Content uploaded by Michelle L Steinauer
Author content
All content in this area was uploaded by Michelle L Steinauer on Feb 25, 2019
Content may be subject to copyright.
Published 15 February 2019
DOI: 10.1645/17-53
Contents and archives available through www.bioone.org or www.jstor.org
Journal of Parasitology
journal homepage: www.journalofparasitology.org
ACANTHOCEPHALAN PARASITES OF THE OARFISH, REGALECUS RUSSELII
(REGALECIDAE), WITH A DESCRIPTION OF A NEW SPECIES OF
GYMNORHADINORHYNCHUS (ACANTHOCEPHALA: GYMNORHADINORHYNCHIDAE)
Michelle L. Steinauer
1
, Ana E. Garcia-Vedrenne
2
, Sara B. Weinstein
2
, and Armand M. Kuris
2
1
Department of Basic Medical Sciences, COMP-NW, Western University of Health Sciences, Lebanon, Oregon 97355.
2
Department of Ecology, Evolution and Marine Biology, and Marine Science Institute, University of California, Santa Barbara, California 93106.
Correspondence should be sent to Michelle L. Steinauer at: msteinauer@westernu.edu
KEY WORDS ABSTRACT
Gymnorhadinorhynchidae
Gymnorhadinorhynchus mari-
serpentis
Bolbosoma
Small Subunit Ribosomal
DNA
Large Subunit Ribosomal
DNA
Sea of Japan
Phylogenetic Analysis
Diplosentis
Oarfish are rarely seen and seldom studied, which makes their parasite fauna even more enigmatic.
Necropsy of 12 oarfish, Regalecus russelii (Regalecidae) (Cuvier, 1816), from Japan yielded 2 species
of acanthocephalans. One species was found in 2 oarfish and a total of 76 specimens was collected,
but only a single, immature specimen of the second species was found. The former represents an
undescribed species from the order Echinorhynchida and is described here. Morphological and
phylogenetic analyses of the small subunit (SSU)rDNA place this species in the family
Gymnorhadinorhynchidae, and genus Gymnorhadinorhynchus which is characterized by a
cylindrical proboscis with longitudinal rows of hooks, basal circle of enlarged hooks, asymmetry
of hook shape, 4 cement glands, and a spineless trunk. Diagnostic characters of this species within
the genus are the number of longitudinal rows of hooks (14), smaller body size (males: 4.8–6.6 mm
and females: 5.3–6.3 mm) and a number of molecular autapomorphies including a number of long
insertions in both the SSU and large subunit rDNA (LSU). A single immature female of Bolbosoma
sp. (Palaeacanthocephala: Plagiorhynchidae) was also found with its anterior end embedded in the
mucosa of the pyloric ceca. The characters of this specimen are not consistent with any other known
species of Bolbosoma; however, because only 1 immature specimen with a partially invaginated
proboscis was recovered, it was not designated as a new species.
Oarfish (Regalecidae) are mesopelagic fishes that have an
elongate, lampriform body. Even though they are widely
distributed throughout the oceans, they are rarely sighted or
captured. Because of their unique body form and large size (.10
m), it is thought that rare sightings of washed up specimens have
spawned sea-serpent tales. Limited, infrequent access to speci-
mens has contributed to a paucity of data regarding their natural
history, including their helminth fauna. Previous necropsies of
oarfish have revealed immature Bolbosoma vasculosum (Rudolphi,
1819) Porta, 1908 (Monticelli, 1900), the adults of which are
typically considered parasites of marine mammals (Costa et al.,
2000), and an unidentifiable partial specimen that appeared to
belong to the family Arythmacanthidae (Yamaguti, 1935) Pichelin
and Cribb, 1999 (Kuris et al., 2015). The present study describes
the acanthocephalan fauna recovered from 12 necropsied oarfish,
Regalecus russelii (Regalecidae) that were collected off the coast
of Japan. Two species of Acanthocephala were recovered, 1
common and 1 rare. Both likely represent undescribed species;
however, adequate material for a formal description was only
obtained from 1 of them. Morphological features and phyloge-
netic analysis of DNA sequences indicated that the newly
designated species belongs to the genus Gymnorhadinorhynchus
(Braicovich et al., 2014) (Echinorhynchida: Gymnorhadinorhyn-
chidae).
MATERIALS AND METHODS
Collection and specimen preparation
Oarfish, R. russelii, were collected over the course of 4 yr by set
net from 3 general locations in the Sea of Japan: (1) Miyazu and
Kyotango in Kyoto Prefecture; (2) Wakamatsu Ward, Kita-
kyushu, Fukuoka; and (3) Oga Peninsula, Akita Prefecture (Table
I). The fish remained frozen or in formalin until necropsied at the
Maizuru Fisheries Research Station. Three of 12 oarfish
contained acanthocephalans. A 440-cm male (KMNH VP
100161) and a 298-cm female (KMNH VP 100160) were collected
in March 2014 from Wakamatsu Ward, Kitakyushu, Fukuoka
Prefecture (33858018.600 N, 130846011.7 00 Eand33856034.0 00 N,
Version of Record, first published online with fixed content and layout,
in compliance with ICZN Arts. 8.1.3.2, 8.5, and 21.8.2 as amended,
2012.
