2018, Royal Society of Chemistry
24 LIANG ET AL.
routes to synthesize battery materials
177–185
and exploring
sustainable battery material substitutes.
177,186–190
The second category focuses on developing cheaper batte-
ries to replace Li-ion batteries. Because of the limited avail-
ability and uneven distribution of Li in the world, alternative
metal-ion batteries using earth-abundant metal elements, such
as Na-ion,
191–193
Zn-ion,
194–196
K-ion,
197–199
Mg-ion,
200–202
and Al-ion batteries,
203–206
have been studied. For instance,
Na accounts for 2.64% of the earth’s crustal reserves, which is
4 to 5 orders of magnitude higher than Li. Besides, Na is
widely distributed and easy to extract, thus resulting in a
lower price (Table 7). Similar circumstances can be found for
Zn and other elements. Despite great efforts and significant
progress made in research labs, many issues have to be over-
come to enable these new battery systems to be cost competi-
tive alternatives to Li-ion batteries.
5.4 |Enabling mechanical flexibility
Wearable electronic devices, especially those with mechanical
flexibility (eg, roll-up displays), represent a new direction for
the electronics industry.
2,209–213
Further, they may be
combined with wearable sensors (eg, smart clothing) to
revolutionize the human’s life. Strong consumer demands
are driving the development of such flexible devices. Some
flexible electronics are already available on the market, for
example, the FlexPai and Samsung Infinity Flex. Flexible
rechargeable batteries have become an active research area in
the last few years to meet the energy storage requirements in
these flexible devices.
To date, various types of flexible batteries including flexible
Li-ion,
214–218
Li-sulfur,
219–221
Li-air,
210,222
Zn-ion,
223–226
and
Zn-air
12,227–233
batteries have been demonstrated. As shown in
Figure 12, some of them have exhibited attractive potentials for
PEDs. Despite these achievements, substantial technical chal-
lenges remain. The electrochemical functions of flexible batte-
ries usually deteriorate under long-term frequent mechanical
deformations, for example, bending, folding, twisting, and
other strain modes.
237
One critical task is to create flexible elec-
trodes with high capacity, fast charge/discharge capability, and
excellent cycling stability, which can be further coupled with
flexible electrolytes and separators.
6|CONCLUSIONS AND
PERSPECTIVES
PEDs are important platforms for realizing efficient informa-
tion collection, processing, and dissemination. They have
experienced a rapid growth during the previous three decades.
The performance of these PEDs has been becoming more and
more sensitive to their energy consumption, which relies on
their energy storage components, that is, batteries. The ever-
increasing demands for high-performance batteries drive the
progresses of commercial battery devices from lead-acid to Ni-
Cd, to Ni-MH, and to Li-ion batteries. The advances in battery
technology, in turn, promote the continuously reinventing of
the PED products to promote our lifestyle changes. For
instance, since the birth of the first cellular phone in 1993, the
mobile phones were upgraded every 2 years on average, which
is closely related to the remarkable process in rechargeable bat-
tery technologies. In order to further satisfy the continually
high requirements of rechargeable batteries in PEDs, signifi-
cant research efforts worldwide have been devoted to improv-
ing existing battery systems using new materials, advanced
techniques, and emerging energy chemistries. Furthermore,
many new battery systems are also being explored. Although
most of recent findings in research labs are far from large-scale
practical applications, in our view, the following directions can
open up new frontiers based on multidisciplinary scientific
investigations.
The current exploration on the energy storage system
enables sustainable developments of the battery technology
with enhanced specific energy, better safety, and lower cost,
especially under the drive of vast demands from PED and
electrical vehicle industry. Among various new battery tech-
nologies, Li metal-based batteries, sodium ion batteries, as
well as those rechargeable batteries with solid electrolytes
are particularly regarded as promising energy storage sys-
tems in the future to replace the current batteries. These
next-generations of advanced batteries will facilitate the
development of new information devices to continuously
push it forward. With the progress in the rechargeable bat-
tery technology, more and more multifunctional PEDs are
highly anticipated in the near future.
The trend for the development of the state-of-the-art
rechargeable battery technologies requires a precision match
between the requirements of the devices and the electro-
chemical indicators of energy storage process, which is also
a long-pursued goal for the customized design of batteries
for specific applications. To realize this point, it is highly
important to establish better marriages among materials,
functionalities, applications, and their innovations. The tar-
geted research activities in such fields can help to efficiently
develop key information energy materials, and thus further
promote the progress of the PEDs.
The current hot topic on AI is the coming innovative
direction in the field of materials science. AI is able to not
only quickly analyze data to deduce general characteristics of
advanced materials, but also filter all possible composition
combinations to precisely predict new compositions and
potential properties that can be used for predesigned applica-
tion areas. In this regards, AI provides a new and promising
tool for the innovation of advanced battery materials, includ-
ing electrode materials, electrolytes, separators, and other
LIANG ET AL.25
components. Consequently, the introduction of AI can signifi-
cantly increase the rates of creating advanced energy materials
when compared to those conventional methods through
highly frequent experiments in the lab. With these efforts, the
performance and cost-effectiveness of battery technologies
are expected to be remarkably optimized in a short time.
Meanwhile, the considerable growth of the energy storage
technologies not only guarantees the continuous operation of
AI equipment, but also meets their future requirements of
ever-increasing energy consumption.
Information energy material is a rising point of interdisci-
plinary. More efforts are required to be highly multidisciplinary
between scientists and engineers in the fields of chemistry,
material science, computer, mathematics, physics, and engi-
neering. Such interactions should be explored systematically
from both theoretical and experimental aspects to boost the
progress. These explorations can shed new lights in the
improvement of electrochemical performance for the coming
battery technologies, which will bring more cheerfulness to
our future life.
ACKNOWLEDGMENTS
This work was supported by National Key Research and Devel-
opment Program (2016YFA0202500 and 2015CB932500),
National Natural Science Foundation of China (21676160,
51602107, 21776019, 21825501, 21808124, and U1801257),
the Tsinghua University Initiative Scientific Research Program,
the China Postdoctoral Science Foundation (2017M620049),
and the Tip-top Scientific and Technical Innovative Youth Tal-
ents of Guangdong Special Support Program (2017TQ04C419).
Y. Chen thanks funding support from Australian Research
Council under the Future Fellowships scheme (FT160100107)
and Discovery Programme (DP180102210).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ORCID
Yuan Chen https://orcid.org/0000-0001-9059-3839
Qiang Zhang https://orcid.org/0000-0002-3929-1541
REFERENCES
1. Barsukov Y, Qian J. Battery Power Management for Portable
Devices. Norwood, MA: Artech House; 2013.
