Content uploaded by Darío Javier Cruz
Author content
All content in this area was uploaded by Darío Javier Cruz on Oct 17, 2017
Content may be subject to copyright.
Chapter 12
Biogeography and Ecology of Tulasnellaceae
Franz Oberwinkler, Darı
´o Cruz, and Juan Pablo Sua
´rez
12.1 Introduction
Schr€
oter (1888) introduced the name Tulasnella in honour of the French physicians,
botanists and mycologists Charles and Louis Rene
´Tulasne for
heterobasidiomycetous fungi with unique meiosporangial morphology. The place-
ment in the Heterobasidiomycetes was accepted by Rogers (1933), and later also by
Donk (1972). In Talbot’s conspectus of basidiomycetes genera (Talbot 1973), the
genus represented an order, the Tulasnellales, in the Holobasidiomycetidae, a view
not accepted by Bandoni and Oberwinkler (1982). In molecular phylogenetic
studies, Tulasnellaceae were included in Cantharellales (Hibbett and Thorn
2001), a position that was confirmed by following studies, e.g. Hibbett et al.
(2007, 2014).
12.2 Systematics and Taxonomy
Most tulasnelloid fungi produce basidiomata on wood, predominantly on the
underside of fallen logs and twigs. Reports on these collections are mostly
published in local floras, mycofloristic listings, or partial monographic treatments.
F. Oberwinkler (*)
Institut für Evolution und O
¨kologie, Universita
¨tTübingen, Auf der Morgenstelle 1, 72076
Tübingen, Germany
e-mail: franz.oberwinkler@uni-tuebingen.de
D. Cruz • J.P. Sua
´rez
Museum of Biological Collections, Section of Basic and Applied Biology, Department of
Natural Sciences, Universidad Te
´cnica Particular de Loja, San Cayetano Alto s/n C.P, 11
01 608 Loja, Ecuador
©Springer International Publishing AG 2017
L. Tedersoo (ed.), Biogeography of Mycorrhizal Symbiosis, Ecological Studies 230,
DOI 10.1007/978-3-319-56363-3_12
237
Cite: Oberwinkler F, Cruz D, Suárez JP. 2017. Biogeography and
ecology of Tulasnellaceae. Ecol. Stud. 230: 237-271.
Unfortunately, the ecological relevance of Tulasnella fruiting on variously decayed
wood or on bark of trees is not understood. It would appear plausible to assume that
Tulasnella species are involved in wood decay, and that they may function in
anamorphic stages as mycobionts in close by habitats. Therefore it seemed imper-
ative to include in this overview of tulasnelloid mycobionts also reports on
basidiomata.
Though some well developed Tulasnella species can be recognized in the field
by the experienced mycologist with some certainty, correct identification of the
genus was only possible microscopically in pre-molecular times. Most tulasnelloid
fungi were sampled by collectors interested in corticiaceous fungi, Reports on these
collections are mostly published in local floras, mycofloristic listings, or partial
monographic treatments. Some of these publications are used to document biogeo-
graphical patterns on continental scales (Table 12.1). Because of considerable
taxonomic difficulties and inaccuracies in traditional microscopic identification of
Tulasnella morphospecies, they cannot be used for an attempt to disentangle their
distribution areas. However, molecular data may help to overcome this bottleneck.
In several Tulasnella species the hymenial surface has a rosy to faintly viola-
ceous tint (Fig. 12.1). Basidiomata consist of a few basal hyphae with or without
clamps. Normally a simple but rarely considerably thickened hymenium is devel-
oped. Subhymenial structures may be lacking, and consequently single generative
hyphae produce meiosporangia. Such growth forms or developmental stages cannot
be detected in the field. These are only detected microscopically by chance,
growing on the surface of other fungi, especially their hymenia. The growth can
be intrahymenial, e.g. in T. inclusa (Gloeotulasnella i., Christiansen 1959), or,
rather exotically, parasitising on amoebae (T. zooctonica, Drechsler 1969).
The anamorphic stage of Tulasnella has been named Epulorhiza (Moore 1987), and
it has been often used in mycorrhiza studies. Since the concept “One fungus ¼one
name” was implemented at the International Botanical Congress XVIII, Melbourne,
July 2011 (McNeill and Turland 2011; McNeil et al. 2012), the name Epulorhiza
became synonymous. Nevertheless, articles dealing with Epulorhiza are included in our
review, even when it appears uncertain in several cases, whether or not Tulasnella is
involved. For the reason of taxonomic clarity in the following text, a short comment on
the Ceratobasidium-Rhizoctonia complex is included here. In various treatments, the
formal taxonomy of the so-called “form genus Rhizoctonia” has been dealt with
(e.g. Gonza
´lez Garcia et al. 2006; Yang and Li 2012). As pointed out by Oberwinkler
et al. (2013), the name Ceratobasidium can only be applied for Ceratobasidium
calosporum and the genera Koleroga,Oncobasidium,Uthatobasidium,and
Ypsilonidium have to be put under synonymy of Rhizoctonia. The latter one has priority
over Thanatephorus. Unfortunately, these taxonomic re-arrangements were widely
ignored in a recent paper by Go
´nzalez et al. (2016).
Micromorphological characteristics of Tulasnella species include unique basidia
with strongly swollen sterigmata (Fig. 12.1), also called epibasidia, which is a
misleading term. After meiosis in the basidium, haploid nuclei and the basidial
cytoplasm migrate through the sterigmata into the terminally developing basidio-
spores. In the basal position, the sterigmata become secondarily septate. Apically
238 F. Oberwinkler et al.
Table 12.1 Compilation of perfect stages of Tulasnellaceaespecies, arranged according to Fig. 12.2
Regions Europe Asia Af America Pac Aus
Subdivisions N W C E S te tr N C S
Species Spores ●
T. eichleriana Globose–elliptical ●● ●●●● ● ●
T. violea ●● ● ● ●
T. zooctonia ●●
T. cystidiophora ●● ●
T. pacifica ●
T. bourdotii ●●
T. subglobispora ●●
T. hyalina ●●
Pseudotulasnella
guatemalensis
●
T. guttulata
T. traumatica ●●
T. conidiata ●●
T. valentini Oblong–elliptical ●
Stilbotulasnella conidiophora ●
T. albida ●●●●●
T. pinicola ●● ●
T. thelephorea ●● ●●●
T. asymmetrica ●
T. pruinosa ●● ● ●
T. dissitispora Phaseoli-form-subcylin-
drical
●
T. tomaculum ●● ●●● ●●
T. andina ●
T. irregularis ●
T. fuscoviolacea ●●●
T. rubropallens ●● ● ●
T. griseorubella ●●
T. bifrons ●●
T. robusta ●
T. cruciata ●●
T. kirschneri ●
T. pallidocremea ●
T. balearica Sigmoid ●
T. deliquescens ●●
T. quasiflorens ●
T. curvispora Allantoid ●
T. permacra ●
T. allantospora ●● ● ● ● ●
T. danica ●● ●
T. saveloides ●● ●
T. aggregata ●
T. anguifera Spiral ●
T. interrogans ●●
T. falcifera ●
T. helicospora ●● ●
T. calospora Fusiform-subfusi-form ●●●● ●● ● ●
T. eremophila ●
T. kongoensis ●
T. brinkmannii ●
T. pallida ●● ●●●
T. echinospora ●● ●
●records arranged geographically. Ccentral, Eeast, Nnorth, Ssouth, te temperate, tr tropical, Wwest. Literature: Europe: Bresadola
(1903), Bourdot and Galzin (1927), Pearson (1928), Strid (1975), Torkelsen (1977), Hjortstam (1978), Wojewoda (1978, 1983, 1986),
Hauerslev (1989), Roberts (1992, 1993a, b, 1994a, b, 1996, 1999, 2003), Due~
nas (1996, 2001, 2005), Van de Put and Antonissen (1996),
Roberts and Pia˛tek (2004), Ordynets (2012), Kunttu et al. (2015), Polemis et al. (2016). Asia: Do
gan and Kurt (2016). Africa: Crous et al.
(2015). North America: Rogers (1933), Olive (1946). Central America: Roberts (2006). South America: Martin (1939), Lopez (1987),
Greslebin and Rajchenberg (2001), Cruz et al. (2011, 2014, 2016), Nouhra et al. (2013). Pacific area: Olive (1957), Bandoni and
Oberwinkler (1982). Australia: Warcup and Talbot (1967, 1971, 1980). Orig
12 Biogeography and Ecology of Tulasnellaceae 239
partly septate basidia have been reported for Pseudotulasnella guatemalensis
(Lowy 1964). Basidiospores germinate by hyphae or secondary ballistospores.
Dolipores with continuous parenthesomes are a constant ultrastructural feature in
Tulasnella (Fig. 12.1). However, parenthesomes could not be found in dolipores of
Stilbotulasnella conidiophora (Bandoni and Oberwinkler 1982). Other apparently
unique ultrastructural features include cell wall expansions filled with amorphous
matrix (Fig. 12.1). It is unknown whether this character is representative in all or
most of Tulasnella species. Morphological and ultrastructural characters were
indicative of a separate systematic position in former heterobasidiomycetous
fungi, but precise phylogenetic position of Tulasnella within Basidiomycota
remained unsettled.
There is a set of micromorphological characters in Tulasnella species, which
appear to be applicable for circumscribing taxa. However, even in the case of very
accurate microscopic work, there remains much uncertainty about the variability of
structural features. This explains at least partly why reliable species identification is
difficult and quite often questionable. This situation became strikingly evident,
when molecular analyses showed that morphospecies were often not verifiable or
included cryptic taxa (Taylor and McCormick 2008; Cruz et al. 2014). Whether the
finding of Linde et al. (2013) in Australian orchid mycorrhizae, that an eight-locus
analysis is broadly congruent with the solely ITS based result, can be generalized,
remains questionable. For taxonomic details and nomenclature of Tulasnella
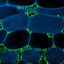
Fig. 12.1 Tulasnella violea (a,d) and Tulasnella spp. (b,c): (a) hymenial surface, bar 5 mm; (b)
dolipore with continuous parenthesomes, bar 0.1 μm; (c) spirally growing hypha with cell wall
extensions (arrows), bar 2 μm; (d) section through basidiome with basidia and basidiospores, one
forming a secondary spore, bar 5 μm. From Oberwinkler (2012)
240 F. Oberwinkler et al.
species we refer to Cruz et al. (2014, 2016). Table 12.1 provides an overview about
the basic morphological features and distribution of Tulasnellaceae morphospecies.
12.3 Phylogenetic Position of Tulasnella
A sequence database for the identification of ectomycorrhizal basidiomycetes
included also Tulasnella (Bruns et al. 1998). Tulasnelloid orchid associates clus-
tered with good support within the cantharelloid clade. In an attempt to identify
single pelotons of Dactylorhiza majalis using single-strand conformation polymor-
phism and mitochondrial ribosomal large subunit DNA sequences, Kristiansen
et al. (2001) found two taxa, Tulasnella, and a second one, distantly related to
Laccaria. As sister of the Tulasnella cluster, Sebacina sp. was found, and both
together appeared in a neighbour position to cantharelloid fungi. An expanded
taxon set of basidiomycetes was used by Bidartondo et al. (2003) to resolve the
phylogenetic placement of Aneura (Cryptothallus) associated fungi (see Sect.