Journal of Parasitology 2019 105(1) 124–132
ÓAmerican Society of Parasitologists 2019
124
130840051.6 00 E), and were kept frozen until necropsy in June 2014.
A third, 282-cm female (FAKU 135806) was collected in January
2014 from Miyazu, Kyoto, Honshu Prefecture (35833000.900 N,
135815041.5 00 E) and preserved in formalin until necropsy in June
2014. The intestinal tracts, including the pyloric ceca, were
examined for parasites. For the male fish (KMNH VP 100161), a
subsample of the pyloric ceca was examined (94 g out of 186 g of
total tissue). Acanthocephalans were saved in either 10% formalin
or ethanol (70% or 95%).
Specimens for morphological analysis were stained with either
Semichon’s acetocarmine or Meyer’s carmalum. Specimens were
then dehydrated in an ethanol series, cleared with xylene, and
mounted in gum damar resin. Measurements were made with the
use of a Leica 1000DM compound microscope (Leica Micro-
systems, Buffalo Grove, Illinois) fitted with an Infinity1 camera
and Infinity Analyze 6.0.0 software (Lumenera Corporation,
Ontario, Canada).
DNA sequencing and phylogenetic analysis
Only the specimens of the new species of Gymnorhadinorhyn-
chus were sequenced, given that the only specimen of Bolbosoma
sp. was preserved in formalin. Specimens stored in ethanol were
washed in deionized water and lysed in a 5% chelex solution at
~99 C for 30 min. The lysate was used directly in a 10 lL PCR
reaction following manufacturer’s instructions (GoTaqt, Prom-
ega, Madison, Wisconsin). Part of the SSU rDNA (1,479 bp) was
amplified with the use of the following primers from Braicovich et
al. (2014): AGATTAAGCCATGCATG and CAAAGGGG
GACTTAATC. A total of 2,892 base pairs (bp) of the LSU
region was amplified and sequenced using the following 5 sets of
primers: (1) CAAGTACCGTGAGGGAAAGTT and CAGC
TATCCTGAGGGAAAC (~800 bp) (Garcia-Varela and Nadler,
2005), (2) GCACCCGCTGAAYTTAAG and CCAGCGC
CATCCATTTTCA (~1,600 bp) (Lockyer et al., 2003), (3)
ACCCGAAAGATGGTGAACTATG and CTTGGA
GACCTGTTGCGG (~1,100 bp) (Braicovich et al., 2014), (4)
CCTGAAAATGGATGGCGCT and GATGTACCGCCC
CAGTCAAACT (~1,000 bp) (Braicovich et al., 2014), and (5)
GGAAAGAAGACCCTGTTG and CCAGCCAGT
TATCCCTGT (~450 bp) (Braicovich et al., 2014). A 651-bp
region of the COI gene was amplified and sequenced with the
following universal primers from Folmer et al. (1994): GGTCAA
CAAATCATAAAGATATTGG and TAAACTTCAGGGT
GACCAAAAAATCA. The internal transcribed spacer regions
1 and 2 of the rDNA (ITS) and the interspaced 5.8s rDNA
sequence (541 bp) were amplified and sequenced with the
following primers: GTCGTAACAAGGTTTCCGTA and
TATGCTTAAATTCAGCGGGT (Luton et al., 1992; Steinauer
et al., 2007). The general thermocycling protocol for the PCR
amplifications above was as follows: initial denature at 94 C for 3
min, 35 cycles of 94 C for 1 min, 50 C annealing for 30 sec, 72 C
for 45 sec, and a final extension step at 72 C for 7 min. However,
the extension time was modified to 1 min for larger fragments.
The samples were visualized along with negative controls on a 1%
agarose gel and positive samples were cleaned up with the
E.Z.N.A. Cycle Pure kit (Omega Bio-tek, Norcross, Georgia) and
submitted to GenScript (Piscataway, New Jersey) for sequencing.
Initial Nucleotide BLASTtsearches of all sequences confirmed
our designation of the specimens into the order Echinorhynchida
and indicated that they were most similar to members of the
Gymnorhadinorhynchidae and Transvenidae. We used a phylo-
genetic analysis to determine the placement of our specimens
within the Echinorhynchida. We downloaded the available
alignment file from a recent phylogenetic analysis of the
Echinorhynchida, which included SSU rDNA sequences from
all the available specimens (24) of the Echinorhynchida and 3
members of the order Polymorphida as outgroup taxa, including
Corynosoma enhydri Morozov, 1940, Ibirhynchus dimorpha
Schmidt, 1973, Southwellina hispida Van Cleave, 1925 (Braicovich
et al., 2014). We aligned sequences of our specimens to this pre-
existing alignment with Aliview 1.17.1 (Larsson, 2014). We
executed a maximum likelihood analysis with the use of a GTR
þIþG evolutionary model as indicated by the AIC criterion of
jModeltest 2.1.7 (Darriba et al., 2012). We performed a heuristic
search with TBR branch swapping and 100 random-addition-
sequence replicates with the use of PAUP*4.0 b 10 (Swofford,
2003). Branch support was estimated with 100 bootstrap
Table I. Collection information, sex, total length, and intensity of acanthocephalan infection in 12 oarfish Regalecus russelii collected from the Sea of
Japan.