2. Li F, Kaiser MR, Ma J, Guo Z, Liu H, Wang J. Free-standing
sulfur-polypyrrole cathode in conjunction with polypyrrole-
coated separator for flexible Li-S batteries. Energy Storage
Mater. 2018;13:312.
3. Brodd RJ, Bullock KR, Leising RA, Middaugh RL, Miller JR,
Takeuchi E. Batteries, 1977 to 2002. J. Electrochem. Soc. 2004;
151:K1.
4. Liu Z, Yang S, Sun B, Chang X, Zheng J, Li X. A peapod-like
CoP@C nanostructure from phosphorization in a low-temperature
molten salt for high-performance lithium-ion batteries. Angew.
Chem. Int. Ed. 2018;57:10187.
5. Li Y, Xu H, Chien P-H, et al. A perovskite electrolyte that is sta-
ble in moist air for lithium-ion batteries. Angew. Chem. Int. Ed.
2018;57:8587.
6. Xie J, Peng H-J, Huang J-Q, Xu W-T, Chen X, Zhang Q. A
supramolecular capsule for reversible polysulfide storage/delivery
in lithium-sulfur batteries. Angew. Chem. Int. Ed. 2017;56:16223.
7. Tarascon JM, Armand M. Issues and challenges facing recharge-
able lithium batteries. Nature. 2001;414:359.
8. Chen W, Gong YF, Liu JH. Recent advances in electrocatalysts
for non-aqueous Li-O
2
batteries. Chin. Chem. Lett. 2017;28:709.
9. Cui C, Wei Z, Xu J, et al. Three-dimensional carbon frameworks
enabling MoS
2
as anode for dual ion batteries with superior
sodium storage properties. Energy Storage Mater. 2018;15:22.
10. Chen L, Fan L-Z. Dendrite-free Li metal deposition in all-solid-
state lithium sulfur batteries with polymer-in-salt polysiloxane
electrolyte. Energy Storage Mater. 2018;15:37.
11. Chen W, Jin Y, Zhao J, Liu N, Cui Y. Nickel-hydrogen batteries
for large-scale energy storage. Proc. Natl. Acad. Sci. U S A.
2018;115:11694.
12. Chen T, Zhang Z, Cheng B, et al. Self-templated formation of
interlaced carbon nanotubes threaded hollow Co
3
S
4
nanoboxes
for high-rate and heat-resistant lithium-sulfur batteries. J. Am.
Chem. Soc. 2017;139:12710.
13. Shi J-L, Tang C, Huang J-Q, Zhu W, Zhang Q. Effective expo-
sure of nitrogen heteroatoms in 3D porous graphene framework
for oxygen reduction reaction and lithium-sulfur batteries.
J. Energy Chem. 2018;27:167.
14. Cheng X-B, Yan C, Zhang X-Q, Liu H, Zhang Q. Electronic and
ionic channels in working interfaces of lithium metal anodes.
ACS Energy Lett. 2018;3:1564.
15. Zhao C-Z, Zhang X-Q, Cheng X-B, et al. An anion-immobilized
composite electrolyte for dendrite-free lithium metal anodes.
Proc. Natl. Acad. Sci. U S A. 2017;114:11069.
16. Liu S, Li J, Yan X, et al. Superhierarchical cobalt-embedded
nitrogen-doped porous carbon nanosheets as two-in-one hosts for
high-performance lithium-sulfur batteries. Adv. Mater. 2018;30:
1706895.
17. Winter M, Barnett B, Xu K. Before Li ion batteries. Chem. Rev.
2018;118(23):11433.
18. Thangadurai V, Narayanan S, Pinzaru D. Garnet-type solid-state
fast Li ion conductors for Li batteries: Critical review. Chem.
Soc. Rev. 2014;43:4714.
19. Dunn B, Kamath H, Tarascon J-M. Electrical energy storage for
the grid: A battery of choices. Science. 2011;334:928.
20. Whittingham MS. Lithium batteries and cathode materials. Chem.
Rev. 2004;104:4271.
21. Scrosati B, Garche J. Lithium batteries: Status, prospects and
future. J. Power Sources. 2010;195:2419.
22. Ji LW, Lin Z, Alcoutlabi M, Zhang XW. Recent developments in
nanostructured anode materials for rechargeable lithium-ion bat-
teries. Energy Environ. Sci. 2011;4:2682.
26 LIANG ET AL.
23. Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Chal-
lenges in the development of advanced Li-ion batteries: A
review. Energy Environ. Sci. 2011;4:3243.
24. Albertus P, Babinec S, Litzelman S, Newman A. Status and chal-
lenges in enabling the lithium metal electrode for high-energy
and low-cost rechargeable batteries. Nat. Energy. 2018;3:16.
25. Hwang J-Y, Myung S-T, Sun Y-K. Sodium-ion batteries: Present
and future. Chem. Soc. Rev. 2017;46:3529.
26. Schmuch R, Wagner R, Horpel G, Placke T, Winter M. Perfor-
mance and cost of materials for lithium-based rechargeable auto-
motive batteries. Nat. Energy. 2018;3:267.
27. Zhou L, Zhang K, Hu Z, et al. Recent developments on and pros-
pects for electrode materials with hierarchical structures for lith-
ium-ion batteries. Adv. Energy Mater. 2018;8:1701415.
28. Borchardt L, Oschatz M, Kaskel S. Carbon materials for lithium
sulfur batteries-ten critical questions. Chem. Eur. J. 2016;22:
7324.
29. Sun Y, Liu N, Cui Y. Promises and challenges of nanomaterials
for lithium-based rechargeable batteries. Nat. Energy. 2016;1:
16071.
30. Cheng X-B, Zhang R, Zhao C-Z, Zhang Q. Toward safe lithium
metal anode in rechargeable batteries: A review. Chem. Rev.
2017;117:10403.
31. Choi S, Wang G. Advanced lithium-ion batteries for practical
applications: Technology, development, and future perspectives.
Adv. Mater. Technol. 2018;3:1700376.
32. http://www.chyxx.com/industry/201804/629104.html. Accessed
November 25, 2018.
33. Goodenough JB. How we made the Li-ion rechargeable battery.
Nat. Electron. 2018;1:204.
34. Pistoia G. Batteries for Portable Devices. Amsterdam, The
Netherlands: Elsevier; 2005.
35. Choi JW, Aurbach D. Promise and reality of post-lithium-ion bat-
teries with high energy densities. Nat. Rev. Mater. 2016;1:16013.
36. Blomgren GE. The development and future of lithium ion batte-
ries. J. Electrochem. Soc. 2017;164:A5019.
37. Bin D, Wen YP, Wang YG, Xia YY. The development in aque-
ous lithium-ion batteries. J. Energy Chem. 2018;27:1521.
38. Yoshino A. The birth of the lithium-ion battery. Angew. Chem.
Int. Ed. 2012;51:5798.