12.5.1). They were phylogenetically well supported with T. asymmetrica as a sister
taxon and T. obscura and T. calospora in the same clade. Similar results were
obtained by Kottke et al. (2003), focusing on the mycobiont of Aneura pinguis, and
Weiß et al. (2004) in an approach covering most of heterobasidiomycetous genera
sequenced at that time. Resupinate homobasidiomycetes were analyzed molecu-
larly by Binder et al. (2005), again fitting Tulasnella species to the cantharelloid
clade but without substantial support. The results of Moncalvo et al. (2006) in
analyzing the cantharelloid clade were also ambiguous concerning Tulasnella in
nuc-rDNA and RPB2 together with mtSSU genes. Shimura et al. (2009) sequenced
the Japanese Cypripedium macranthos mycobiont and found a weakly supported
sister relationship to Cantharellus spp. and related taxa, including Sistotrema sp., in
a very limited sampling. In a comprehensive analysis of publicly available
sequences of Ceratobasidiaceae s.l. and related taxa, Veldre et al. (2013) included
also some anamorphic tulasnelloid strains and T. cystidiophora. Both groups
clustered in a sister relationship and were positioned in the Cantharellales. Also
in the review on Agaricomycetes of Hibbett et al. (2014), the Tulasnellaceae are
included in the Cantharellales.
12.4 The Presumable Age of Tulasnella and Evolution
of Plant Associations
Taylor and Berbee (2006) dated Basidiomycota between 1489 and 452 Mya, the
huge timespan resulting from the uncertainty in determining the age of the asco-
mycetous fossil Paleopyrenomycites. A maximum age of the evolutionary root in
Marchantiophyta is calculated for 450 Mya by Clarke et al. (2011), 520–470 Mya
12 Biogeography and Ecology of Tulasnellaceae 241
by Cooper et al. (2012), and 475 Mya by Sun et al. (2014). In a detailed time scale,
Cooper et al. (2012) mark a divergence time of 100–50 Mya for Aneura pinguis and
A. mirabilis. It may be concluded that Tulasnella mycobionts share the same age of
their liverwort photobionts. The second calibration approach of Taylor and Berbee
(2006) was used by Garnica et al. (2016) to determine divergence times in
Sebacinales and other taxa of Basidiomycota. For Cantharellales they found
317–128 Mya with an average of 203 Mya. With some caution, a similar age
interval may be adopted for Tulasnellaceae. Orchids originated approximately
100–80 Mya before present (Givnish et al. 2015), thus indicating a similar age of
their mycobionts, including Tulasnella.
Yukawa et al. (2009) summarized the occurrence of ORM mycobionts in major
clades of the Orchidaceae. Tulasnellaceae were reported from Apostasioideae,
Vanillinae, Cypripedioideae, Disinae, Orchidinae, Goodyerinae, Prasophyllinae,
Diuridinae, Caladeniinae, Neottieae, Dendrobiinae, Malaxideae, Calypsoeae,
Pleurothallidinae, and Cymbidiinae.
12.5 Biotrophic Associations of Tulasnella
12.5.1 Tulasnella Associated with Liverworts
Liverwort mycobionts were examined in the course of an extensive study of
biodiversity in a tropical cloud forest in South Ecuador (Kottke et al. 2003). Aneura
pinguis was associated with Tulasnella species related to T. asymmetrica
(Fig. 12.2), while Jungermanniales (Lophozia spp. and Calypogeia muelleriana)
involved sebacinoid mycobionts. The same sequence group of T. asymmetrica
(AY152406) was recovered in a study on the enigmatic hepatic Aneura mirabilis
(as Cryptothallus mirabilis, Wickett and Goffinet 2008) mycobionts in Europe by
Bidartondo et al. (2003). Aneura mirabilis is a mycoheterotrophic liverwort and
specialized as an epiparasite on Tulasnella species that form ectomycorrhizae with
surrounding trees like Alnus glutinosa,Betula pubescens,Pinus pinaster,
P. muricata or Salix aurita and S. cinerea (Bidartondo et al. 2003). In a geograph-
ically strongly expanded study on liverwort-fungal symbioses, Bidartondo and
Duckett (2010) reported Aneuraceae-associated Tulasnella from Europe, North
and South America, East Asia and New Zealand.
Thallose European and Andean species of Aneuraceae (Metzgeriales) host
Tulasnella mycobionts of high diversity especially in the European samples
(Nebel et al. 2004; Pressel et al. 2010; Preußing et al. 2010). These interactions
were considered by Krause et al. (2011) as a model of early evolved symbiotic
associations. It is most likely that specific Tulasnella species occur together with
the hosts throughout their distribution range.
242 F. Oberwinkler et al.
Fig. 12.2 Dendrogram of Tulasnellaceae species inferred by Jaccard analysis of all available
structures from 48 taxa, including the new species Tulasnella andina and T. kirschneri. Names of
species presented in detail by Cruz et al. (2016) are written in bold. Seven groups are defined,
based on basidiospore morphology. Other characters are indicated by symbols: clamp connections
(unfilled circles), cystidia ( filled circles), chlamydospores ( filled stars). From Cruz et al. (2016)
12 Biogeography and Ecology of Tulasnellaceae 243
12.5.2 Ectomycorrhiza (EcM)
The ectomycorrhizal lifestyle in fungi, including Tulasnella, and dealing with
diversity, distribution and evolution, was reviewed by Tedersoo et al. (2010). In a
study on ectomycorrhizal liaisons between forest orchids and trees in the Bavarian
northern Frankenalb, Bidartondo et al. (2004) mention Tulasnella and tulasnelloid
fungi as “lineages that contain some ectomycorrhizal strains”, however, without
further explanation.
In a wet Tasmanian sclerophyll forest, Tedersoo et al. (2008a) report several
unidentified Tulansella species associated with Eucalyptus regnans (Myrtaceae),
Nothofagus cunninghamii (Nothofagaceae), and Pomaderris apetala
(Rhamnaceae). The authors mention that Tulasnella is commonly observed in
Tasmania but seldom recorded in the Northern Hemisphere as EcM mycobionts.
This comment appears hardly probable for the real ECM occurrence of Tulasnella,
but matches literature information at present. Nevertheless, when studying the
community composition of Picea abies and Betula pendula seedlings in three
Estonian old-growth forests, Tedersoo et al. (2008b) found that “ordination ana-
lyses suggested that decay type determined the composition of EcM fungal com-
munity in dead wood”. In fact, in this study, Tulasnella EcMs were verified for the
first time in the Northern Hemisphere besides the experimental synthesis study of
Bidartondo et al. (2003).
12.5.3 Tulasnella Orchid Mycorrhiza (OM)
In seed germination experiments of orchids, Bernard (1899, 1909) and Burgeff (1909,
1932, 1936) detected the importance of fungal mycobionts during the early develop-
mental stages. At that time, identification of the mycobionts was impossible. In
addition, Burgeff (1932) treated the biology of symbiosis in tropical orchids exten-
sively. After a review of OMs by Rasmussen (2002), Dearnaley (2007) updated new
publications in this field. The trophic relationships in orchid mycorrhizae, including
Tulasnellaceae, and their implications for conservation were summarized by Rasmus-
sen and Rasmussen (2007). In a review on mutualistic, root-inhabiting fungi of orchids,
Kottke and Sua
´rez (2009) compiled also reports of tulasnelloid mycobionts, some of
them associated with epiphytic tropical orchids. The complex of requirements of
germination and seedling establishment in orchids, including tulasnelloid mycobionts,
were comprehensively treated by Rasmussen et al. (2015). Sua
´rez and Kottke (2016)
summarized their overview on ORMs in tropical mountain forests in Ecuador that main
fungal partners, including Tulasnella, correspond to findings in other biomes. Partial
genome sequences of two Tulasnella mycobionts, originating from Australian
Chiloglottis and Drakaea orchid species, may allow to obtain insight in evolutionary
trends of tulasnelloid OM (Ruibal et al. 2013).
244 F. Oberwinkler et al.
12.6 Biogeography of Tulasnella
12.6.1 Europe
Europe has the most abundant records of Tulasnella as fruit-bodies and in molec-
ular identification events from plant roots (Fig. 12.3). Hadley (1970) reported no
specificity of Tulasnella calospora in symbioses tests with European orchids,
Coeloglossum viride,Dactylorhiza purpurella,Goodyera repens and the tropical
Cymbidium canaliculatum,Epidendrum radicans,Laeliocattleya cv., Spathoglottis
plicata, and considered it as a potential universal orchid symbiont. Dijk et al. (1997)
stated that “Epulorhiza repens has been isolated from a vast amount of terrestrial
orchids, and is considered a ubiquitous orchid endophyte”. Tulasnella was the
predominant mycobiont in 59 root samples of seven European and North American
Cypripedium species (Shefferson et al. 2005). In addition, mycorrhizal specificity
of 90 populations of 15 Cypripedium taxa across Europe, Asia, and North America
was quantified by Shefferson et al. (2007). The orchids were associated almost
exclusively with Tulasnellaceae mycobionts.
The mycobiont septal structure of native terrestrial French Dactylorhiza majalis
(Strullu and Gourret 1974) and Italian D. maculata,D. sambucina,andPlatanthera
bifolia (Filipello Marchisio et al. 1985) was studied with the transmission electron
microscope. They authors found dolipores with continuous parenthesomes, suggesting
Sebacina and/or Tulasnella mycobionts, which were finally identified by Andersen
(1990) as T. deliquescens and T. calospora, respectively. A remarkable experimental
approach was carried out by Smreciu and Currah (1989), who studied symbiotic and
asymbiotic germination of seeds of north temperate terrestrial orchids in Europe and
Fig. 12.3 Sampling localities for Tulasnella spp., extracted from literature. Tulasnelloid associ-
ates with liverworts are marked with green dots. Orchid mycorrhizae (red dots) summarize isolates
of Tulasnella from orchid roots and molecularly identified samples. Tulasnelloid ectomycorrhizae
are marked with yellow dots. Lignicolous (blue dots) means that basidiomata were collected on
wood
12 Biogeography and Ecology of Tulasnellaceae 245
North America. The European species included Dactylorhiza maculata,D. sambucina,
Epipactis palustris,E. purpurata,Gymnadenia conopsea,G. odoratissima,Neottia
nidus-avis,Nigritella nigra,andOrchis morio. It appears that mycobionts of these
mostly widespread orchids were predominantly tulasnelloid fungi, except in N. nidus-
avis and E. purpurata. Rasmussen and Rasmussen (1991) tried to identify experimen-
tally the environmental conditions for germination and seedling development in
D. majalis together with T. calospora. A stimulating effect of Tulasnella (Epulorhiza
repens)andRhizoctonia (Ceratorhiza sp.) on the growth of Dutch Dactylorhiza spp.
and Orchis morio was reported by Dijk and Eck (1995). Single-strand conformation
polymorphism and mitochondrial ribosomal large subunit DNA sequences were used
by Kristiansen et al. (2001) to identify T. deliquescens and Laccaria sp. as D. majalis
mycobionts from single pelotons. Various fungal strains, isolated from non orchid
sources were used to test symbiotic germination of British D. fuchsii (Salman et al.
2001). Besides Ceratobasidium cornigerum,alsoT. helicospora stimulated germina-
tion of the orchid seeds and promoted seedling growth. From a wetland of Bavaria,
Bidartondo et al. (2004) reported Tulasnella as a mycobiont of D. majalis. Unidentified
Tulasnella OM symbionts were found in D. baltica,E. atrorubens,andO. militaris in
Estonian mine tailing hills and pristine sites (Shefferson et al. 2008). Most likely the
seed germination experiments of the boreal-alpine D. lapponica, collected from the
Solendet Nature Reserve in Central Norway, were enhanced by tulasnelloid
mycobionts (Øien et al. 2008). In analyzing the mycobionts of five Dactylorhiza
species in Belgium, Jacquemyn et al. (2012) concluded that orchid rarity is related to
mycorrhizal specificity and fungal distribution. In an extensive study of 114 sampled
individuals from one to three populations of 14 species of Dactyorhiza in Belgium,
France, Italy, Portugal, Sweden and the United Kingdom, Jacquemyn et al. (2016b)
suggested that habitat-driven variation occurs in mycorrhizal communities in which
Tulasnella plays an essential role.