Fish no. Nearby city Coordinates Date Sex Total length A†
FAKU 132285 Miyazu 35835020.900 N 135814013.700 E 4 Jan 10 M .238 0
FAKU 135753 Miyazu 35835020.900 N 135814013.700 E 17 Dec 13 349 0
FAKU 135805 Miyazu 35833000.900 N 135815041.500 E 31 Jan 14 F 162 0
FAKU 135806 Miyazu 35833000.900 N 135815041.500 E 29 Jan 14 F 282 1‡
FAKU 135807 Miyazu 35835020.900 N 135814013.700 E 3 Feb 14 M 281 0
FAKU 135808 Miyazu 35835020.900 N 135814013.700 E 19 Jan 14 M 215 0
FAKU 135809 Miyazu 35835020.900 N 135814013.700 E 30 Jan 14 M 261 0
FAKU 135988 Kyotango 35839026.3 00 N 135818015.1 00 E 19 Dec 13 M 0
FAKU 135989 Kyotango 35839026.3 00 N 135818015.1 00 E 19 Dec 13 M 220 0
KMNH VP 100160 Wakamatsu 33856034.000 N 130840051.600 E 17 Mar 14 F 298 2
KMNH VP 100161 Wakamatsu 33858018.600 N 130846011.700 E 19 Mar 14 M 440 76§
N/A* Oga 39850051.200 N 139850046.800 E 28 Jan 14 F 305 0
* No catalog number. Obtained from Oga Aquarium.
† Abundance of acanthocephalan Gymnorhadinorhynchus mariserpentis unless otherwise noted.
‡Bolbosoma sp.
§ Only half of the cecae were examined; thus this number represents a subsample.
STEINAUER ET AL.—ACANTHOCEPHALA OF OARFISH 125
replicates with 100 random-addition-sequence replicates per
bootstrap replicate. We also performed a phylogenetic analysis
with LSU rDNA sequences; however, data were only available for
18 of the 27 specimens. Because the results of the LSU rDNA
analysis did not differ significantly in the placement of the new
species the results are not presented here.
RESULTS
Two species of acanthocephalans were found. A total of 76
specimens of the new species of Gymnorhadinorhynchus were
collected from the subsample of the male oarfish (KMNH VP
100161) and 2 were collected from the female oarfish (KMNH VP
100160). Specimens with their proboscides embedded in the
mucosa (indicating infection site) were found primarily in the
pyloric ceca, although 1 was found in the intestine. A single
specimen of Bolbosoma sp. was recovered from the pyloric ceca of
the second female oarfish (FAKU 135806), and the entire
specimen was found surrounded by host tissue.
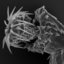
The specimen of Bolbosoma sp. was an immature female with
no developing ovarian balls and measured 5.5 mm in length (Fig.
1). The anterior bulb, which is characteristic of the genus
Bolbosoma, was less distinct than that of other members of the
genus. The bulb width was 0.86 mm and the body width below the
bulb was 0.65 mm. The proboscis was not fully evaginated and
was 335 lm wide and estimated to be 630 lm long. It contained 22
oppositely arranged longitudinal rows of hooks of an estimated
7–9 hooks per row (5–6 could be seen). The hooks that could be
seen gradually decreased in length yet increased in width from the
anterior to the posterior. The largest hook was 59 317 lm. The
roots were large, posteriorly directed, and sometimes extended
beyond the tip of the hook. The most basal hooks were unrooted
and spine-like and were greatly reduced in size, measuring 27 39
lm. The spines on the anterior portion of the trunk, or bulb,
appeared to be distributed asymmetrically. On the ventral side,
body spines extended from the neck to about 2/3 of the bulb. On
the dorsal side, spines were apparent only on the neck and were
not observed on the bulb. The proboscis receptacle was double
walled and measured 689 lm long by 268 lm wide. The combined
characters and morphometrics of the specimens of Gymnorhadi-
norhynchus were not consistent with any other described species of
acanthocephalan. The specimens matched the description for the
family Gymnorhadinorhynchidae Braicovich et al. (2004), includ-
ing: Elongate trunk lacking spines. Neck present. Proboscis
cylindrical with hooks arranged in longitudinal rows. Basal hooks
form a circle and are larger than those just anterior. Proboscis
receptacle double-walled; ganglion not observed. Lemnisci, 2,
variable in length. Testes 2. Cement glands 4, tubular.