39. Zhu G, Wen K, Lv W, et al. Materials insights into low-temperature
performances of lithium-ion batteries. J. Power Sources. 2015;
300:29.
40. Yan ZL, Hu QY, Yan GC, et al. Co
3
O
4
/Co nanoparticles
enclosed graphitic carbon as anode material for high performance
Li-ion batteries. Chem. Eng. J. 2017;321:495.
41. Liang Y, Zhang W, Wu D, Ni Q-Q, Zhang MQ. Interface engi-
neering of carbon-based nanocomposites for advanced electro-
chemical energy storage. Adv. Mater. Interfaces. 2018;5:1800430.
42. https://www.apple.com/macbook-pro/. Accessed December 3, 2018.
43. Sumboja A, Liu J, Zheng WG, Zong Y, Zhang H, Liu Z. Electro-
chemical energy storage devices for wearable technology: A
rationale for materials selection and cell design. Chem. Soc. Rev.
2018;47:5919.
44. Pu X, Hu W, Wang ZL. Toward wearable self-charging power
systems: The integration of energy-harvesting and storage
devices. Small. 2018;14:1702817.
45. Gao M, Li L, Song Y. Inkjet printing wearable electronic devices.
J. Mater. Chem.C. 2017;5:2971.
46. An BW, Shin JH, Kim S-Y, et al. Smart sensor systems for wear-
able electronic devices. Polymers. 2017;9:303.
47. https://www.apple.com/au/watch/battery.html. Accessed December
3, 2018.
48. https://arstechnica.com/apple/2015/05/review-the-absolutely-optional-
apple-watch-and-watch-os-1-0/. Accessed December 1, 2018.
49. Mardonova M, Choi Y. Review of wearable device technology
and its applications to the mining industry. Energies. 2018;11:547.
50. http://it.sohu.com/20130613/n378655557.shtml. Accessed December
5, 2018.
51. https://en.wikipedia.org/wiki/Google_Glass. Accessed December
5, 2018.
52. https://9to5google.com/2017/07/24/google-glass-enterprise-edition-
specs/. Accessed December 5, 2018.
53. https://www.vuzix.com/Products/m100-smart-glasses. Accessed
December 5, 2018.
54. https://thefutureofthings.com/5137-omsignal-smart-shirt-to-wirelessly-
measure-your-vitals/. Accessed December 4, 2018.
55. https://sneakerdon.com/nike-air-mag-back-to-the-future-417744-
001. Accessed December 3, 2018.
56. https://technode.com/2018/01/03/worlds-top-drone-seller-dji-made-
2-7-billion-2017/. Accessed December 7, 2018.
57. Placke T, Kloepsch R, Duehnen S, Winter M. Lithium ion, lith-
ium metal, and alternative rechargeable battery technologies: The
odyssey for high energy density. J. Solid State Electrochem.
2017;21:1939.
58. Shen X, Liu H, Cheng X-B, Yan C, Huang J-Q. Beyond lithium
ion batteries: Higher energy density battery systems based on
lithium metal anodes. Energy Storage Mater. 2018;12:161.
59. Goodenough JB, Kim Y. Challenges for rechargeable Li batte-
ries. Chem. Mater. 2010;22:587.
60. Liang Y, Chen L, Cai L, et al. Strong contribution of pore mor-
phology to the high-rate electrochemical performance of lithium-
ion batteries. Chem. Commun. 2016;52:803.
61. Liu Z, Yu Q, Zhao Y, et al. Silicon oxides: A promising family
of anode materials for lithium-ion batteries. Chem. Soc. Rev.
2018;48:285.
62. Liang YR, Cai LF, Chen LY, et al. Silica nanonetwork confined
in nitrogen-doped ordered mesoporous carbon framework for
high-performance lithium-ion battery anodes. Nanoscale. 2015;7:
3971.
63. Liu H, Li Z, Liang Y, Fu R, Wu D. Facile synthesis of MnO
multi-core@nitrogen-doped carbon shell nanoparticles for high
performance lithium-ion battery anodes. Carbon. 2015;84:419.
64. Jiang N, Li B, Ning F, Xia D. All boron-based 2D material as
anode material in Li-ion batteries. J. Energy Chem. 2018;27:1651.
65. Wu H, Zheng GY, Liu NA, Carney TJ, Yang Y, Cui Y. Engi-
neering empty space between Si nanoparticles for lithium-ion bat-
tery anodes. Nano Lett. 2012;12:904.
66. Feng K, Li M, Liu WW, et al. Silicon-based anodes for lithium-
ion batteries: From fundamentals to practical applications. Small.
2018;14:33.
67. Winter M, Besenhard JO. Electrochemical lithiation of tin and tin-based
intermetallics and composites. Electrochim. Acta. 1999;45:31.
68. Zhu ZQ, Wang SW, Du J, et al. Ultrasmall Sn nanoparticles
embedded in nitrogen-doped porous carbon as high-performance
anode for lithium-ion batteries.Nano Lett. 2014;14:153.
69. Yi Z, Wang Z, Cheng Y, Wang L. Sn-based intermetallic com-
pounds for Li-ion batteries: Structures, lithiation mechanism, and
LIANG ET AL.27
electrochemical performances. Energy Environ. Mater. 2018;
1:132.
70. Jiang B, He Y, Li B, et al. Polymer-templated formation of
polydopamine-coated SnO
2
nanocrystals: anodes for cyclable lith-
ium-ion batteries. Angew. Chem. Int. Ed. 2017;56:1869.
71. Dong W, Xu J, Wang C, et al. A robust and conductive black tin
oxide nanostructure makes efficient lithium-ion batteries possi-
ble. Adv. Mater. 2017;29:1700136.
72. Zhu JX, Yin ZY, Yang D, et al. Hierarchical hollow spheres com-
posed of ultrathin Fe
2
O
3
nanosheets for lithium storage and photo-
catalytic water oxidation. Energy Environ. Sci. 2013;6:987.
73. Yu AP, Park HW, Davies A, Higgins DC, Chen ZW, Xiao XC.
Free-standing layer-by-layer hybrid thin film of graphene-MnO
2
nanotube as anode for lithium ion batteries. J. Phys. Chem. Lett.
2011;2:1855.
74. Yan N, Hu L, Li Y, et al. Co
3
O
4
nanocages for high-performance
anode material in lithium-ion batteries. J. Phys. Chem. C. 2012;
116:7227.
75. Zheng XF, Wang HE, Wang C, et al. 3D interconnected macro-
mesoporous electrode with self-assembled NiO nanodots for high-
performance supercapacitor-like Li-ion battery. Nano Energy.
2016;22:269.