Tulasnelloid mycobionts of Epipactis palustris were reported from Northeast
Bavarian wetlands (Bidartondo et al. 2004). Multiple independent colonization
events of former lignite mining areas in Eastern Germany by E. palustris were
documented by Esfeld et al. (2008) and observed in different rockgarden areas of
Tuebingen Botanical Garden by the first author between 1975 and 1995 (unpubl). In
a comparative study of E. helleborine,E. neerlandica, and E. palustris in Belgium,
Tulasnella was only retrieved from the latter photobiont (Jacquemyn et al. 2016a).
In ten North American and European Goodyera species, Tulasnella was only found
in G. pubescens and G. repens in the USA (McCormick et al. 2004; Shefferson et al.
2010). In their study on carbon and nitrogen exchange in Goodyera repens, Liebel
et al. (2015) found Tulasnella and Ceratobasidium as the most frequent mycobionts
of the orchid species.
Fungi from the roots of the common terrestrial orchid Gymnadenia conopsea
included typical ORMs of the Tulasnellaceae and Ceratobasidiaceae as well as several
ectomycorrhizal taxa of the Pezizales (Stark et al. 2009). In this orchid, Te
ˇs
ˇitelova
´et al.
(2013) found evidence that polyploidization can be associated with a shift in their
tulasnelloid mycorrhizal symbionts. Among a variety of ascomycetous and
246 F. Oberwinkler et al.
basidiomycetous associates of Himantoglossum adriaticum, Tulasnellaceae were iden-
tified in two protected areas of Central Italy (Pecoraro et al. 2013).
Liparis loeselii and Hammarbya paludosa are wetland specialists associated
with tulasnelloid mycobionts in Hungary (Illye
´s 2011). In situ and in vitro germi-
nation of L. loeselii were studied by Illye
´s et al. (2005). They found Tulasnella
(Epulorhiza) and Ceratobasidium (Rhizoctonia) as mycorrhizal partners. Broader
samplings with Dactylorhiza incarnata,Epipactis palustris,Gymnadenia
conopsea,Ophrys oestrifera,Op. sphegodes, and Orchis militaris,Or. palustris,
and Or. purpurea indicated Tulasnella associations to prefer wetter habitats (Illye
´s
et al. 2009), or to tolerate a wide spectrum of water availability (Illye
´s et al. 2010).
Here, the question arises, what constrains the distribution of orchid populations
(McCormick and Jacquemyn 2014), a question that should better be modified into
what constrains the distribution of orchid-mycobiont associations. Recently
Jacquemyn et al. (2015b) reported Tulasnellaceae in the roots and the soil of the
green Neottia ovata (Listera ovata) in eastern Belgium. It is noteworthy to mention
that tulasnelloid mycobionts have not been found in the achlorophyllous N. nidus-
avis (e.g. Selosse et al. 2002).
The mycorrhizal fungal diversity of Orchis militaris, including tulasnelloid
associates, detected in some Hungarian habitats, is considered to be essential for
the wide ecological range of the orchid species (Ouanphanivanh et al. 2007). In a
multidisciplinary approach of the simultaneously investigated mediterranean
Orchis simia,O. anthropophora, and their hybrid O. bergonii, Schatz et al.
(2010) compared leaf growth, seed viability, emitted scent, and mycorrhizal species
and their rate of infection. The mycobionts were unidentified Tulasnella species.
Five Orchis species, O. anthropophora,O. mascula,O. militaris,O. purpurea, and
O. simia, sampled from the Netherlands to Italy by Jacquemyn et al. (2010),
contained a majority of Tulasnella mycobionts. In three closely related and hybrid-
izing species, O. anthropophora,O. militaris, and O. purpurea, the influence of
mycorrhizal associations on reproductive isolation of the orchids appeared to be of
minor importance (Jacquemyn et al. (2011a). Girlanda et al. (2011) reported
Tulasnella calospora mycobionts in the mediterranean meadow orchids Ophrys
fuciflora,Anacamptis laxiflora,O. purpurea, and Serapias vomeracea. In a com-
prehensive survey of 16 European and Mediterranean Orchis species, Jacquemyn
et al. (2011b) found dominating Tulasnella OMs from the Netherlands, Belgium,
France, Portugal, Italy, Cyprus, and Israel. For the persistence and rarity of A. morio
and Dactylorhiza fuchsii in Belgian habitats, Bailarote et al. (2012) suggested that
fungal diversity with dominating Tulasnella are not necessarily related. Studies
conducted in the Gargano National Park in southern Italy by Jacquemyn et al.
(2014, 2015a) comprised Anacamptis pyramidalis,A. (Orchis)morio,
A. papilionacea,Neotinea maculata,N. ustulata,Orchis anthropophora,
O. italica,O. pauciflora,O. provincialis,O. quadripunctata,Ophrys apulica,Op.
biscutella,Op. bombyliflora,Op. sphegodes,Op. sicula,Op. tenthredinifera,
Serapias bergonii,S. cordigera,S. lingua, and S. vomeracea. The mycobionts of
coexisting orchid species had distinct mycorrhizal communities and were predom-
inantly recruited by Tulasnella and Rhizoctonia (“Ceratobasidiaceae”). A broad
12 Biogeography and Ecology of Tulasnellaceae 247
spectrum of mycobionts, including Tulasnella, were found to be associated with
O. tridentata in Central Italy by Pecoraro et al. (2012). The temporal variation in
mycorrhizal diversity of A. morio from North Italian meadows was analysed by
Ercole et al. (2014). The fungi, manually isolated from pelotons, were common
Tulasnella in autumn and winter, the pezizacean clade very frequent in spring, and
Ceratobasidium more frequent in summer. In 16 Mediterranean orchid species of
the genera Anacamptis,Ophrys,Orchis, and Serapias, Pellegrino et al. (2014)
found 18 operational taxonomic units (OTUs) of Tulasnella and
“Ceratobasidiaceae”. Mycobiont analyses of the mediterranean Op. bertolonii
revealed Tulasnella as the dominant fungal partner (Pecoraro et al. 2015). The
fine-scale spatial distribution of OM fungi, including Tulasnella, in soils of host-
rich mediterranean grasslands of northern Italy was screened by Voyron et al.
(2016) and found to be extremely sporadic. The spatially tight dependency of
tulasnelloid associates of orchids was clearly documented in populations of
A. morio,Gymnadenia conopsea, and O. mascula in Southern Belgium (Waud
et al. 2016a). Also in Belgium, the majority of mycobionts of O. mascula and
O. purpurea appeared to be Tulasnella (Waud et al. 2016b).
Bidartondo et al. (2004) reported Tulasnella as mycobiont of Platanthera
chlorantha from the Bavarian Frankenalb. In a study on the evolution of endemic
Azorean orchids, also ORMs were analyzed, and T. calospora and Tulasnella spp.
were found in Platanthera species (Bateman et al. 2014). Kohout et al. (2013)
studied the fungal communities associated with Pseudorchis albida in the S
ˇumava
National Park, Czech Republic. The mycobionts of the orchid were four unnamed
Tulasnella strains. In protocorms of P. albida, also from this country, and in
Serapias parviflora from Sardinia, Tulasnella spp. were detected by St€
ockel et al.
(2014). Protocorms of the mediterranean orchid Serapias vomeracea were colo-
nized by Tulasnella calospora in an experimental study of Balestrini et al. (2014).
12.6.2 Temperate Asia
Whole rDNA analyses of roots and leaves of Bletilla ochracea from a mountain
near Guiyang in Guizhou Province, China, provided a high number of fungal OTUs,
dominated by ascomycetes (Tao et al. 2008). In addition, also Epulorhiza sp. could
be identified. Eom (2012) isolated T. calospora,T. irregularis, and Tulasnella
sp. from terrestrial Korean Bletilla striata,Calanthe discolor,Cymbidium
goeringii, and Pogonia minor. Eom (2015) identified T. calospora and Tulasnella
sp. in Cephalanthera falcata,C. longibracteata,Platanthera chlorantha, and
P. mandarinorum in Korea. Jiang et al. (2011) isolated Tulasnella spp. from
Changnienia amoena, an orchid distributed in various provinces of Central China.
Lee and You (2000) identified Tulasnella repens in the native Korean Cymbid-
ium goeringii. Korean species of Cymbidium were successfully inoculated with
Tulasnella repens by Lee et al. (2001). In a comparative study, Ogura-Tsujita et al.
(2012) tried to find a correlation in mycobiont’s association in Cymbidium during
248 F. Oberwinkler et al.
the evolution of autotrophy to mycoheterotrophy. Tulasnella dominated in the
autotrophic C. dayanum, were less frequent in mixotrophic C. goeringii and
C. lancifolium and absent in mycoheterotrophic C. macrorhizon and C. aberrans.
In five Korean terrestrial orchids, C. goeringii,Spiranthes sinensis,Calanthe
discolor,Bletilla striata, and Pogonia minor, Youm et al. (2012) identified
Tulasnella calospora,T. irregularis,T. sp., and Sebacina vermifera.
The mycobiont of the threatened orchid Cypripedium macranthos var.
rebunense, from Rebun Island northwest of Hokkaido was identified as Tulasnella
(Shimura et al. 2009). Mycobionts of six endangered slipper orchid species from
Southwestern China, Paphiopedilum micranthum,P. armeniacum,P. dianthum,
Cypripedium flavum,C. guttatum, and C. tibeticum, were identified as Tulasnella
spp. by Yuan et al. (2010). Hayakawa et al. (1999) isolated Tulasnella deliquescens
from naturally occurring protocorms, seedlings, and adult Japanese Dactylorhiza
aristata. Most of the OM fungi in Dendrobium fimbriatum and D. officinale from
Guangxi were identified as members of the Tulasnellaceae by Xing et al. (2013).
Tan et al. (2014) used their Tulasnella isolates of D. officinale from Yunnan to carry
out seed germination experiments. They found different interactive capacities in
two fungal strains.
As mycobionts of Epipactis thunbergii, Eom and Kim (2013) identified i. a.
T. calospora and Tulasnella sp. E. thunbergii and Habenaria radiata were colo-
nized by the ecologically adapted, associated with various mycobionts in manmade
wetlands in the Hiroshima Prefecture, Japan (Cowden and Shefferson 2013). While
a diverse suite of fungal symbionts was found in H. radiata,E. palustris was nearly
exclusively inhabited by Tulasnella spp. Based on the morphology and cultures of
isolates with anastomoses, Uetaka et al. (1999) identified Epulorhiza repens in the
Japanese terrestrial orchids Gymnadenia camtschatica,Platanthera tipuloides and
Pogonia japonica. In nine species of the genus Holcoglossum from Yunnan and
Guangxi, T. calospora and the anamorphic tulasnelloid Epulorhiza were found (Tan
et al. 2012). From different populations of Liparis japonica in Northeast China,
Ding et al. (2014) identified fungi of the T. calospora species group. In situ and
in vitro specificity between mycobionts and Spiranthes sinensis var. amoena was
analyzed by Masuhara and Katsuya (1994). The germination was mainly induced
by Tulasnella (as Rhizoctonia repens).
12.6.3 Subtropical and Tropical Asia
Apostasioideae are considered the basal group of the Orchidaceae (Chase et al.