The phylogenetic analysis also supported the placement of the
specimen into this family and genus. The SSU rDNA data set
included 28 taxa and 1,511 characters. Both the SSU and LSU
rDNA sequences contained several long insertions that were not
present in the other taxa. Maximum-likelihood analysis indicated
that the new species fell into a well-supported clade (100%
bootstrap support), which includes 2 species belonging to the
family Transvenidae along with Gymnorhadinorhynchus decapteri
and Rhadinorhynchus sp. (Fig. 2). If our taxonomic placement is
correct, a clade with 85% bootstrap represents the genus
Gymnorhadinorhynchus. However, this clade includes a specimen
identified as Rhadinorhynchus sp. (accession number AY062433.1)
from fishes of the family Sciaenidae from the Pacific Ocean North
of Mexico (Garcia-Varela et al., 2002; and Garcia-Varela, pers.
comm.). This specimen of Rhadinorhynchus sp. was probably
misidentified because it is not included in a monophyletic group
with other species of Rhadinorhynchus in the SSU rDNA tree
(Braicovich et al., 2014). This assertion is plausible because the
proboscis morphology of Rhadinorhynchus is similar to that of
Gymnorhadinorhynchus, and at the time the Rhadinorhynchus
specimen was identified, Gymnorhadinorhynchus had not yet been
named. If our hypothesis regarding the taxonomy of this group is
correct, the putative Rhadinorhynchus specimen would actually be
a new species of Gymnorhadinorhynchus.
DESCRIPTION
Measurements are given in micrometers unless otherwise stated.
The range of each character is given and is followed by the mean.
Gymnorhadinorhynchus mariserpentis n. sp.
(Figs. 3–5)
General: Body long, cylindrical, without spines. Proboscis long,
cylindrical, curved, with hooks arranged in longitudinal rows,
Figure 1. Bolbosoma sp. recovered from the pyloric ceca of Regalecus
russelii.(A) Whole specimen. Note the small size of the anterior bulb. (B)
Proboscis. Note the small hooks at the base of the proboscis.
126 THE JOURNAL OF PARASITOLOGY, VOL. 105, NO. 1, FEBRUARY 2019
typically 14 (1 specimen had 13). Hooks on opposite sides of
proboscis shaped differently so that those on inner curve of
proboscis (ventral) are themselves more curved and thicker than
hooks on outer curve of proboscis (dorsal). Hooks in longitudinal
rows alternating except basal hooks, which are enlarged and
positioned in a circle around base of proboscis. Hooks within a
longitudinal row of similar size (except basal circle of hooks), with
largest hooks located in anterior third of proboscis. Hooks
gradually decrease in size anteriorly and posteriorly from this
point with an increase in size of basal hook, which is of similar
size to longest hooks on proboscis or are longest hooks. Body wall
contains numerous nuclei.
Males (based on 11 specimens): Trunk 4.81–6.58 mm (5.30)
long, 0.40–0.73 mm (0.69) wide at widest point, usually between
anterior one-third and one-half of trunk. Proboscis 1.25–1.43 mm
(1.34) long by 193–240 (217) wide at maximum width. Proboscis
armed with 14 longitudinal rows of 24–29 (26.7) hooks. Basal
hook on inner curve of proboscis 40–49 (45) long, 8–12 (10) wide.
Longest hooks that were not basal hooks 43–52 (47) long, 11–15
(13) wide. Neck 213–285 (236) long by 222–274 (245) wide at
junction with trunk. Proboscis receptacle 1,120–2,080 (1,582)
long, 169–258 (221) wide at widest point. Lemnisci long, slender,
typically extending just past proboscis receptacle, but sometimes
shorter. Testes ovoid and contiguous, anterior testis 321–486
(384) long, 199–333 (271) wide; posterior testis 300–461 (355)
Figure 2. Maximum likelihood phylogenetic tree of 28 acanthocephalan species including Gymnorhadinorhynchus mariserpentis n. sp. Analysis is
based on SSU ribosomal DNA. Bootstrap support values are indicated at nodes. Results indicate that the new species is closely related to the type species
of Gymnorhadinorhynchus and an unidentified species that was putatively misidentified as a species of Rhadinorhynchus.
STEINAUER ET AL.—ACANTHOCEPHALA OF OARFISH 127
long, 202–360 (287) wide. Reproductive organs fill 50.7–53.2%
(52.8) of trunk. Four tubular cement glands arranged in pairs with
2 immediately posterior to testes and 2 positioned further
posteriorly; posterior pair difficult to detect in some specimens.
S¨
afftigen’s pouch long and sinuous, anteriorly extending nearly to
the testes, with the cement glands arranged around the pouch,
991–1,140 (1,081) long, 141–230 (186) wide.
Females (based on 8 gravid specimens): Trunk 5.25–6.30 (5.72)
mm long, 5.6–8.2 (5.7) mm wide at widest point, usually between
midpoint and anterior one-third of trunk. Proboscis 1,360–1,710
(1,560) long, 173–218 (193) wide at widest point, usually at base of
proboscis. Proboscis armed with 14 rows (rarely 13) of 26–28 (27)
hooks. Longest hooks (that were not the basal hooks) 52–60 (56)
long, 12–17 (15) wide, usually occur at anterior one-third of
proboscis. Basal hooks 51–63 (55) by 11–17 (13) wide. Neck 246–
294 (265) long, 236–301 (276) wide at junction with trunk.