76. Sun XL, Si WP, Liu XH, et al. Multifunctional Ni/NiO hybrid
nanomembranes as anode materials for high-rate Li-ion batteries.
Nano Energy. 2014;9:168.
77. Soundharrajan V, Sambandam B, Song J, et al. A one-pot strat-
egy to NiO nanoparticles with excellent anode properties for lith-
ium ion batteries. J. Energy Chem. 2018;27:300.
78. Manthiram A, Knight JC, Myung S-T, Oh S-M, Sun Y-K.
Nickel-rich and lithium-rich layered oxide cathodes: Progress and
perspectives. Adv. Energy Mater. 2016;6:1501010.
79. Qiu B, Zhang M, Xia Y, Liu Z, Meng YS. Understanding and con-
trolling anionic electrochemical activity in high-capacity oxides for
next generation Li-ion batteries. Chem. Mater. 2017;29:908.
80. Noh H-J, Youn S, Yoon CS, Sun Y-K. Comparison of the struc-
tural and electrochemical properties of layered Li[Ni
x
Co
y
Mn
z
]O
2
(x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-
ion batteries. J. Power Sources. 2013;233:121.
81. Kraytsberg A, Ein-Eli Y. Higher, stronger, better …a review of
5 volt cathode materials for advanced lithium-ion batteries. Adv.
Energy Mater. 2012;2:922.
82. Ma J, Hu P, Cui G, Chen L. Surface and interface issues in spinel
LiNi
0.5
Mn
1.5
O
4
: Insights into a potential cathode material for
high energy density lithium ion batteries. Chem. Mater. 2016;28:
3578.
83. Zhang Z, Dong S, Cui Z, Du A, Li G, Cui G. Rechargeable mag-
nesium batteries using conversion-type cathodes: A perspective
and minireview. Small Methods. 2018;2:1800020.
84. Zhao S, Fang R, Sun Z, et al. A 3D multifunctional architecture
for lithium-sulfur batteries with high areal capacity. Small
Methods. 2018;2:1800067.
85. Zhang X, Mu X, Yang S, et al. Research progress for the devel-
opment of Li-air batteries: Addressing parasitic reactions arising
from air composition. Energy Environ Mater. 2018;1:61.
86. Guo W, Fu Y. A perspective on energy densities of rechargeable
Li-S batteries and alternative sulfur-based cathode materials.
Energy Environ Mater. 2018;1:20.
87. Tang M, Li H, Wang E, Wang C. Carbonyl polymeric electrode
materials for metal-ion batteries. Chin. Chem. Lett. 2018;29:232.
88. Cui J, Zhan T-G, Zhang K-D, Chen D. The recent advances in
constructing designed electrode in lithium metal batteries. Chin.
Chem. Lett. 2017;28:2171.
89. Yang D, Zhang L, Yan X, Yao X. Recent progress in oxygen
electrocatalysts for zinc-air batteries. Small Methods. 2017;1:
1700209.
90. Zhang X-Q, Cheng X-B, Zhang Q. Advances in interfaces
between Li metal anode and electrolyte. Adv. Mater. Interfaces.
2018;5:1701097.
91. Fan X, Chen L, Ji X, et al. Highly fluorinated interphases enable
high-voltage Li-metal batteries. Chem. 2018;4:174.
92. Li T, Liu H, Shi P, Zhang Q. Recent progress in carbon/lithium
metal composite anode for safe lithium metal batteries. Rare
Metals. 2018;37:449.
93. Zhang X-Q, Cheng X-B, Chen X, Yan C, Zhang Q. Fluoroethy-
lene carbonate additives to render uniform Li deposits in lithium
metal batteries. Adv. Funct. Mater. 2017;27:1605989.
94. Zhang X-Q, Chen X, Cheng X-B, et al. Highly stable lithium
metal batteries enabled by regulating the solvation of lithium ions
in nonaqueous electrolytes. Angew. Chem. Int. Ed. 2018;57:5301.
95. Cheng X-B, Hou T-Z, Zhang R, et al. Dendrite-free lithium depo-
sition induced by uniformly distributed lithium ions for efficient
lithium metal batteries. Adv. Mater. 2016;28:2888.
96. Xin S, Chang ZW, Zhang XB, Guo YG. Progress of rechargeable
lithium metal batteries based on conversion reactions. Natl. Sci.
Rev. 2017;4:54.
97. Wang H, Yang Y, Liang Y, et al. Graphene-wrapped sulfur parti-
cles as a rechargeable lithium-sulfur battery cathode material with
high capacity and cycling stability. Nano Lett. 2011;11:2644.
98. Su Y-S, Manthiram A. A new approach to improve cycle perfor-
mance of rechargeable lithium-sulfur batteries by inserting a free-
standing MWCNT interlayer. Chem. Commun. 2012;48:8817.
99. Manthiram A, Fu Y, Chung S-H, Zu C, Su Y-S. Rechargeable
lithium-sulfur batteries. Chem. Rev. 2014;114:11751.
100. Nayak PK, Yang L, Brehm W, Adelhelm P. From lithium-ion
to sodium-ion batteries: Advantages, challenges, and surprises.
Angew. Chem. Int. Ed. 2018;57:102.
101. Chen K, Sun Z, Fang R, Li F, Cheng H. Development of gra-
phene-based materials for lithium-sulfur batteries. Acta Phys.
Chim. Sin. 2018;34:377.
102. Xu R, Sun Y, Wang Y, Huang J, Zhang Q. Two-dimensional ver-
miculite separator for lithium sulfur batteries. Chin. Chem. Lett.
2017;28:2235.
103. Kong L, Yan C, Huang J-Q, et al. A review of advanced energy
materials for magnesium-sulfur batteries. Energy Environ. Mater.
2018;1:100.
104. Liu J, Wang M, Xu N, Qian T, Yan C. Progress and perspective
of organosulfur polymers as cathode materials for advanced lith-
ium-sulfur batteries. Energy Storage Mater. 2018;15:53.
105. Song K, Agyeman DA, Park M, Yang J, Kang Y-M. High-
energy-density metal-oxygen batteries: Lithium-oxygen batteries
vs sodium-oxygen batteries. Adv. Mater. 2017;29:1606572.
106. Varma SJ, Kumar KS, Seal S, Rajaraman S, Thomas J. Fiber-type
solar cells, nanogenerators, batteries, and supercapacitors for
wearable applications. Adv. Sci. 2018;5:1800340.
107. Pan J, Xu YY, Yang H, Dong Z, Liu H, Xia BY. Advanced
architectures and relatives of air electrodes in Zn-air batteries.
Adv. Sci. 2018;5:1700691.
28 LIANG ET AL.
108. Li B-Q, Zhang S-Y, Wang B, Xia Z-J, Tang C, Zhang Q. A por-
phyrin covalent organic framework cathode for flexible Zn-air
batteries. Energy Environ. Sci. 2018;11:1723.