2003). Five studied Apostasia species had Botryobasidium and Ceratobasidium
mycobionts, and the related Neuwiedia veratrifolia was associated with
Ceratobasidium and Tulasnella (Yukawa et al. 2009). Most of the mycobiont
isolates of Neuwiedia veratrifolia, collected in Borneo, could be assigned to
Tulasnella by Kristiansen et al. (2004).
12 Biogeography and Ecology of Tulasnellaceae 249
The mycobiont of the “Chinese King Medicine Orchid”, Anoectochilus
roxburghii, was identified as Epulorhiza sp. and was successfully used in
co-culture experiments to improve the growth of the host plant (Li et al. 2012).
Dan et al. (2012) found that eight of 42 OM fungal strains tested including three
Epulorhiza spp. enhanced the growth of the host plantlets. The endophyte promot-
ing the growth and contents of kinsenosides and flavonoids of A. formosanus was
identified as Epulorhiza sp. by Zhang et al. (2013). Likewise, in seven localities of
Taiwan, Jiang et al. (2015) isolated mycobionts of this medicinally used orchid. No
increase in orchid seed germination was found when Tulasnella strains were
applied that clustered in clade III of their study. Mycobionts of the Chinese
medicinal orchid Dendrobium officinale were identified as Epulorhiza sp. and
inoculation of the fungus resulted in promoted seedling growth (Jin et al. 2009).
For symbiotic seed germination of D. draconis and Grammatophyllum speciosum,
native orchids of Thailand, the anamorph of Tulasnella calospora proved to be most
effective to stimulate protocorm development (Nontachaiyapoom et al. 2011). In
contrast, Salifah et al. (2011) found that seed germination rates in this orchid were
best when co-cultured with Fusarium sp. Five Tulasnella isolates of four
Dendrobium species from Chiang Rai Province of Thailand showed different
promoting effects on seed germination (Swangmaneecharern et al. 2012). The in
situ seed baiting of the epiphytic D. aphyllum from the Xishuangbanna tropical
Botanical Garden in South Yunnan, studied by Zi et al. (2014), revealed Tulasnella
spp. as mycobionts. In contrast, Agustini et al. (2016) isolated Rhizoctonia-like
fungi from D. lancifolium var. papuanum and Calanthe triplicata from Papua,
which was considered of “Ceratobasidium” relationship. Khamchatra et al.
(2016a) isolated T. violea and Epulorhiza repens from the Thai epiphytic
D. friedricksianum. Under in vitro culture conditions, Wang et al. (2016) found
promoted D. catenatum seedling growth from Hainan with dual inoculation of
Epulorhiza and Enterobacter or Herbaspirillum bacteria.
Commercially grown Thai species and hybrids of Cymbidium,Dendrobium, and
Paphiopedilum were used by Nontachaiyapoom et al. (2010) for isolation of
mycobionts. They identified Tulasnella anamorphs. Tulasnella spp., isolated from
wild and horticulturally grown Cymbidium spp. in SW-China, were used to test
growth differences in co-cultures with C. hybridum, an important pot ornamental
orchid (Zhao et al. 2014a). In addition, deep sequencing-based comparative tran-
scriptional profiles of these photo- and mycobionts were carried out (Zhao et al.
2014b). The positive experiments were indicative for application in Cymbidium’s
commercial cultivation. Mycobionts of C. faberi,C. goeringii, and C. goeringii var.
longibracteatum, also from SW-China, included Tulasnella spp. (Huang and Zhang
2015). Yu et al. (2015) isolated and identified endophytes, and Tulasnella ORMs
from roots of C. goeringii and C. faberi.
The germination and development of the terrestrial Arundina chinensis,
Spathoglottis pubescens, and Spiranthes hongkongensis from various locations of
Hong Kong were found to be strongly stimulated by Epulorhiza isolates (Shan et al.
2002). Isolated E. repens from the Thai terrestrial S. plicata enhanced seed germi-
nation in vitro considerably (Athipunyakom et al. 2004a). From this orchid species
250 F. Oberwinkler et al.
of Papua, Sufaati et al. (2012) reported Tulasnella mycobionts. In a study on
mycorrhizal associations and root morphology of 31 terrestrial and epiphytic
orchids species of the Western Ghats, southern India, also S. spicata was included
(Sathiyadash et al. 2012). Regarding the mycobionts, there is only the single remark
that the orchids “had moniliform structures resembling those of Tulasnella
calospora (Epulorhiza repens) in the cortical and root hair cells”.
In the endangered epiphytic Thai slipper orchid Paphiopedilum villosum,
Tulasnella sp. could be identified as mycobiont (Khamchatra et al. 2016b). A highly
compatible Epulorhiza strain was used to demonstrate promotion of seed germina-
tion and protocrom development in Papilionanthe teres from Xishuangbanna,
South China (Zhou and Gao 2016). In seed germination and seedling development
of the Thai terrestrial orchid Pecteilis susannae, the incubation of Tulasnella
enhanced growth considerably (Chutima et al. 2011). Isolates from the tropical
orchids Arachnis sp., Arundina graminifolia,Dendrobium crumenatum,
Diplocaulobium enosmum,Oncidium hybr., Vanda hybr., and Spathoglottis plicata
in Singapore comprised both Sebacina and Tulasnella mycobionts (Ma et al. 2003).
Mycobionts isolated from pelotons of Calanthe rubens, Ca. rosea,Cymbidium
sinense,Cy. tracyanum,Goodyera procera,Ludisia discolor,Paphiopedilum
concolor,P. exul,P. godefroyae,P. niveum and P. villosum were identified as
Epulorhiza calendulina,E. repens,and Tulasnella sp. among multiple mycobionts
(Athipunyakom et al. 2004b). Suryantini et al. (2015) reported on Epulorhiza and
Tulasnella spp. associated with epiphytic Ca. vestita and Bulbophyllum beccarii
from West Kalimantan. Seed germination of the epiphytic, therapeutically valuable
orchid Coelogyne nervosa, endemic to south India, was higher when inoculated
with Epulorhiza sp. (Sathiyadash et al. 2014).
12.6.4 North America
Rhizoctonia anaticula was described by Currah (in Currah et al. 1987), based on
five isolates of native Alberta orchids, and later transferred into the tulasnelloid
anamorphic genus Epulorhiza (Currah et al. 1990). The same mycobiont was also
isolated from Calypso bulbosa and Platanthera obtusata sampled in various loca-
tions of Alberta (Currah and Sherburne 1992; Currah et al. 1988). The TEM
micrographs indicate tulasnelloid fungi (Currah and Sherburne 1992). Smreciu
and Currah (1989) recovered potentially high percentage of tulasnelloid
mycobionts in symbiotic and asymbiotic germination of seeds of north temperate
terrestrial orchids Amerorchis rotundifolia, Ca. bulbosa,Coeloglossum viride,
Corallorhiza maculata,Co. trifida,Cypripedium calceolus,Goodyera repens,
Platanthera hyperborea,P. obtusata, and P. orbiculata, four of them also occurring
in Europe. So far, it remains unsettled what Ceratobasidium cereale, a mycobiont
of G. repens, is (Peterson and Currah 1990). In germination experiments of
P. hyperborea seeds, mycobionts of uncertain taxonomic position, like Rhizoctonia
cerealis or Ceratorhiza goodyerae-repentis, were used (Richardson et al. 1992).
12 Biogeography and Ecology of Tulasnellaceae 251
The orchid–mycobiont association was studied in detail in Goodyera repens,a
terrestrial orchid of the eastern United States (McCormick et al. 2006). It was found
that protocorms and adult orchids were able to switch with closely related
Tulasnella fungi. In germination tests of seeds of Goodyera discolor,Liparis
liliifolia and Tipularia discolor, McCormick et al. (2012) used fungal strains
isolated from adult orchids and found that Tulasnella was involved in all cases.
Shefferson et al. (2005) detected Tulasnella spp. in root samples of Cypripedium
californicum,C. fasciculatum and C. montanum in California; C. candidum and
C. parviflorum in Illinois and Kentucky, C. guttatum in Alaska. Whitridge and
Southworth (2005) reported Tulasnellaceae associated with Cypripedium
fasciculatum, and with Piperia sp. One of the rarest North American terrestrial
orchids, Piperia yadonii, showed non-specific ORMs, including Tulasnellaceae
(Pandey et al. 2013). In Encyclia tampensis of South Florida, Zettler et al.
(2013), reported T. irregularis as mycobiont and essential fungal partner during
seed germination. The symbiotic germination of Spiranthes lacera, with a naturally
occurring endophyte, Ceratorhiza cf. goodyerae-repentis, and with Epulorhiza
repens was tested by Zelmer and Currah (1997). The orchid occurs in the eastern,
northern and central parts of North America. The symbiotic germination of
S. brevilabris showed Epulorhiza mycobionts, and the reintroduction of the endan-
gered orchid, native to Florida, was discussed by Stewart et al. (2003).
In an integrated approach to Rhizoctonia taxonomy, Mordue et al. (1989)
succeeded in taxonomically separating orchid isolates, i.e. tulasnelloid mycobionts
from other Rhizoctonia-like fungi. A key and notes for the genera of fungi,
mycorrhizal with orchids, and a new species in the genus Epulorhiza, was provided
by Currah and Zelmer (1992). Ceratorhiza pernacatena and Epulorhiza
calendulina were described as mycorrhizal fungi of terrestrial orchids in the
Canadian prairies by Zelmer and Currah (1995), tulasnelloid mycobionts at least
in one case. Epulorhiza inquilina was proposed for the mycobiont of the mature
orchids Platanthera clavellata,P. cristata and P. integrilabia in Canada (Currah
et al. 1997). For the propagation of the auto-pollinated terrestrial P. clavellata in the
southern Appalachians, Epulorhiza spp. strains were applied in vitro by Zettler and
Hofer (1998). In P. praeclara of midwestern prairies, Epulorhiza and Ceratorhiza
were found and used in symbiotic seed germination and coinoculations by Sharma
et al. (2003a, b). Also in the endangered Hawaiian endemic Platanthera
leucophaea,Epulorhiza was found as mycobiont (Zettler et al. 2005).
Seeds of the endangered epiphytic orchid Epidendrum nocturnum from Florida
were germinated in vitro with Epulorhiza repens (Massey and Zettler 2007; Zettler
et al. 2007). Mycorrhized seedlings could successfully be reintroduced in the
Florida Panther National Wildlife Refuge. Symbiotic seed germinations of three
semi-aquatic orchids, Habenaria macroceratitis,H. quniqueseta, and H. repens
from Florida had Epulorhiza mycobionts (Stewart and Zettler 2002). Later, in
H. macroceratitis, Stewart and Kane (2006) isolated six Epulorhiza strains.
Epulorhiza sp. was present in seed germination of H. repens in situ beyond its
range in southern North America (Keel et al. 2011).
252 F. Oberwinkler et al.
12.6.5 Central and South America
Unfortunately, in their study on basidiomycetous endophytes from the roots of
epiphytic orchids in La Selva, Costa Rica, Richardson et al. (1993) use the generic
names Moniliopsis and Ceratorhiza for the isolates. Though it is most likely that
Tulasnella is included in these fungi, verification is impossible. Otero et al. (2002)
isolated Rhizoctonia-like fungi inclusive of Tulasnella from orchids in Puerto Rico.
They included the epiphytic species Campylocentrum fasciola,C. filiforme,
Ionopsis satyrioides,I. utricularioides,Psychilis monensis,Tolumnia variegata,
and the terrestial Erythrodes plantaginea,Oeceoclades maculata, and Oncidium
altissimum. In Brazil, Epulorhiza epiphytica was isolated from mycorrhizal roots of
epiphytic orchids and described as a new tulasnelloid anamorph by Pereira et al.