Proboscis receptacle 1,330–2,070 (1,723) long, 159–272 (223) wide
at widest point. Eggs with prominent polar prolongations, 40–52
(44) long, 5–12 (8) wide. Genital pore terminal.
Taxonomic summary
Host: Regalecus russelii (Cuvier, 1816).
Site of infection: Gastrointestinal tract, primarily the pyloric
ceca.
Type locality: Hibiki-nada Sea near Wakamatsu Ward,
Kitakyushu, Fukuoka Prefecture, Kyushu Island, Japan
(33858018.6 00 N, 130846011.7 00 E).
Specimens deposited: Smithsonian National Museum of Natu-
ral History Invertebrate Zoology Collection. Holotype: 1480200,
allotype: 1480201, and paratypes: 1480202–1480207. GenBank
accession numbers: MK014834, MK014866, MK014867,
MK012665, MK012666, MK012667.
ZooBank registration: urn:lsid:zoobank.org:ast:8907476-52FC-
4608-8145-B2CD6A023C64
Etymology: The specific epithet, ‘‘mariserpentis,’’ is combina-
tion of the Latin words for sea, ‘‘mare,’’ and serpent, ‘‘serpentis,’’
in the genitive form. Thus, its meaning is ‘‘the sea serpent’s
Gymnorhadinorhynchus.’’
Remarks
This species most closely resembles G. decapteri Braicovich et
al. (2014), the type and only species currently recognized in this
genus. It can be distinguished from G. decapteri, a parasite of the
round scad, Decapterus punctatus (Carangidae), by the number of
longitudinal rows of hooks (14 vs. 10) and smaller body size. This
species also resembles members of a genus referred to as
Neorhadinorhynchus by Amin (2013) or Diplosentis,which
includes the synonymized genera Neorhadinorhynchus and Scle-
rocollum (Pichelin and Cribb, 2001). As defined by Pichelin and
Cribb (2001), members of Diplosentis have hooks of similar shape
on the proboscis, which is inconsistent with G. mariserpentis.
However, it should be noted that the original description, and
redescription of Diplosentis nudus Hassanine, 2003 (described as
Rhadinorhynchus nudus Harada, 1938) describe this species as
having different or ‘‘slightly different’’ hooks on the dorsal and
ventral sides of the proboscis. Gymnorhadinorhynchus mariser-
pentis differs from the original description and redescription of
this species in its enlarged basal circle of hooks on the proboscis,
and the length and extent of S ¨
afftigen’s pouch, which is much
longer and extends anteriorly to the first pair of cement glands.
DISCUSSION
The family Gymnorhadinorhynchidae was recently erected with
the description of G. decapteri in 2014. The justification for this
new family is that the combination of characters including an
enlarged basal circle of hooks, dorsal-ventral hook shape
asymmetry, spineless trunk, and 4 tubular cement glands is not
accommodated by any other existing family as presently
described. We agree with this assessment, but note the striking
similarity of D. nudus (Harada, 1938) Hassanine 2003 (Acantho-
cephala: Cavisomidae). The taxonomy of this species has a
complex history. It was originally described as R. nudus from
Trachurus japonicus (Japanese horse mackerel, Carangidae) from
Keelung, Taiwan. Rhadinorhynchus nudus was moved to the
subgenus Neorhadinorhynchus (Yamaguti, 1939), which was later
erected to full genus status (Yamaguti, 1963), and later
synonymized with Diplosentis (Pichelin and Cribb, 2001). The
specimens from oarfish do not fit the generic description of
Figure 3. Proboscis of Gymnorhadinorhynchus mariserpentis n. sp.
Note that the basal hooks are arranged in a circle and that they are
somewhat larger than the hooks located just anterior.
128 THE JOURNAL OF PARASITOLOGY, VOL. 105, NO. 1, FEBRUARY 2019
Figure 4. Gymnorhadinorhynchus mariserpentis n. sp. (A) Proboscis and proboscis receptacle. (B) Female reproductive tract; ub, uterine bell, sa,
sorting apparatus; ut, uterus. (C) Male reproductive tract; t, testis; cg, cement glands; Sp, S ¨
afftigen’s pouch; cb, copulatory bursa. (D) Male reproductive
system; p, penis; cb, copulatory bursa.
STEINAUER ET AL.—ACANTHOCEPHALA OF OARFISH 129
Figure 5. Proboscis of Gymnorhadinorhynchus mariserpentis n. sp. (A) Hooks and hook roots of the proboscis; r, retractor muscle. (B) Arrow
indicates enlarged basal hook of the proboscis; r, retractor muscle. (C) Internal base of proboscis; pr, proboscis receptacle; r, retractor muscles. (D)
Arrow indicates sensory pit.