109. Wang H-F, Tang C, Wang B, Li B-Q, Zhang Q. Bifunctional
transition metal hydroxysulfides: Room-temperature sulfurization
and their applications in Zn-air batteries. Adv. Mater. 2017;29:
1702327.
110. Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research develop-
ment on sodium-ion batteries. Chem. Rev. 2014;114:11636.
111. Bin D-S, Lin X-J, Sun Y-G, et al. Engineering hollow carbon
architecture for high-performance K-ion battery anode. J. Am.
Chem. Soc. 2018;140:7127.
112. Wang Q, Xu J, Zhang W, et al. Research progress on vanadium-
based cathode materials for sodium ion batteries. J. Mater. Chem.
A. 2018;6:8815.
113. Kim H, Kim JC, Bianchini M, Seo D-H, Rodriguez-Garcia J,
Ceder G. Recent progress and perspective in electrode materials
for K-ion batteries. Adv. Energy Mater. 2018;8:1702384.
114. Li T, Zhang Q. Advanced metal sulfide anode for potassium ion
batteries. J. Energy Chem. 2018;27:373.
115. Zhang C, Lu C, Zhang F, Qiu F, Zhuang X, Feng X. Two-dimen-
sional organic cathode materials for alkali-metal-ion batteries.
J. Energy Chem. 2018;27:86.
116. Chen R, Luo R, Huang Y, Wu F, Li L. Advanced high energy
density secondary batteries with multi-electron reaction materials.
Adv. Sci. 2016;3:1600051.
117. Zhang X-Q, Huang J-Q, Zhang Q. Recent advances in energy
chemical engineering of next-generation lithium batteries. Engi-
neering. 2018;4:831.
118. Liu X, Zhang Q, Huang J, Zhang S, Peng H, Wei F. Hierarchical
nanostructured composite cathode with carbon nanotubes as con-
ductive scaffold for lithium-sulfur batteries. J. Energy Chem.
2013;22:341.
119. Liu S, Yao L, Zhang Q, et al. Advances in high-performance lith-
ium-sulfur batteries. Acta Phys. Chim. Sin. 2017;33:2339.
120. Choi NS, Chen Z, Freunberger SA, et al. Challenges facing lith-
ium batteries and electrical double-layer capacitors. Angew.
Chem. Int. Ed. 2012;51:9994.
121. Patil A, Patil V, Shin DW, Choi J-W, Paik D-S, Yoon S-J. Issue
and challenges facing rechargeable thin film lithium batteries.
Mater. Res. Bull. 2008;43:1913.
122. Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M. Li-O
2
and Li-S batteries with high energy storage. Nat. Mater. 2012;
11:19.
123. Liu B, Fang R, Xie D, et al. Revisiting scientific issues for indus-
trial applications of lithium-sulfur batteries. Energy Environ.
Mater. 2018;1:196.
124. Shen X, Tian Z, Fan R, et al. Research progress on silicon/carbon
composite anode materials for lithium-ion battery. J. Energy
Chem. 2018;27:1067.
125. Huo H, Xing Y, Pecht M, Zuger BJ, Khare N, Vezzini A. Safety
requirements for transportation of lithium batteries. Energies.
2017;10:793.
126. Kong L, Li C, Jiang J, Pecht MG. Li-ion battery fire hazards and
safety strategies. Energies. 2018;11:793.
127. Balakrishnan PG, Ramesh R, Kumar TP. Safety mechanisms in
lithium-ion batteries. J. Power Sources. 2006;155:401.
128. Liu K, Liu Y, Lin D, Pei A, Cui Y. Materials for lithium-ion bat-
tery safety. Sci. Adv. 2018;4:eaas9820.
129. Hyung YE, Vissers DR, Amine K. Flame-retardant additives for
lithium-ion batteries. J. Power Sources. 2003;119:383.
130. Granzow A. Flame retardation by phosphorus-compounds. Acc.
Chem. Res. 1978;11:177.
131. Pires J, Castets A, Timperman L, et al. Tris(2,2,2-trifluoroethyl)
phosphite as an electrolyte additive for high-voltage lithium-ion
batteries using lithium-rich layered oxide cathode. J. Power
Sources. 2015;296:413.
132. Choi J-A, Sun Y-K, Shim E-G, Scrosati B, Kim D-W. Effect of
1-butyl-1-methylpyrrolidinium hexafluorophosphate as a flame-
retarding additive on the cycling performance and thermal prop-
erties of lithium-ion batteries. Electrochim. Acta. 2011;56:10179.
133. Wang Q, Ping P, Sun J, Chen C. Improved thermal stability of
lithium ion battery by using cresyl diphenyl phosphate as an elec-
trolyte additive. J. Power Sources. 2010;195:7457.
134. Arbizzani C, Gabrielli G, Mastragostino M. Thermal stability and
flammability of electrolytes for lithium-ion batteries. J. Power
Sources. 2011;196:4801.
135. Seki S, Kobayashi Y, Miyashiro H, et al. Lithium secondary bat-
teries using modified-imidazolium room-temperature ionic liquid.
J. Phys. Chem. B. 2006;110:10228.
136. Lewandowski A, Swiderska-Mocek A. Ionic liquids as electro-
lytes for Li-ion batteries-An overview of electrochemical studies.
J. Power Sources. 2009;194:601.
137. Chen Z, Ren Y, Jansen AN, Lin C-K, Weng W, Amine K. New
class of nonaqueous electrolytes for long-life and safe lithium-ion
batteries. Nat. Commun. 2013;4:1513.
138. Zhang SS. A review on electrolyte additives for lithium-ion batte-
ries. J. Power Sources. 2006;162:1379.
139. Yu X, Manthiram A. Electrode-electrolyte interfaces in lithium-
based batteries. Energy Environ. Sci. 2018;11:527.
140. Chen W, Lei T, Wu C, et al. Designing safe electrolyte systems
for a high-stability lithium-sulfur battery. Adv. Energy Mater.
2018;8:1702348.
141. Wu F, Fitzhugh W, Ye L, Ning J, Li X. Advanced sulfide solid
electrolyte by core-shell structural design. Nat. Commun. 2018;9:
4037.
142. Lu Y, Li L, Zhang Q, Niu Z, Chen J. Electrolyte and interface
engineering for solid-state sodium batteries. Joule. 2018;2:1747.
143. Fu K, Gong Y, Hitz GT, et al. Three-dimensional bilayer garnet
solid electrolyte based high energy density lithium metal -sulfur
batteries. Energy Environ. Sci. 2017;10:1568.
144. Armand M. Polymer solid electrolytes-an overview. Solid State
Ion. 1983;9-10:745.