(2003), and additional ORMs from neotropical orchids were characterized mor-
phologically and molecularly by Pereira et al. (2005b), and for Laeliinae by
Almeida et al. (2007).
Kottke et al. (2008) used sequence data of Tulasnella and other mycobionts to
interprete fungal networks between diverse photobionts, including epiphytic
orchids and Aneuraceae. Mosquera-Espinosa et al. (2010) studied 12 fungal isolates
of eight Colombian orchids and reported Ceratobasidium spp. as mycobionts.
However, a proper taxonomic identification was not achieved. Mycorrhizal net-
works with prominent Tulasnella OM mycobionts were considered to promote and
stabilize the neotropical mountain rain forest (Kottke et al. 2013). Cruz et al. (2014)
analyzed the variability of micromorphological features of basidiomata and the
genomic polymorphism of Tulasnella ORMs in South Ecuadorian orchid species of
the genera Elleanthus,Maxillaria,Pleurothallis,Prostechea, and Stelis. From five
terrestrial orchids of Co
´rdoba, Argentina, Aa achalensis,Cyclopogon elatus,
Habenaria hexaptera,Pelexia bonariensis, and Sacoila australis, Ferna
´ndez Di
Pardo et al. (2015) isolated various mycobionts, including Epulorhiza.Sua
´rez and
Kottke (2016) summarized main mycobionts, including Tulasnella, and their spec-
ificities in neotropical orchids of South Ecuadorian rain forests. In an Andean cloud
forest of South Ecuador, Sua
´rez et al. (2006) found that diverse tulasnelloid fungi
form mycorrhizae with epiphytic Pleurothallis lilijae,Stelis concinna,S. hallii, and
S. superbiens. A study of Sua
´rez et al. (2016) in Ecuador revealed that Teagueia
spp. were associated with members of Tulasnellaceae, corresponding to four OTUs.
All detected mycobionts had a wide geographical distribution.
Experiments for a symbiotic propagation to reintroduce endangered Mexican
terrestrial Bletia urbana,B. campanulata, and Dichromanthus aurantiacus were
carried out by Ortega-Larrocea and Rangel-Villafranco (2007), applying anamor-
phic Tulasnella strains. Ovando et al. (2005) isolated and screened endophytic fungi
from the roots of the epiphytic orchids Brassavola nodosa,Cattleya skinneri, and
C. aurantiaca from Tuzanta
´n, South Mexico. The isolated strains were assigned to
11 fungal genera. Eight strains, used for germination experiments, did not show any
promoting effects. However, three strains, including Epulorhiza, provided mycor-
rhizal characteristics in C. aurantiaca. A new tulasnelloid anamorph, Epulorhiza
12 Biogeography and Ecology of Tulasnellaceae 253
amonilioides, lacking monilioid hyphae in pure culture, was isolated from
Brassavola and Encyclia species and described by Almeida et al. (2014) from
Bahia, Brazil. When analyzing three sympatric epiphytic Cymbidieae, Cyrtochilum
flexuosum,C. myanthum, and Maxillaria calantha from two sites of South Ecua-
dorian mountain rain forests, Cevallos et al. (2016) concluded that these orchids
have site-adjusted OM communities with keystone mycobionts, including
Tulasnella. In testing seed germination and protocorm development of
Cyrtopodium glutiniferum from Brazil, Pereira et al. (2015) found promotion by
mycorrhizal fungi of the tulasnelloid anamorphs Epulorhiza spp. In roots of four
Vanilla species from Puerto Rico, Costa Rica and Cuba, Porras-Alfaro and Bayman
(2007) found mycobionts of Ceratobasidium,Thanatephorus and Tulasnella.
Epulorhiza spp. was isolated from various Brazilian Epidendrum species (Pereira
2009, Pereira et al. 2009, 2011a, b, 2014a). From the epiphytic E. stamfordianum,
Erycina crista-galli,andStelis quadrifida from Southeast Chiapas, Mexico,
Ceratorhiza and Epulorhiza mycobionts were reported by Cruz Blası
´(2007). Two
different Tulasnella species were found to be associated with South Ecuadorian
E. rhopalostele, an orchid preferably growing on dead trees (Riofrı
´o et al. 2013).
Populations of E. firmum in Costa Rica had highly diverse and spatially heterogeneous
mycobionts, including six Tulasnella strains (Kartzinel et al. 2013). The mycobionts of
E. secundum, a widespread Brazilian orchid, were identified as Tulasnella spp. by
Pereira et al. (2014a) and as T. calospora by Nogueira et al. (2014). In vitro seed
germination and protocorm development of Brazilian Oncidium flexuosum was studied
with mycobionts of Epulorhiza and Ceratorhiza, earlier isolated from this orchid
(Pereira et al. 2005a, c), and Da Silva Coelho et al. (2010) reported regeneration and
production of the fungal protoplasts.
Epulorhiza epiphytica was isolated from Polystachya concreta and the African
Oeceoclades maculata, naturalized in the Neotropics, by Pereira et al. (2005b).
Nine unnamed morphotypes of fungi, associated with O. maculata, were isolated
from the understory of Avocado in Brazil by Teixeira et al. (2015).
In the mycorrhizal association of the terrestrial Chilean orchid Bipinnula
fimbriata also tulasnelloid ORMs were present (Steinfort et al. 2010). Mujica
et al. (2016) found that mycorrhizal diversity, including Tulasnella, decreased in
habitats of B. fimbriata and B. plumosa with higher N, but increased with P
availability in B. fimbriata. Morphological and molecular characterization con-
firmed that Chilean Chloraea collicensis and C. gavilu mycorrhizal partners belong
to Tulasnella (Pereira et al. 2014b). In contrast, Atala et al. (2015) reported
mycobionts with possible Thanatephorus teleomorphs from the critically endan-
gered Chilean C. cuneata. However, the data presented cannot exclude tulasnelloid
associates. In a study by Herrera et al. (2016), in six Chloraea species and Bipinnula
fimbriata from Chilean Coastal Range and Andes. Tulasnella spp. were found as
dominating mycobionts. Fracchia et al. (2014) found promoted see germination
through tulasnelloid and Ceratobasidium-like fungi in Gavilea australis, an endan-
gered terrestrial orchid from south Patagonia.
254 F. Oberwinkler et al.
12.6.6 Africa
Martos et al. (2012) identified a bipartite network including 73 orchid species and
95 taxonomic units of mycorrhizal fungi across the natural habitats of Reunion
Island. 58 tulasnellaceous OTUs were found in 73 orchid species, thus representing
the most frequent OM mycobionts. In their study on the evolution of endemic
Azorean orchids, Bateman et al. (2014) reported also the mycorrhizal association of
Tulasnella aff. Calospora with Platanthera algeriensis in Morocco. Most of the
OM fungi of the Itremo region in the Central Highlands of Madagascar were
identified as Tulasnella (Yokoya et al. 2015). The symbiotic seedling development
of the terrestrial Cynorkis purpurea, also from the Itremo area, has been tested
experimentally by Rafter et al. (2016). Though epiphyte-derived Sebacina cultures
had the strongest influence, also Tulasnella appeared as an advantageous
mycobiont. Disa bracteata of South Africa was associated with Tulasnella spp. in
West and South Australia as in its country of origin (Bonnardeaux et al. 2007). In an
attempt to elucidate the impact of above- and belowground mutalisms in
South African orchid diversification, an irregular pattern of fungal associates,
including 35, unspecified Tulasnella individuals, were detected (Waterman et al.
2011). The authors concluded that “shifts in fungal partner are important for
coexistence but not for speciation” of the host plants.
12.6.7 Australia
When Warcup and Talbot (1967) succeeded to isolate and cultivate OM fungi from
terrestrial Australian orchids, and finally obtained perfect states of Rhizoctonias, a new
era of experimental mycology and especially of studies in symbiotic systems began.
Tulasnella calospora was found to be the perfect state of three cultures considered to be
Rhizoctonia repens. Isolates were obtained from South Australia (Acianthus exsertus,
Caladenia reticulata,Cymbidium canaliculatum,Dendrobium sp., Diuris longifolia,
D. maculata,andThelymitra antennifera). Tulasnella asymmetrica was described as a
new species and as mycobiont of Thelymitra luteocilium from the Australian Mt. Lofty
Range. In a second contribution of the authors (Warcup and Talbot 1971), the
description of Tulasnella asymmetrica was emended and further orchid hosts were
reported from the Mt. Lofty Range: Thelymitra aristata (also Cape Jervis),
T. grandiflora,andT. pauciflora. Additional hosts were Th. epipactoides (Eyre
Peninsula), and Dendrobium tetragonum from North Queensland. The basidial stage
of the morphotype of T. allantospora with clamps was obtained from Mt. Lofty isolates
of Corybas dilatatus, and basidiocarp samples without clamps were collected on fallen
Eucalyptus wood in the same locality. The perfect stage of T. violea developed from an
isolate obtained from Th. aristata, collected in Uley, Eyre Peninsula. Tulasnella
cruciata was introduced as new to science, isolated from the Mt. Lofty Range orchids
Acianthus caudatus and Th. pauciflora, while the strain of Th. fusco-lutea originated
12 Biogeography and Ecology of Tulasnellaceae 255
from Pomonal, Victoria. In the third joint effort of Warcup and Talbot (1980) to obtain
perfect states of OM mycobionts they succeeded with T. irregularis sp. nov., isolated
from Dendrobium dicuphum, sampled near Darwin, Northern Territory. In studying
the specificity of ORMs in Australian terrestrial orchids, Warcup (1971) reported that
Th. aristata is at least associated with three species of Tulasnella. In the “Orchids of
South Australia” (Bates and Weber 1990), T. calospora is listed as mycobiont in orchid
species of the genera Acianthus,Diuris,Orthoceras,andThelmytra. For the latter one
and Acianthus,alsoT. cruciata is mentioned. The symbiotic germination of some
Australian terrestrial orchids was analyzed by Warcup (1973) who reported that various
isolates of T. calospora differed markedly in the efficiency with which they stimulated
germination of the Diuris and Thelymitra photobionts. A close association of this
mycobiont with Diuris and Orthoceras orchids was confirmed by Warcup (1981).
The mycorrhizal specificity of D. fragrantissima with Tulasnella spp. and persistence
in a reintroduced population west of Melbourne was studied by Smith et al. (2007,
2010). In D. magnifica and Prasophyllum giganteum,T. calospora was found, and in
Pyrorchis nigricans isolates T. danica were identified (Bonnardeaux et al. 2007).
A narrow group of monophyletic Tulasnella symbiont lineages is associated
with multiple species of Chiloglottis in New South Wales and the Australian
Capital Territory (Roche et al. 2010). For Tulasnella OM species delimitation in
the Australian orchid genera Chiloglottis,Drakaea,Paracaleana and Arthrochilus,
Linde et al. (2013) used six nuclear loci, two mitochondrial loci, the photo- and
mycobiont association and sampling locations in an integrated approach. They
found that the Chiloglottis isolates belong to one species, and those from Drakaea
and Paracaleana to a sister taxon, a result in accordance with previous ITS
analyses. Boddington and Dearnaley (2009) reported a putative mycorrhizal
Tulasnella-like fungus in the tropical epiphytic Dendrobium speciosum of
Queensland. In studies of Drakaea species in Southwest Australia, Phillips et al.
(2011, 2014) found no evidence that Tulasnella specificity contributed to the rarity
of the orchids.