130 THE JOURNAL OF PARASITOLOGY, VOL. 105, NO. 1, FEBRUARY 2019
Diplosentis (sensu Pichelin and Cribb, 2001) because of dorsal-
ventral hook asymmetry present in the specimens from oarfish.
The original description of D. nudus (as R. nudus) only included
immature females (no eggs) and it is unclear how many specimens
were examined and measured. Nevertheless, the specimens differ
from G. mariserpentis in that the basal circle of hooks was not
described as enlarged and a detailed proboscis drawing was not
included. Diplosentis nudus (Neorhadinorhynchus nudus in Amin
[2013]) was redescribed in 2006 from Rastrelliger kanagurta
(Indian mackerel, Scombridae) from the Red Sea near Egypt
(Hassanine, 2006). Although these specimens appear quite similar
in morphology to the original description, their body size and
many characteristics are much larger. Body size also clearly
separates G. mariserpentis from the redescribed D. nudus. Males of
D. nudus range from 8.6 to 12.7 mm and females from 12.3 to 18.4
mm, whereas males of G. mariserpentis range from 4.8 to 6.6 mm
and females from 5.3 to 6.3 mm. Furthermore, the basal circle of
hooks of the redescribed D. nudus is not enlarged. Thus, they are
clearly different species. Amin and Nahhas (1994) reported on a
collection of D. nudus (as Neorhadinorhynchus nudum) from
Euthynnus affinis (Cantor, 1849) (mackerel tuna, Scombridae) off
the Fiji Islands. Measurements of a single male and single
immature female were given and the authors deemed the
morphology ‘‘practically identical’’ to the original description;
however, it was noted that the specimens from Fiji contained an
enlarged basal circle of hooks. Therefore, the specimens reported
by Amin and Nahhas appear to belong to the family Gymno-
rhadinorhynchidae. Unfortunately, no specimens were deposited
in a museum, so they are not available for further examination.
An additional recent morphological study of D. nudus (reported
as Neorhadinorhynchus nudus) from Auxis thazard (frigate tuna)
from the South China Sea, reports additional morphological
variation in the species, but G. mariserpentis still differs from these
specimens by the enlarged basal circle of hooks and the extent of
S¨
afftigen’s pouch (Li et al., 2018).
The phylogenetic analysis indicated that the Gymnorhadino-
rhynchidae are most closely related to members of the Trans-
venidae (Pararhadinorhynchus sp. and Transvena annulospinosa);
however, taxon sampling is sparse. Additional sequencing of
members of the Echinorhynchida is required given the disagree-
ment about phylogenetic relationships of these families (Pichelin
and Cribb, 2001; Amin, 2013).
Prevalence of G. mariserpentis was 17% (2 out of 12), with the
worms highly aggregated in 1 host fish, a finding even more
notable because only half of the pyloric ceca were examined in the
heavily infected oarfish. Oarfish become infected by ingesting an
intermediate host, which is likely to be krill (Euphasiacea)
(Roberts, 2012). Euphausiids such as krill comprise the primary
diet of oarfish, and are typical intermediate hosts for echino-
rhynchid acanthocephalans (Schmidt, 1985), The degree of
aggregation found in this study is surprising, given the observed
low densities and prevalences of acanthocephalan cystacanths in
euphausiids (e.g., Shimazu, 1975; Go
´mez-Guti ´
errez et al., 2010);
however, this might be explained by seasonal transmission. Both
infected oarfish were collected in March, and the uninfected
individuals were collected December through February of varying
years. Also, the female worms were in a similar state of
development with their eggs just maturing. Other Acanthoceph-
ala, including D. nudus in the Red Sea, have a strong seasonal
cycle in which adult worms are absent from the definitive host for
specific parts of the year (Hassanine, 2006). An alternative
explanation for aggregation is that the life cycle incorporates a
paratenic host that accumulates juvenile worms and transmits
them together as a package to the oarfish host, but this seems less
likely because oarfish feed almost exclusively on krill (Roberts,
2012), as confirmed by recovering only krill in the guts of the
oarfish we examined. Thus, the extent of aggregation may have
more to do with the timing of sampling of such rare specimens.
The recovered specimen of Bolbosoma was immature and was
surrounded by host tissue, suggesting that it was not likely to
develop into an adult within the oarfish. Species of Bolbosoma are
typically parasites of marine mammals, and no species are known
to develop into adults within fishes. It is possible that oarfish serve
as a paratenic hosts. Oarfish might be preyed upon by sperm
whales and beaked whales, which are potential definitive hosts
(e.g., Hoberg et al., 1993; Cappozzo et al., 2005). There has been 1
previous report of Bolbosoma from an oarfish, B. vasculosum
(Rudolphi, 1819) Porta, 1908 (Monticelli, 1900; Kuris et al.,
2015), however, it was also immature, supporting the hypothesis
that the oarfish is not an appropriate definitive host for this group
of acanthocephalans and instead a potential paratenic host. The
specimen of Bolbosoma described here does not resemble B.