145. Zeng X-X, Yin Y-X, Li N-W, Du W-C, Guo Y-G, Wan L-J.
Reshaping lithium plating/stripping behavior via bifunctional
polymer electrolyte for room-temperature solid Li metal batteries.
J. Am. Chem. Soc. 2016;138:15825.
146. Porcarelli L, Gerbaldi C, Bella F, Nair JR. Super soft all-ethylene
oxide polymer electrolyte for safe all-solid lithium batteries. Sci.
Rep. 2016;6:19892.
147. Chen S, Wen K, Fan J, Bando Y, Golberg D. Progress and future
prospects of high-voltage and high-safety electrolytes in advanced
lithium batteries: From liquid to solid electrolytes. J. Mater.
Chem. A. 2018;6:11631.
148. Ma Q, Hu Y, Li H, Chen L, Huang X, Zhou Z. An sodium bis
(trifluoromethanesulfonyl)imide-based polymer electrolyte for
solid-state sodium batteries. Acta Phys. Chim. Sin. 2018;34:213.
LIANG ET AL.29
149. Luo W, Gong Y, Zhu Y, et al. Reducing interfacial resistance
between garnet-structured solid-state electrolyte and li-metal
anode by a germanium layer. Adv. Mater. 2017;29:1606042.
150. Chua S, Fang R, Sun Z, et al. Hybrid solid polymer electrolytes
with two-dimensional inorganic nanofillers. Chem. Eur. J. 2018;
24:18180.
151. McOwen DW, Xu S, Gong Y, et al. 3D-printing electrolytes for
solid-state batteries. Adv. Mater. 2018;30:1707132.
152. Dai J, Yang C, Wang C, Pastel G, Hu L. Interface engineering
for garnet-based solid-state lithium-metal batteries: Materials,
structures, and characterization. Adv. Mater. 2018;30:1802068.
153. Han X, Gong Y, Fu K, et al. Negating interfacial impedance in
garnet-based solid-state Li metal batteries. Nat. Mater. 2017;
16:572.
154. Manthiram A, Yu X, Wang S. Lithium battery chemistries
enabled by solid-state electrolytes. Nat. Rev. Mater. 2017;2:
16103.
155. Xu R-C, Xia X-H, Wang X-L, Xia Y, Tu J-P. Tailored Li
2
S-P
2
S
5
glass-ceramic electrolyte by MoS
2
doping, possessing high ionic
conductivity for all-solid-state lithium-sulfur batteries. J. Mater.
Chem. A. 2017;5:2829.
156. Luo W, Gong Y, Zhu Y, et al. Transition from superlithiophobi-
city to superlithiophilicity of garnet solid-state electrolyte. J. Am.
Chem. Soc. 2016;138:12258.
157. Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J. Recent advances
in all-solid-state rechargeable lithium batteries. Nano Energy.
2017;33:363.
158. Wang C, Gong Y, Liu B, et al. Conformal, nanoscale ZnO sur-
face modification of garnet-based solid-state electrolyte for lith-
ium metal anodes. Nano Lett. 2017;17:565.
159. Yamane H, Shibata M, Shimane Y, et al. Crystal structure of a
superionic conductor, Li
7
P
3
S
11
.Solid State Ion. 2007;178:1163.
160. Ohtomo T, Hayashi A, Tatsumisago M, Tsuchida Y, Hama S,
Kawamoto K. All-solid-state lithium secondary batteries using
the 75Li
2
S25P
2
S
5
glass and the 70Li
2
S30P
2
S
5
glass-ceramic as
solid electrolytes. J. Power Sources. 2013;233:231.
161. Kamaya N, Homma K, Yamakawa Y, et al. A lithium superionic
conductor. Nat. Mater. 2011;10:682.
162. Wu H, Zhuo D, Kong D, Cui Y. Improving battery safety by
early detection of internal shorting with a bifunctional separator.
Nat. Commun. 2014;5:5193.
163. Zhang SS, Xu K, Jow TR. An inorganic composite membrane
as the separator of Li-ion batteries. J. Power Sources. 2005;
140:361.
164. Lin D, Zhuo D, Liu Y, Cui Y. All-integrated bifunctional separa-
tor for Li dendrite detection via novel solution synthesis of a ther-
mostable polyimide separator. J. Am. Chem. Soc. 2016;138:
11044.
165. Zhang J, Yue L, Kong Q, et al. Sustainable, heat-resistant and
flame-retardant cellulose-based composite separator for high-per-
formance lithium ion battery. Sci. Rep. 2014;4:3935.
166. Orendorff CJ, Lambert TN, Chavez CA, Bencomo M, Fenton
KR. Polyester separators for lithium-ion cells: improving thermal
stability and abuse tolerance. Adv. Energy Mater. 2013;3:314.
167. Arora P, Zhang ZM. Battery separators. Chem. Rev. 2004;104:
4419.
168. Chen Z, Hsu P-C, Lopez J, et al. Fast and reversible thermore-
sponsive polymer switching materials for safer batteries. Nat.
Energy. 2016;1:15009.
169. Zheng G, Lee SW, Liang Z, et al. Interconnected hollow carbon
nanospheres for stable lithium metal anodes. Nat. Nanotechnol.
2014;9:618.
170. Li N-W, Yin Y-X, Yang C-P, Guo Y-G. An artificial solid elec-
trolyte interphase layer for stable lithium metal anodes. Adv.
Mater. 2016;28:1853.
171. Zhang R, Chen X-R, Chen X, et al. Lithiophilic sites in doped
graphene guide uniform lithium nucleation for dendrite-free lith-
ium metal anodes. Angew. Chem. Int. Ed. 2017;56:7764.
172. Yan C, Cheng X-B, Tian Y, et al. Dual-layered film protected
lithium metal anode to enable dendrite-free lithium deposition.
Adv. Mater. 2018;30:1707629.
173. Zhou F, Zhao X, Dahn JR. Impact of Al or Mg substitution on
the thermal stability of Li
(1.05)
Mn
(1.95-z)
M
z
O
(4)
(M=Al or Mg).
J. Electrochem. Soc. 2010;157:A798.
174. Cho J, Kim YW, Kim B, Lee JG, Park B. A breakthrough in the
safety of lithium secondary batteries by coating the cathode mate-
rial with AIPO
4
nanoparticles. Angew. Chem. Int. Ed. 2003;42:
1618.
175. Xia L, Li S-L, Ai X-P, Yang H-X, Cao Y-L. Temperature-sensi-
tive cathode materials for safer lithium-ion batteries. Energy
Environ. Sci. 2011;4:2845.
176. Poizot P, Dolhem F, Gaubicher J. Progress in all-organic
rechargeable batteries using cationic and anionic configurations:
Toward low-cost and greener storage solutions? Curr. Opin.