According to Brundrett (2007), most West Australian orchids studied have
highly specific mycorrhizal associations with fungi in the Rhizoctonia alliance,
most likely including Tulasnella spp. The nutrient-acquisition patterns of ORMs,
inclusive of Tulasnella, appear to explain the diversification in terrestrial orchids in
this biodiversity hotspot (Nurfadilah et al. 2013).
Milligan and Williams (1988) obtained 27 tentatively identified Tulasnella
calospora isolates from Microtis spp. at seven sites in the Sydney region. The
specificity of associations between M. parviflora and Epulorhiza spp. was studied
by Perkins et al. (1995). The compatibility webs of brief encounters, lasting
relationships and alien invasions of West Australian terrestrial orchids were studied
by Bonnardeaux et al. (2007), documenting that M. media, together with the
invasive Disa bracteata, had the most ORMs. Mycorrhizal preference apparently
promotes habitat invasion of M. media in Western Australia (De Long et al. 2013).
When studying the effects of endophytic fungi on New Zealand terrestrial
M. unifolia,Spiranthes novae-zelandiae, and Thelymitra longifolia, Frericks
256 F. Oberwinkler et al.
(2014) obtained Tulasnella calospora isolations and compared them with strains of
various geographical origins.
The rare subterranean, achlorophyllous orchid Rhizanthella gardneri from
western Australia lives in a more than triple association with autotrophic and
heterotrophic partners in which, apparently, two Tulasnella species are involved
(Warcup 1985). In a taxonomic study and an experimental approach to grow
Rhizanthella gardneri together with Melaleuca scalena (Myrtaceae), Bougoure
et al. (2009a, b) used as mycobiont an unidentified, so-called “Ceratobasidium”
with the positive result that 5% of carbon fed to Melaleuca as
13
CO
2
was transferred
to R. gardneri. Further studies are needed to clarify the taxonomy and whether
diverse mycobionts are involved in this association.
12.7 Conclusions
Our literature search for Tulasnella on a global scale confirmed that distribution
patterns are biased by sampling. Nevertheless, there is unequivocal documentation
that Tulasnella as a group and certain morphological species have global distribu-
tion. Furthermore, it appears obvious that the world-wide distribution of orchids
may reflect a similar occurrence of their mycobionts, for which Tulasnella species
play a crucial role. The same may be true for Tulasnella associates of certain
liverworts. In addition, lignicolous basidiomata of Tulasnella are reported from
collecting areas of mycologists, interested in corticioid fungi. Apart from these
restrictions, a more adequate interpretation of Tulasnella’s biogeography is the
distribution pattern of suited habitats which appear to occur in a nearly world-wide
range.
Acknowledgements We thank Leho Tedersoo for the invitation to contribute to the book on
Biogeography of Mycorrhizae, Roland Kirschner and two anonymous reviewers for critical
comments. Gratefully acknowledged are the copyright permissions for Fig. 12.1 (Staatliches
Museum für Naturkunde Karlsruhe), and Fig. 12.2 (J. Cramer in Gebr. Borntraeger
Verlagsbuchhandlung, Stuttgart).
References
Agustini V, Sufaati S, Suharno, Suwannasai N (2016) Rhizoctonia-like fungi isolated from roots of
Dendrobium lancifolium var. papuanum and Calanthe triplicata in Papua, Indonesia.
Biodiversity 17:377–383
Almeida PR, van den Berg C, Goes-Neto A (2007) Morphological and molecular characterization
of species of Tulasnella (Homobasidiomycetes) associated with Neotropical plants of
Laeliinae (Orchidaceae) occuring in Brazil. Lankesteriana 7:22–27
Almeida PRM, van den Berg C, Go
´es-Neto A (2014) Epulorhiza amonilioides sp. nov.: a new
anamorphic species of orchid mycorrhiza from Brazil. Neodiversity 7:1–10
12 Biogeography and Ecology of Tulasnellaceae 257
Andersen TF (1990) A study of hyphal morphology in the form genus Rhizoctonia. Mycotaxon
37:25–46
Atala C, Pereira G, Romero C, Mu~
noz-Tapia L, Vargas R, Suz LM (2015) Orchidioid fungi of the
form-genus Rhizoctonia associated with the roots of Chloraea cuneata Lindl. from Araucaria,
Chile. Gayana Bot 72:145–148
Athipunyakom P, Manoch L, Piluck C, Artjariyasripong S, Tragulrung S (2004a) Mycorrhizal
fungi from Spathoglottis plicata and the use of these fungi to germinate seeds of S. plicata
in vitro. Kasetsart J (Nat Sci) 37:83–93
Athipunyakom P, Manoch L, Piluek C (2004b) Isolation and identification of mycorrhizal fungi
from eleven terrestrial orchids. Kasetsart J (Nat Sci) 38:216–228
Bailarote BC, Lievens B, Jacquemyn H (2012) Does mycorrhizal specificity affect orchid decline
and rarity? Am J Bot 99:1655–1665
Balestrini R, Nerva L, Sillo F, Girlanda M, Perotto S (2014) Plant and fungal gene expression in
mycorrhizal protocorms of the orchid Serapias vomeracea colonized by Tulasnella calospora.
Planta 239:1337–1349
Bandoni RJ, Oberwinkler F (1982) Stilbotulasnella: a new genus in the Tulasnellaceae. Can J Bot
60:875–1879
Bateman RM, Rudall PJ, Bidartondo MI, Cozzolino S, Tranchida-Lombardo V, Carine MA,
Moura M (2014) Speciation via floral heterochrony and presumed mycorrhizal host switching
of endemic butterfly orchids on the Azorean archipelago. Am J Bot 101:979–1001
Bates RJ, Weber JZ (1990) Orchids of South Australia. Caudell AB, Government Printer, South
Australia, Adelaide
Bernard N (1899) Sur la germination de Neottia nidus-avis. C R Acad Sci 128:1253–1255
Bernard N (1909) L’e
´volution dans la symbiose des orchide
´es et leurs champignons commensaux.
Ann Sci Nat Bot 9:1–196
Bidartondo MI, Duckett JG (2010) Conservative ecological and evolutionary patterns in liverwort-
fungal symbioses. Proc R Soc B 277:485–492
Bidartondo MI, Bruns TD, Weiß M, Se
´rgio C, Read DJ (2003) Specialized cheating of the
ectomycorrhizal symbiosis by an epiparasitic liverwort. Proc R Soc Lond B 270:835–842
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the
dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and
trees. Proc R Soc Lond B 271:1799–1806
Binder M, Hibbett DS, Larsson KH, Larsson E, Langer E, Langer G (2005) The phylogenetic
distribution of resupinate forms across the major clades of mushroom-forming fungi
(Homobasidiomycetes). Syst Biodivers 3:113–157
Boddington M, Dearnaley JDW (2009) Morphological and molecular identification of fungal
endophytes from roots of Dendrobium speciosum. Proc R Soc Queensland 114:13–17
Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K (2007) Diversity of
mycorrhizal fungi of terrestrial orchids: compatilbility webs, brief encounters, lasting relation-
ships and alien invasions. Mycol Res 111:51–61
Bougoure JJ, Ludwig M, Brundrett MC, Grierson PF (2009a) Identity and specificity of the fungi
forming mycorrhizas with rare, mycoheterotrophic Rhizanthella gardneri (Orchidaceae).
Mycol Res 113:1097–1106
Bougoure JJ, Brundrett MC, Grierson PF (2009b) Carbon and nitrogen supply to the underground
orchid Rhizanthella gardneri. New Phytol 186:947–956
Bourdot H, Galzin A (1927) Hyme
´nomyce
`tes de France. Hete
´robasidie
´s—Homobasidie
´s
gymnocarpes. Soc Myc France, Sceaux
Bresadola J (1903) Fungi polonici a cl. Viro B. Eichler lecti. Ann Mycol 1:65–131
Brundrett MC (2007) Scientific approaches to Australian temperate terrestrial orchid conservation.
Aust J Bot 55:293–307
Bruns TD, Szaro TM, Gardes M et al (1998) A sequence database for the identification of
ectomycorrhizal basidiomycetes by phylogenetic analyses. Mol Ecol 7:257–272
258 F. Oberwinkler et al.
Burgeff H (1909) Die Wurzelpilze der Orchideen, ihre Kultur und ihr Leben in der Pflanze. Gustav
Fischer, Jena
Burgeff H (1932) Saprophytismus und Symbiose, Studien an tropischen Orchideen. Gustav
Fischer, Jena
Burgeff H (1936) Samenkeimung der Orchideen und Entwicklung ihrer Keimpflanzen. Gustav
Fischer, Jena
Cevallos S, Sa
´nchez-Rodrı
´guez A, Decock C, Declerck S, Suarez JP (2016) Are there keystone
mycorrhizal fungi associated to tropical epiphytic orchids? Mycorrhiza. doi:10.1007/s00572-
016-0746-8
Chase MW, Freudenstein JV, Cameron KM (2003) DNA data and Orchidaceae systematics: a new
phylogenetic classification. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ (eds) Orchid
conservation. Nat Hist Publ, Kota Kinabalu, pp 69–89
Christiansen MP (1959) Danish resupinate fungi. I. Ascomycetes and Heterobasidiomycetes.
Dansk Bot Arkiv 19:20–34
Chutima R, Dell B, Lumyong S (2011) Effects of mycorrhizal fungi on symbiotic seed germina-
tion of Pecteilis susannae (L.) Rafin. (Orchidaceae), a terrestrial orchid in Thailand. Symbiosis
53:149–156
Clarke JT, Warnock RCM, Donoghue PCJ (2011) Establishing a time-scale for plant evolution.
New Phytol 192:266–301
Cooper ED, Henwood MJ, Brown EA (2012) Are the liverworts really that old? Cretaceous origins
and Cenozoic diversifications in Lepidoziaceae reflect a recurrent theme in liverwort evolution.
Biol J Linn Soc 107:425–441
Cowden CC, Shefferson RP (2013) Diversity of root-associated fungi of mature Habenaria
radiata and Epipactis thunbergii colonizing manmade wetlands in Hiroshima prefecture,
Japan. Mycoscience 54:327–334
Crous PW, Wingfield MJ, Guarro J et al (2015) Fungal planet description sheets: 320–370.