vasculosum (Rudolphi, 1819) Porta, 1908. Our specimen clearly
differs in hook morphology. The hooks of B. vasculosum
gradually decrease in size toward the basal hooks, and diminutive
spine-like hooks are not present on the proboscis (Petrochenko,
1956). Furthermore, the trunk spines of B. vasculosum are not
continuous. A large area without spines forms a band separating
the spined regions (Petrochenko, 1956). The specimen of
Bolbosoma reported here most closely resembles Bolbosoma
turbinella (Diesing, 1851) Porta 1908, the type species of the
genus. However, all of the characters on our specimens are much
smaller than those reported elsewhere (Measures, 1992) including
the hooks, which are thought to be less variable in size than soft
tissue characteristics. The basal hooks of B. turbinella are greatly
reduced in size (Measures, 1992). This character was not seen in
the specimen of Bolbosoma recovered from these oarfish.
Furthermore, electron micrographs of B. turbinella show hooks
on the dorsal portion of the bulb, which were not observable on
our specimen (Measures, 1992). However, body spines are
notoriously difficult to interpret with a light microscope.
ACKNOWLEDGMENTS
We thank Tyson Roberts for arranging and coordinating the
work in Japan and sharing his knowledge of oarfish biology, and
Dr. Yoshiaki Yabumoto of Kitakyushu Museum of Natural
History for access to the fish for dissection. We also thank Dr.
Yoshiaki Kai and the Maizuru Fisheries Research Station of the
University of Kyoto for gathering together most of the oarfish
material, and Dr. Yuko Tagekawa of Kagawa University for
facilitating the project. We also thank Anindo Choudhury for
generation of Figures 3–5. This article benefited from support
from a National Science Foundation Ecology of Infectious
Diseases grant (OCE-1115965).
LITERATURE CITED
AMIN, O. M. 2013. Classification of the Acanthocephala. Folia
Parasitologica 60: 273–305.
STEINAUER ET AL.—ACANTHOCEPHALA OF OARFISH 131
AMIN, O. M., AND F. M. NAHHAS. 1994. Acanthocephala of
marine fishes off Fiji Islands, with descriptions of Filisoma
longcementglandatus n. sp., and gravid females of Rhadino-
rhynchus johnstoni (Rhadinorhynchidae); and keys to species
of the genera Filisoma and Neorhadinorhynchus. Journal of
Parasitology 80: 768–774.
BRAICOVICH, P. E., A. L. LANFRANCHI,M.D.FARBER,A.E.
MARVALDI,J.L.LUQUE,AND J. T. TIMI. 2014. Genetic and
morphological evidence reveals the existence of a new family,
genus and species of Echinorhynchida (Acanthocephala).
Folia Parasitologica 61: 377–384.
CAPPOZZO, H. L., M. F. NEGRI,B.MAHLER,V.V.LIA,P.
MARTINEZ,A.GIANGGIOBE,AND A. SAUBIDET. 2005. Biological
data on two Hector’s beaked whales, Mesoplodon hectori,
stranded in Buenos Aires province, Argentina. Latin Amer-
ican Journal of Aquatic Mammals 4: 113–128.
COSTA, G., J. C. CHUBB,AND C. J. VELTKAMP. 2000. Cystacanths of
Bolbosoma vasculosum in the black scabbard fish Aphanopus
carbo, oceanic horse mackerel Trachurus picturatus and
common dolphin Delphinus delphis from Madeira, Portugal.
Journal of Helminthology 74: 113–120.
DARRIBA, D., G. L. TABOADA,R.DOALLO,AND D. POSADA. 2012.
jModelTest 2: more models, new heuristics and parallel
computing. Nature Methods 9: 772–772.
FOLMER, O., M. BLACK,W.HOEH,R.LUTZ,AND R. VRIJENHOEK.
1994. DNA primers for amplification of mitochondrial
cytochrome C oxidase subunit I from diverse metazoan
invertebrates. Molecular Marine Biology and Biotechnology
3: 294–299.
GARCIA-VARELA, M., M. P. CUMMINGS,G.P.P.DE LEON,S.L.
GARDNER,AND J. P. LACLETTE. 2002. Phylogenetic analysis
based on 18S ribosomal RNA gene sequences supports the
existence of class polyacanthocephala (acanthocephala).
Molecular Phylogenetics and Evolution 23: 288–292.
GARCIA-VARELA, M., AND S. A. NADLER. 2005. Phylogenetic
relationships of Palaeacanthocephala (Acanthocephala) in-
ferred from SSU and LSU rDNA gene sequences. Journal of
Parasitology 91: 1401–1409.
GO
´MEZ-GUTI ´
ERREZ, J., C. J. ROBINSON,S.KAWAGUCHI,AND S.
NICOL. 2010. Parasite diversity of Nyctiphanes simplex and
Nematoscelis difficilis (Crustacea: Euphausiacea) along the
northwestern coast of Mexico. Diseases of Aquatic Organ-
isms 88: 249–266.