Electrochem. 2018;9:70.
177. Yang T, Qian T, Wang M, et al. A sustainable route from bio-
mass byproduct okara to high content nitrogen-doped carbon
sheets for efficient sodium ion batteries. Adv. Mater. 2016;
28:539.
178. Zhuo L, Wu Y, Wang L, Yu Y, Zhang X, Zhao F. One-step
hydrothermal synthesis of SnS
2
/graphene composites as anode
material for highly efficient rechargeable lithium ion batteries.
RSC Adv. 2012;2:5084.
179. Recham N, Armand M, Laffont L, Tarascon JM. Eco-Efficient
Synthesis of LiFePO
4
with different morphologies for Li-ion bat-
teries. Electrochem. Solid State Lett. 2009;12:A39.
180. Liu Y, Uchaker E, Zhou N, Li J, Zhang Q, Cao G. Facile synthe-
sis of nanostructured vanadium oxide as cathode materials for
efficient Li-ion batteries. J. Mater. Chem. 2012;22:24439.
181. Ji H, Yang G, Miao X, Hong A. Efficient microwave hydrother-
mal synthesis of nanocrystalline orthorhombic LiMnO
2
cathodes
for lithium batteries. Electrochim. Acta. 2010;55:3392.
182. Yang K, Guo Q, Li H, et al. Highly efficient sol-gel synthesis for
ZnS@N, S co-doped carbon nanosheets with embedded hetero-
structure for sodium ion batteries. J. Power Sources. 2018;
402:340.
183. Wang Q, Miao H, Sun S, Xue Y, Liu Z. One-pot synthesis of
Co
3
O
4
/Ag nanoparticles supported on N-doped graphene as effi-
cient bifunctional oxygen catalysts for flexible rechargeable zinc-
air batteries. Chem. Eur. J. 2018;24:14816.
184. Cheng X-B, Tian G-L, Liu X-F, et al. Robust growth of herring-
bone carbon nanofibers on layered double hydroxide derived cat-
alysts and their applications as anodes for Li-ion batteries.
Carbon. 2013;62:393.
185. Dang R, Jia X, Wang P, Zhang X, Wang D, Wang G. Hydrother-
mal synthesis of peony-like CuO micro/nanostructures for high-
performance lithium-ion battery anodes. Chin. Chem. Lett. 2017;
28:2263.
30 LIANG ET AL.
186. Chen L, Yang S, Huang J, et al. Two-dimensional porous carbon
nanosheets from exfoliated nanopaper-like biomass. Mater. Lett.
2018;232:187.
187. Zhang J, Xiang J, Dong Z, et al. Biomass derived activated car-
bon with 3D connected architecture for rechargeable lithium-
sulfur batteries. Electrochim. Acta. 2014;116:146.
188. Hong K-L, Qie L, Zeng R, et al. Biomass derived hard carbon used
as a high performance anode material for sodium ion batteries.
J. Mater. Chem. A. 2014;2:12733.
189. Chen H, Armand M, Demailly G, Dolhem F, Poizot P, Tarascon
J-M. From biomass to a renewable Li
x
C
6
O
6
organic electrode for
sustainable Li-ion batteries. ChemSusChem. 2008;1:348.
190. Deng B, Shen L, Liu Y, et al. Porous Si/C composite as anode
materials for high-performance rechargeable lithium-ion battery.
Chin. Chem. Lett. 2017;28:2281.
191. Liu S, Shao L, Zhang X, Tao Z, Chen J. Advances in electrode
materials for aqueous rechargeable sodium-ion batteries. Acta
Phys. Chim. Sin. 2018;34:581.
192. Fang Y, Chen Z, Xiao L, Ai X, Cao Y, Yang H. Recent progress
in iron-based electrode materials for grid-scale sodium-ion batte-
ries. Small. 2018;14:1703116.
193. Yang F, Gao H, Chen J, Guo Z. Phosphorus-based materials
as the anode for sodium-ion batteries. Small Methods. 2017;1:
1700216.
194. Wu B, Zhang G, Yan M, et al. Graphene scroll-coated alpha-
MnO
2
nanowires as high-performance cathode materials for
aqueous Zn-ion battery. Small. 2018;14:1703850.
195. Li W, Wang K, Cheng S, Jiang K. A long-life aqueous Zn-ion
battery based on Na
3
V
2
(PO
4
)
2
F
3
cathode. Energy Storage Mater.
2018;15:14.
196. Zhang N, Cheng F, Liu Y, et al. Cation-deficient spinel ZnMn
2
O
4
cathode in Zn(CF
3
SO
3
)
2
electrolyte for rechargeable aqueous Zn-
ion battery. J. Am. Chem. Soc. 2016;138:12894.
197. Zhang W, Mao J, Li S, Chen Z, Guo Z. Phosphorus-based alloy
materials for advanced potassium-ion battery anode. J. Am.
Chem. Soc. 2017;139:3316.
198. Wang W, Zhou J, Wang Z, et al. Short-range order in mesopor-
ous carbon boosts potassium-ion battery performance. Adv.
Energy Mater. 2018;8:1701648.
199. Pramudita JC, Sehrawat D, Goonetilleke D, Sharma N. An initial
review of the status of electrode materials for potassium-ion bat-
teries. Adv. Energy Mater. 2017;7:1602911.
200. Singh N, Arthur TS, Ling C, Matsui M, Mizuno F. A high
energy-density tin anode for rechargeable magnesium-ion batte-
ries. Chem. Commun. 2013;49:149.
201. Ichitsubo T, Adachi T, Yagi S, Doi T. Potential positive elec-
trodes for high-voltage magnesium-ion batteries. J. Mater. Chem.
2011;21:11764.
202. Rastgoo-Deylami M, Chae MS, Hong S-T. H
2
V
3
O
8
as a high
energy cathode material for nonaqueous magnesium-ion batteries.
Chem. Mater. 2018;30:7464.
203. Zhang X, Tang Y, Zhang F, Lee C-S. A novel aluminum-graphite
dual-ion battery. Adv. Energy Mater. 2016;6:1502588.
204. Wang S, Jiao S, Wang J, et al. High-performance aluminum-ion
battery with CuS@C microsphere composite cathode. ACS Nano.
2017;11:469.
205. Chen H, Guo F, Liu Y, et al. A defect-free principle for advanced
graphene cathode of aluminum-ion battery. Adv. Mater. 2017;29:
1605958.
206. Das SK. Graphene: A cathode material of choice for aluminum-
ion batteries. Angew. Chem. Int. Ed. 2018;57:16606.
207. Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-
Gonzalez J, Rojo T. Na-ion batteries, recent advances and present
challenges to become low cost energy storage systems. Energy
Environ. Sci. 2012;5:5884.