Persoonia 34:167–266
Cruz Blası
´J (2007) Colonizacio
´n micorrı
´zica y diversidad de hongos micorrı
´zicos de algunas
especies de orquı
´deas epifitas tropicales en el Sureste de Chiapas, Me
´xico. Tesis para Maestro
en Ciencias, Montecillo, Texococo, Edo. De Me
´xico
Cruz DJ, Sua
´rez JP, Kottke I, Piepenbring M, Oberwinker F (2011) Defining species in Tulasnella
by correlating morphology and nrDNA ITS-5.8s sequence data of basidiomata from a tropical
Andean forest. Mycol Prog 10:229–238
Cruz DJ, Sua
´rez JP, Kottke I, Piepenbring M (2014) Cryptic species revealed by molecular
phylogenetic analysis of sequences obtained from basidiomata of Tulasnella. Mycologia 106
(4):708–722
Cruz DJ, Sua
´rez JP, Piepenbring M (2016) Morphological revision of Tulasnellaceae, with two
new species of Tulasnella and new records of Tulasnella spp. for Ecuador. Nova Hedwigia
102:279–338
Currah RS, Sherburne R (1992) Septal ultrastructure of some fungal endophytes from boreal
orchid mycorrhizas. Mycol Res 96:583–587
Currah RS, Zelmer C (1992) A key and notes for the genera of fungi mycorrhizal with orchids and
a new species in the genus Epulorhiza. Rep Tottori Mycol Inst 30:43–59
Currah RS, Sigler L, Hambleton S (1987) New records and new taxa of fungi from mycorrhizae of
terrestrial orchids of Alberta. Can J Bot 65:2473–2482
Currah RS, Hambleton S, Smerciu EA (1988) Mycorrhizae and mycorrhizal fungi of Calypso
bulbosa. Am J Bot 75:739–752
Currah RS, Smerciu EA, Hambleton S (1990) Mycorrhizae and mycorrhizal fungi of boreal
species of Platanthera and Coeloglossum (Orchidaceae). Can J Bot 68:1171–1181
Currah RS, Zettler LW, McInnis TM (1997) Epulorhiza inquilina sp. nov. from Platanthera
(Orchidaceae) and a key to Epulorhiza species. Mycotaxon 61:335–342
12 Biogeography and Ecology of Tulasnellaceae 259
Da Silva Coelho I, Vieira de Queiroz M, Dutra Costa M, Kasuya MCM, Fernandes de Arau
´ji E
(2010) Production and regeneration of protoplasts from orchid mycorrhizal fungi Epulorhiza
repens and Ceratorhiza sp. Braz Arch Biol Technol 53:153–159
Dan Y, Yu X-M, Guo S-X, Meng Z-X (2012) Effects of forty-two strains of orchid mycorrhizal
fungi on growth of plantlets of Anoectochilus roxburghii. Afr J Microbiol Res 6:1411–1416
De Long JR, Swarts ND, Dixon KW, Egerton-Warburton LM (2013) Mycorrhizal preference
promotes habitat invasion by a native Australien orchird: Microtis media. Ann Bot
111:409–418
Dearnaley JDW (2007) Further advances in orchid mycorrhizal research. Mycorrhiza 17:475–486
Dijk E, Eck ND (1995) Effects of mycorrhizal fungi on in vitro nitrogen response of some Dutch
indigenous orchid species. Can J Bot 73:1203–1211
Dijk E, Willems JH, van Andel J (1997) Nutrients responses as a key factor to the ecology of
orchid species. Acta Bot Neerl 46:229–363
Ding R, Chen X-H, Zhang L-J, Yu X-D, Qu B, Duan R, Xu Y-F (2014) Identity and specificity of
Rhizoctonia-like fungi from different populations of Liparis japonica (Orchidaceae) in
Northeast China. PLoS One 9(8):e105573
Do
gan HH, Kurt F (2016) New macrofungi records from Turkey and macrofungal diversity of
Pozantı-Adana. Turk J Bot 40:209–217
Donk MA (1972) The Heterobasidiomycetes: a reconnaissance. I. Proc K Ned Akad Wet Ser C
75:365–375
Drechsler C (1969) A Tulasnella parasitic on Amoeba terricola. Am J Bot 56:1217–1220
Due~
nas M (1996) Tremellales and Tulasnellales of Menorca (Balearic Islands, Spain). Nova
Hedwigia 62:467–476
Due~
nas M (2001) Iberian intrahymenial Platygloeales, Tremellales and Tulasnellales. Nova
Hedwigia 72:441–459
Due~
nas M (2005) New and interesting Iberian heterobasidiomycetous fungi. 1. Nova Hedwigia
81:177–198
Eom A-H (2012) Identification of orchid mycorrhizal fungi isolated from five species of terrestrial
orchids in Korea. Korean J Mycol 40:132–135
Eom A-H (2015) Identification of orchid mycorrhizal fungi isolated from terrestrial orchids in
Mt. Hambaek, Korea. Korean J Mycol 43:129–132
Eom A-H, Kim D-S (2013) Identification of orchid mycorrhizal fungi isolated from Epipactis
thunbergii in Korea. Korean J Mycol 41:9–13
Ercole E, Adamo M, Rodda M, Gebauer G, Girlanda M, Perotto S (2014) Temporal variation in
mycorrhizal diversity and carbon and nitrogen stable isotope abundance in the wintergreen
meadow orchid Anacamptis morio mycorrhiza. New Phytol 205:1308–1319
Esfeld K, Hensen I, Wesche K, Jakob SS, Tischew S, Blattner FR (2008) Molecular data indicate
multiple independent colonizations of former lignite mining areas in Eastern Germany by
Epipactis palustris (Orchidaceae). Biodivers Conserv 17:2441–2453
Ferna
´ndez di Pardo A, Chiocchio VM, Barrera V, Colombo RP, Martinez AE, Gasoni L, Godeas
AM (2015) Mycorrhizal fungi isolated from native terrestrial orchids of pristine regions in
Co
´rdoba (Argentina). Rev Biol Trop 63:275–283
Filipello Marchisio V, Berta G, Fontana A, Marzetti Mannina F (1985) Endophytes of wild orchids
native to Italy: their morphology, caryology, ultrastructure and cytochemical characterization.
New Phytol 100:623–641
Fracchia S, Aranda-Rickert A, Flachsland E, Terada G, Sede S (2014) Mycorrhizal compatibility
and symbiotic reproduction of Gavilea australis, an endangered terrestrial orchid from South
Patagonia. Mycorrhiza 24:627–634
Frericks J (2014) The effects of endophytic fungi of NZ terrestrial orchids: developing methods for
conservation. MSc thesis, Victoria University of Wellington
Garnica S, Riess K, Sch€
on ME, Oberwinkler F, Setaro SD (2016) Divergence times and phyloge-
netic patterns of Sebacinales, a highly diverse and widespread fungal lineage. PLoS One 11(3):
e0149531. doi:10.1371/journal.pone.0149531
260 F. Oberwinkler et al.
Girlanda M, Segreto R, Cafasso D, Liebel HB, Rodda M, Ercole E, Cozzolino S, Gebauer G,
Perotto S (2011) Photosynthetic mediterranean meadow orchids feature partial
mycoheterotrophy and specific mycorrhizal associations. Am J Bot 98:1148–1163
Givnish TJ, Spalnik D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, Iles WJD, Clements MA, Arroyo
MTK, Leebens-Mack J, Endara L, Kriebel R, Neubig KM, Whitten WM, Williams NH,
Cameron KM (2015) Orchid phylogenomics and multiple drivers of their extraordinary
diversification. Proc R Soc B 282:20151553
Gonza
´lez Garcia V, Portal Onco MA, Rubio Susan V (2006) Biology and systematics of the form
genus Rhizoctonia. Span J Agric Res 4:55–79
Go
´nzalez D, Rodriguez-Carres M, Boekhout T, Stalpers J, Kuramae EE, Nakatani AK, Vilgalys R,
Cubeta MA (2016) Phylogenetic relationships of Rhizoctonia fungi within the Cantharellales.
Fungal Biol 120:603–619
Greslebin GA, Rajchenberg M (2001) The genus Tulasnella with a new species in the Patagonian
Andes forests of Argentina. Mycol Res 105:1149–1151
Hadley G (1970) Non-specificity of symbiotic infection in orchid mycorrhiza. New Phytol
69:1015–1023
Hauerslev K (1989) Two new tremellaceous fungi from Denmark. Opera Bot 100:113–114
Hayakawa S, Uetake Y, Ogoshi A (1999) Identification of symbiotic Rhizoctonias from naturally
occurring protocorms and roots of Dactylorhiza aristata (Orchidaceae). Jour Fac Agric
Hokkaido Univ 69:129–141
Herrera H, Valadares R, Contreras D, Bashan Y, Arriagada C (2016) Mycorrhizal compatibility
and symbiotic seed germination of orchids from the coastal range and Andes in south Central
Chile. Mycorrhiza. doi:10.1007/s00572-016-0733-0
Hibbett D, Thorn RG (2001) Basidiomycota: Homobasidiomycetes. In: McLaughlin DJ,
McLaughlin EG, Lemke PA (eds) The Mycota. VIIB. Systematics and Evolution. Springer,
Berlin, pp 121–168
Hibbett DS, Binder M, Bischoff JF et al (2007) A higher-level phylogenetic classification of the
fungi. Mycol Res 111:509–547
Hibbett DS, Bauer R, Binder M, Giachini AJ, Hosaka K, Justo A, Larsson E, Larsson KH, Lawrey
JD, Miettinen O, Nagy LG, Nilsson RH, Weiß M, Thorn RG (2014) Agaricomycetes. In:
McLaughlin DJ, Spatafora JW (eds) Systematics and evolution. The Mycota XII Part A, 2nd
edn. Springer, Berlin, pp 373–429
Hjortstam K (1978) Wood inhabiting fungi in the nature reserve Raback on mount Kinnekulle
Sweden. Sven Bot Tidskr 72:321–326
Huang F, Zhang C (2015) Diversity, host- and habitat-preferences on the fungi communities from
the roots of Cymbidium spp. at two sites in China. J Anim Plant Sci 25:270–277
Illye
´s (2011) Hazai la
´pi kosborfajok aktı
´vve
´delme
´t megalapozo
´e
´l€
ohelyi e
´s laborato
´riumi
vizsga
´latok, kül€
on€
os tekintettel a hagymaburok (Liparis loeselii)e
´sat
€
ozegorchidea
(Hammarbya paludosa) fajokra. Doctoral thesis, Budapest
Illye
´s Z, Rudnoy S, Bratek Z (2005) Aspects of in situ, in vitro germination and mycorrhizal
partners of Liparis loeselii. Acta Biol Szeged 49:137–139
Illye
´s Z, Hala
´sz K, Rudno
´y S, Ouanphanivanh N, Garay T, Bratek Z (2009) Changes in the
diversity of mycorrhizal fungi of orchids as a function of the water supply of the habitat. J Appl
Bot Food Qual 83:28–36
Illye
´s Z, Ouanphanivanh N, Rudno
´y S, Orcza
´n K, Bratek Z (2010) The most recent results on
orchid mycorrhizal fungi in Hungary. Acta Biol Hung 61(Suppl):88–96
Jacquemyn H, Honnay O, Cammue BPA, Brys R, Lievens B (2010) Low specificity and nested
subset structure characterize mycorrhizal associations in five closely related species of the
genus Orchis. Mol Ecol 19:4086–4095
Jacquemyn H, Brys R, Cammue BPA, Honnay O, Lievens B (2011a) Mycorrhizal associations and
reproductive isolation in three closely related Orchis species. Ann Bot 107:347–356
12 Biogeography and Ecology of Tulasnellaceae 261
Jacquemyn H, Merckx V, Brys R, Tyteca D, Cammue BPA, Honnay O, Lievens B (2011b)
Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity
in the genus Orchis (Orchidaceae). New Phytol 192:518–528
Jacquemyn H, Deja A, De hert K, Cachapa Bailarote B, Lievens B (2012) Variation in mycorrhizal
associations with tulasnelloid fungi among populations of five Dactylorhiza species. PLoS One
7(8):e42212. doi:10.1371/journal.pone.0042212
Jacquemyn H, Brys R, Merckx VSFT, Waud M, Lievens B, Wiegand T (2014) Coexisting orchid
species have distinct mycorrhizal communities and display strong spatial segregation. New
Phytol 202:616–627
Jacquemyn H, Brys R, Waud M, Busschaert P, Lievens B (2015a) Mycorrhizal networks and
coexistence in species-rich orchid communities. New Phytol 206:1127–1134
Jacquemyn H, Waud M, Merckx VSFT, Lievens B, Brys R (2015b) Mycorrhizal diversity, seed
germination and long-term changes in population size across nine populations of the terrestrial
orchid Neottia ovata. Mol Ecol 24:3269–3280
Jacquemyn H, Waud M, Lievens B, Brys R (2016a) Differences in mycorrhizal communities
between Epipactis palustris,E. helleborine and its presumed sister species E. neerlandica. Ann
Bot 118:105–114
Jacquemyn H, Waud M, Merckx VSFT, Brys R, Tyteca D, Hedre
´n M, Lievens B (2016b) Habitat-
driven variation in mycorrhizal communities in the terrestrial orchid genus Dactylorhiza. Sci
Rep 6:37182. doi:10.1038/srep37182
Jiang WM, Yang GM, Zhang CL, Fu CX (2011) Species composition and molecular analysis of
symbiotic fungi in roots of Changnienia amoena (Orchidaceae). Afr J Microbiol Res
5:222–228
Jiang JH, Lee Y-I, Cubeta MA, Chen L-C (2015) Characterization and colonization of
endomycorrhizal Rhizoctonia fungi in the medicinal herb Anoectochilus formosanus
(Orchidaceae). Mycorrhiza 25:431–445
Jin H, Xu Z-X, Chen J-H, Han S-F, Ge S, Luo Y-B (2009) Interaction between tissue-cultured
seedlings of Dendrobium officinale and mycorrhizal fungus (Epulorhiza sp.) during symbiotic
culture. Chin J Plant Ecol 33:433–441
Kartzinel TR, Trapnell DW, Shefferson RP (2013) Highly diverse and spatially heterogeneous
mycorrhizal symbiosis in a rare epiphyte is unrelated to broad biogeographic or environmental
features. Mol Ecol 22:5949–5961
Keel BG, Zettler LW, Kaplin BA (2011) Seed germination of Habenaria repens (Orchidaceae) in
situ beyond its range, and its potential for assisted migration imposed by climate change.