HASSANINE, R. M. E. S. 2006. Acanthocephalans from Red Sea
fishes. Family Cavisomidae Meyer, 1932: The seasonal cycle
of Diplosentis nudus (Harada, 1938) Pichelin et Cribb, 2001 in
a definitive fish host, and a comment on Sclerocollum Schmidt
et Paperna, 1978. Acta Parasitologica 51: 123–129.
HOBERG, E. P., P. Y. DAOUST,AND S. MCBURNEY. 1993. Bolbosoma
capitatum and Bolbosoma sp. (Acanthocephala) from sperm
whales (Physeter macrocephalus) stranded on Prince Edward
Island, Canada. Journal of the Helminthological Society of
Washington 60: 205–210.
KURIS, A. M., A. G. JARAMILLO,J.P.MCLAUGHLIN,S.B.
WEINSTEIN,A.E.GARCIA-VEDRENNE,G.O.POINAR,M.
PICKERING,M.L.STEINAUER,M.ESPINOZA,J.E.ASHFORD,
AND G. L. P. DUNN. 2015. Monsters of the sea serpent:
Parasites of an oarfish, Regalecus russellii. Journal of
Parasitology 101: 41–44.
LARSSON, A. 2014. AliView: a fast and lightweight alignment
viewer and editor for large datasets. Bioinformatics 30: 3276–
3278.
LI, L., H. X. CHEN,AND Y. YANG. 2018. Morphological and
molecular study of Neorhadinorhynchus nudus (Harada, 1938)
(Acanthocephala: Cavisomidae) from Auxis thazard Lacepede
(Perciformes: Scombridae) in the South China Sea. Acta
Parasitologica 63: 479–485.
LOCKYER, A. E., P. D. OLSON,P.OSTERGAARD,D.ROLLINSON,D.
A. JOHNSTON,S.W.ATTWOOD,V.R.SOUTHGATE,P.HORAK,S.
D. SNYDER,T.H.LE,T.AGATSUMA,D.P.MCMANUS,A.C.
CARMICHAEL,S.NAEM,AND D. T. J. LITTLEWOOD. 2003. The
phylogeny of the Schistosomatidae based on three genes with
emphasis on the interrelationships of Schistosoma Weinland,
1858. Parasitology 126: 203–224.
LUTON, K., D. H. WALKER,AND D. BLAIR. 1992. Comparisons of
ribosomal internal transcribed spacers from two congeneric
species of flukes (Platyhelminthes: Trematoda: Digenea).
Molecular and Biochemical Parasitology 56: 323–327.
MEASURES, L. N. 1992. Bolbosoma turbinella (Acanthocephala) in
a Blue whale, Balaenoptera musculus, stranded in the St-
Lawrence Estuary, Quebec. Journal of the Helminthological
Society of Washington 59: 206–211.
MONTICELLI, F. S. 1900. Sui parasitti del Regalecus glesne.
Monitore Zoologico Italiano, Firenze 11: 36–37.
PETROCHENKO, V. I. 1956. Acanthocephala of domestic and wild
animals. Israel Program for Scientific Translations, Ltd.,
Jerusalem, 465 p.
PICHELIN,S.,AND T. H. CRIBB. 2001. The status of the
Diplosentidae (Acanthocephala: Palaeacanthocephala) and
a new family of acanthocephalans from Australian wrasses
(Pisces: Labridae). Folia Parasitologica 48: 289–303.
ROBERTS, T. R. 2012. Systematics, biology, and distribution of the
species of the oceanic oarfish genus Regalecus (Teleostei,
Lampridiformes, Regalecidae). M ´
emoires du Mus ´
eum Na-
tional d’Histoire Naturelle 202: 1–268.
SCHMIDT, G. D. (1985). Development and life cycles. In Biology of
the Acanthocephala. D. W. T. Crompton and B. B. Nickol
(eds.). Cambridge University Press, Cambridge, U.K., pp.
273–305.
SHIMAZU, T. 1975. Some cestode and acanthocephalan larvae
from euphausiid crustaceans collected in the northern North
Pacific Ocean. Bulletin of the Japanese Society of Scientific
Fisheries 41: 813–821.
STEINAUER, M. L., B. B. NICKOL,AND G. ORTI. 2007. Cryptic
speciation and patterns of phenotypic variation of a highly
variable acanthocephalan parasite. Molecular Ecology 16:
4097–4109.
SWOFFORD, D. L. (2003). PAUP*: Phylogenetic analysis using
parsimony (*and other methods), version 4.0b10. Sinauer
Associates, Inc., Sunderland, Massachusetts.
YAMAGUTI, S. 1939. Studies on the helminth fauna of Japan. Part
29. Acanthocephala, II. Japanese Journal of Parasitology 8:
317–351.
YAMAGUTI, S. 1963. Systema Helminthum. Volume V: Acantho-
cephala. Interscience Publishers, New York, New York, 423
p.
132 THE JOURNAL OF PARASITOLOGY, VOL. 105, NO. 1, FEBRUARY 2019