208. Song M, Tan H, Chao D, Fan HJ. Recent advances in Zn-ion bat-
teries. Adv. Funct. Mater. 2018;28:1802564.
209. Yang Q, Wang Y, Li X, et al. Recent progress of MXene-based
nanomaterials in flexible energy storage and electronic devices.
Energy Environ. Mater. 2018;1:183.
210. Liu Q, Chang Z, Li Z, Zhang X. Flexible metal-air batteries:
Progress, challenges, and perspectives. Small Methods. 2018;2:
1700231.
211. Jin Z, Li P, Jin Y, Xiao D. Superficial-defect engineered nicke-
l/iron oxide nanocrystals enable high-efficient flexible fiber bat-
tery. Energy Storage Mater. 2018;13:160.
212. Hu XF, Li ZF, Chen J. Flexible Li-CO
2
batteries with liquid-free
electrolyte. Angew. Chem. Int. Ed. 2017;56:5785.
213. Zhao F, Zhao X, Peng B, et al. Polyimide-derived carbon nanofi-
ber membranes as anodes for high-performance flexible lithium
ion batteries. Chin. Chem. Lett. 2018;29:1692.
214. Liu Z, Li H, Zhu M, et al. Towards wearable electronic devices: A
quasi-solid-state aqueous lithium-ion battery with outstanding stabil-
ity, flexibility, safety and breathability. Nano Energy. 2018;44:164.
215. Xia H, Xia Q, Lin B, Zhu J, Seo JK, Meng YS. Self-standing
porous LiMn
2
O
4
nanowall arrays as promising cathodes for
advanced 3D microbatteries and flexible lithium-ion batteries
Nano Energy. 2016;22:475.
216. Kim S-H, Choi K-H, Cho S-J, Yoo J, Lee S-S, Lee S-Y. Flexi-
ble/shape-versatile, bipolar all-solid-state lithium-ion batteries
prepared by multistage printing. Energy Environ. Sci. 2018;
11:321.
217. Gao Z, Song N, Zhang Y, Li X. Cotton-textile-enabled, flexible
lithium-ion batteries with enhanced capacity and extended life-
span. Nano Lett. 2015;15:8194.
218. Chen L, Zhou G, Liu Z, et al. Scalable clean exfoliation of high-
quality few-layer black phosphorus for a flexible lithium ion bat-
tery. Adv. Mater. 2016;28:510.
219. Peng H-J, Huang J-Q, Zhang Q. A review of flexible lithium-
sulfur and analogous alkali metal-chalcogen rechargeable batte-
ries. Chem. Soc. Rev. 2017;46:5237.
220. Yao M, Wang R, Zhao Z, Liu Y, Niu Z, Chen J. A flexible all-in-
one lithium-sulfur battery. ACS Nano. 2018;12:12503.
221. Chang J, Shang J, Sun Y, et al. Flexible and stable high-energy
lithium-sulfur full batteries with only 100% oversized lithium.
Nat. Commun. 2018;9:4480.
222. Lei X, Liu X, Ma W, Cao Z, Wang Y, Ding Y. Flexible lithium-
air battery in ambient air with an in-situ formed gel electrolyte.
Angew. Chem. Int. Ed. 2018;57:16131.
223. Li H, Han C, Huang Y, et al. An extremely safe and wearable
solid-state zinc ion battery based on a hierarchical structured
polymer electrolyte. Energy Environ. Sci. 2018;11:941.
224. Ma Y, Xie X, Lv R, Na B, Ouyang J, Liu H. Nanostructured
polyaniline-cellulose papers for solid-state flexible aqueous Zn-
ion battery. ACS Sustain. Chem. Eng. 2018;6:8697.
225. Wang Z, Ruan Z, Ng WS, et al. Integrating a triboelectric nano-
generator and a zinc-ion battery on a designed flexible 3D spacer
fabric. Small Methods. 2018;2:1800150.
LIANG ET AL.31
226. Wang Z, Ruan Z, Liu Z, et al. A flexible rechargeable zinc-ion
wire-shaped battery with shape memory function. J. Mater.
Chem. A. 2018;6:8549.
227. Tan P, Chen B, Xu H, et al. Flexible Zn- and Li-air batteries:
Recent advances, challenges, and future perspectives. Energy
Environ. Sci. 2017;10:2056.
228. Zhu L, Zheng D, Wang Z, et al. A confinement strategy for stabi-
lizing ZIF-derived bifunctional catalysts as a benchmark cathode
of flexible all-solid-state zinc-air batteries. Adv. Mater. 2018;30:
1805268.
229. Su C-Y, Cheng H, Li W, et al. Atomic modulation of FeCo-nitrogen-
carbon bifunctional oxygen electrodes for rechargeable and flexible
all-solid-state zinc-air battery. Adv. Energy Mater. 2017;7:1602420.
230. Ji D, Fan L, Li L, et al. Hierarchical catalytic electrodes of
cobalt-embedded carbon nanotubeicarbon flakes arrays for flexi-
ble solid-state zinc-air batteries. Carbon. 2019;142:379.
231. Guan C, Sumboja A, Zang W, et al. Decorating Co/CoN
x
nano-
particles in nitrogen-doped carbon nanoarrays for flexible and re-
chargeable zinc-air batteries. Energy Storage Mater. 2019;16:243.
232. Guan C, Sumboja A, Wu H, et al. Hollow Co
3
O
4
nanosphere
embedded in carbon arrays for stable and flexible solid-state
zinc-air batteries. Adv. Mater. 2017;29:1704117.
233. Wang ZQ, Meng XY, Wu ZQ, Mitra S. Development of flexible
zinc-air battery with nanocomposite electrodes and a novel sepa-
rator. J. Energy Chem. 2017;26:129.
234. Hu L, Wu H, La Mantia F, Yang Y, Cui Y. Thin, flexible second-
ary Li-ion paper batteries. ACS Nano. 2010;4:5843.
235. Sun Q, Fang X, Weng W, et al. An aligned and laminated nano-
structured carbon hybrid cathode for high-performance lithium-
sulfur batteries. Angew. Chem. Int. Ed. 2015;54:10539.
236. Liu Q-C, Xu J-J, Xu D, Zhang X-B. Flexible lithium-oxygen
battery based on a recoverable cathode. Nat. Commun. 2015;6:
7892.
237. Wang H-G, Li W, Liu D-P, et al. Flexible electrodes for sodium-
ion batteries: Recent progress and perspectives. Adv. Mater.
2017;29:1704117.
How to cite this article: Liang Y, Zhao C-Z,
Yuan H, et al. A review of rechargeable batteries for
portable electronic devices. InfoMat. 2019;1:6–32.
https://doi.org/10.1002/inf2.12000
32 LIANG ET AL.
Available via license: CC BY
Content may be subject to copyright.