Castanea 76:43–54
Khamchatra N, Dixon K, Chayamarit K, Apisitwanich S, Tantiwiwat S (2016a) Using in situ
baiting technique to isolate and identify endophytic and mycorrhizal fungi from seed of a
threatened epiphytic orchid, Dendrobium friedericksianum Rchb. f. (Orchidaceae). Agric Nat
Resour 50:8–13
Khamchatra N, Dixon KW, Tantiwiwat S, Piapukiew J (2016b) Symbiotic seed germination of an
endangered epiphytic slipper orchid, Paphiopedilum villosum (Lindl.) Stein. from Thailand. S
Afr J Bot 104:76–81
Kohout P, Te
ˇs
ˇitelova
´T, Roy M, Vohnı
´k M, Jersa
´kova
´J (2013) A diverse fungal community
associated with Pseudorchis albida (Orchidaceae) roots. Fungal Ecol 6:50–64
Kottke I, Sua
´rez JP (2009) Mutualistic, root-inhabiting fungi of orchids identification and func-
tional types. In: Pridgeon AM, Sua
´rez JP (eds) Proceedings of the second scientific conference
on Andean Orchids. Universidad Te
´cnica Particular de Loja, Loja, Ecuador, pp 84–99
Kottke I, Beiter A, Weiß M, Haug I, Oberwinkler F, Nebel M (2003) Heterobasidiomycetes form
symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while
Aneura pinguis (Metzgeriales) is associated with a Tulasnella species. Mycol Res 107:957–968
Kottke I, Haug I, Setaro S, Sua
´rez JP, Weiß M, Preußing M, Nebel M, Oberwinkler F (2008)
Guilds of mycorrhizal fungi and their relation to trees, ericads, orchids and liverworts in a
neotropical mountain rain forest. Basic Appl Ecol 9:13–23
262 F. Oberwinkler et al.
Kottke I, Setaro S, Haug I, Herrera P, Cruz D, Fries A, Gawlik J, Homeier J, Werner FA,
Gerique A, Sua
´rez JP (2013) Mycorrhiza networks promote biodiversity and stabilize the
tropical mountain rain forest ecosystem: perspectives for understanding complex communities.
In: Bendix J, Beck E, Bra
¨uning A, Makeschin F, Mosandl R, Scheu S, Wilcke W (eds)
Ecosystem services, biodiversity and environmental change in a tropical mountain ecosystem
of South Ecuador. Springer, Berlin, pp 187–203
Krause C, Garnica S, Bauer R, Nebel M (2011) Aneuraceae (Metzgeriales) and tulasnelloid fungi
(Basidiomycota)—a model for early steps in fungal symbiosis. Fungal Biol 115:839–851
Kristiansen KA, Tayler DL, Kjøller R, Rasmussen N, Rosendahl S (2001) Identification of
mycorrhizal fungi from single pelotons of Dactylorhiza majalis (Orchidaceae) using single-
strand conformation polymorphism and mitochondrial ribosomal large subunit DNA
sequences. Mol Ecol 10:2089–2093
Kristiansen KA, Freudenstein JV, Rasmussen FN, Rasmussen HN (2004) Molecular identification
of mycorrhizal fungi in Neuwiedia veratrifolia (Orchidaceae). Mol Phylogenet Evol
33:251–158
Kunttu P, Kulju M, Kotiranta H (2015) Contributions to the Finnish aphyllophoroid funga
(Basidiomycota): new and rare species. Czech Mycol 67:137–156
Lee S-S, You JH (2000) Identification of the orchid mycorrhizal fungi isolated from the roots of
Korean native orchid. Mycobiology 28:17–26
Lee S-S, Lee J-G, Lee J-W et al (2001) Effect of orchid symbiotic fungus on young plant growth of
Cymbidium misericores and C. rubrigemmum in greenhouse. J Korean Hortic Sci 42:223–226
Li B, Tang MJ, Tang K, Zhao LF, Guo SX (2012) Screening for differentially expressed genes in
Anoectochilus roxburghii (Orchidaceae) during symbiosis with the mycorrhizal fungus
Epulorhiza sp. Sci China Life Sci 55:164–171
Liebel HT, Bidartondo M, Gebauer M (2015) Are carbon and nitrogen exchange between fungi
and the orchid Goodyera repens affected by irradiance? Ann Bot 115:251–261
Linde CC, Phillips RD, Crisp MD, Peakall R (2013) Congruent species delineation of Tulasnella
using multiple loci and methods. New Phytol 201:6–12
Lopez SE (1987) Contribution to the study of Argentina xylophilous fungi III. Basidiomycetous
jelly fungi. Darwin 28:271–282
Lowy B (1964) A new genus of the Tulasnellaceae. Mycologia 56:696–700
Ma M, Tan TK, Wong SM (2003) Identification and molecular phylogeny of Epulorhiza isolates
from tropical orchids. Mycol Res 107:1041–1049
Martin GW (1939) New or noteworthy fungi from Panama and Colombia. III. Mycologia
31:239–249
Martos F, Munoz F, Pailler T, Kottke I, Gonneau C, Selosse MA (2012) The role of epiphytism in
architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol
Ecol 21:5098–5109
Massey EE, Zettler LW (2007) An expanded role for in vitro symbiotic seed germination as a
conservation tool: two case studies in North America (Platanthera leucophaea and
Epidendrum nocturnum). Proc 3rd Int Orchid Conserv Congr. Lankesteriana 7:303–308
Masuhara G, Katsuya K (1994) In situ and in vitro specificity between Rhizoctonia spp. and
Spiranthes sinensis (Persoon) Ames var. amoena (M. Bieberstein) Hara. New Phytol
127:711–718
McCormick MK, Jacquemyn H (2014) What constrains the distribution of orchid populations?
New Phytol 202:392–400
McCormick MK, Whigham DF, O’Neill J (2004) Mycorrhizal diversity in photosynthetic
terrestrial orchids. New Phytol 163:425–438
McCormick MK, Whigham DF, Sloan D, O’Malley K, Hodkinson B (2006) Orchid—fungus
fidelity: a marriage meant to last? Ecology 87:903–911
McCormick MK, Taylor DL, Juhaszova K, Burnet RK Jr, Whigham DF, O’Neill JP (2012)
Limitations on orchid recruitment: not a simple picture. Mol Ecol 21:1511–1523
12 Biogeography and Ecology of Tulasnellaceae 263
McNeill J, Turland NJ (2011) Major changes to the Code of Nomenclature—Melbourne, July
2011. Taxon 60:14959–11497
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter D, Hawksworth DL, Herendeen PS,
Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GF, Wiersema, JH, Turland
NJ, Members of the editorial committee (2012) International Code of Nomenclature for algae,
fungi, and plants (Melbourne Code). Koeltz Sci Books, K€
onigstein, pp 1–14
Milligan MJ, Williams PG (1988) The mycelial relationship of multinucleate rhizoctonias from
non-orchids with Microtis (Orchidaceae). New Phytol 108:205–209
Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, Porter TM,
Margaritescu S, Weiß M, Garnica S, Danell E, Langer G, Langer E, Larsson E, Larsson
K-H, Vilgalys R (2006) The cantharelloid clade: dealing with incongruent gene trees and
phylogenetic reconstruction methods. Mycologia 98:937–948
Moore RT (1987) The genera of Rhizoctonia-like fungi: Ascorhizctonia,Ceratorhiza gen. nov,
Epulorhiza gen. nov., Moniliopsis, and Rhizoctonia. Mycotaxon 29:91–99
Mordue JEM, Currah RS, Bridge PD (1989) An integrated approach to Rhizoctonia taxonomy:
cultural, biochemical and numerical techniques. Mycol Res 92:78–90
Mosquera-Espinosa AT, Bayman P, Otero JT (2010) Ceratobasidium como hongo micorrı
´zico de
orquı
´deas en Colombia. Acta Agro 59:316–326
Mujica MI, Saez N, Cisternas M, Manzano M, Armesto JJ, Pe
´rez F (2016) Relationship between
soil nutrients and mycorrhizal associations of two Bipinnula species (Orchidaceae) from
Central Chile. Ann Bot. doi:10.1093/aob/mcw082
Nebel M, Kreier HP, Preußing M, Weiß M, Kottke I (2004) Symbiotic fungal associations of
liverworts are the possible ancestors of mycorrhizae. In: Agerer R, Piepenbring M, Blanz P
(eds) Frontiers in Basidiomycote mycology. IHW-Verlag, Eching, pp 339–360
Nogueira RE, van den Berg POL, Kasuya MCM (2014) Isolation and molecular characterization
of Rhizoctonia-like fungi associated with orchid roots in the Quadrila
´tero Ferrı
´fero and Zona
da Mata regions of the state of Minas Gerais, Brazil. Acta Bot Brasilica 28:298–300
Nontachaiyapoom S, Sasirat S, Manoch L (2010) Isolation and identification of Rhizoctonia-like
fungi from roots of three orchid genera, Paphiopedilum,Dendrobium, and Cymbidium,
collected in Chiang Rai and Chiang Mai provinces of Thailand. Mycorrhiza 20:459–471
Nontachaiyapoom S, Sasirat S, Manoch L (2011) Symbiotic seed germination of Grammatophyllum
speciosum Blume and Dendrobium draconis Rchb.f., native orchids of Thailand. Sci Hortic
130:303–308
Nouhra E, Urcelay C, Longo S, Tedersoo L (2013) Ectomycorrhizal fungal communities
associated to Nothofagus species in Northern Patagonia. Mycorrhiza 23:487–496
Nurfadilah S, Swarts ND, Dixon KW, Lambers H, Merritt DJ (2013) Variation in nutrient-
acquisition patterns by mycorrhizal fungi of rare and common orchids explains diversification
in a global biodiversity hotspot. Ann Bot 111:1233–1241
Oberwinkler F (2012) Mykologie am Lehrstuhl Spezielle Botanik und Mykologie der Universita
¨t
Tübingen, 1974–2011. Andrias 19:23–110, additional 16 plates
Oberwinkler F, Riess K, Bauer R, Kirschner R, Garnica S (2013) Taxonomic re-evaluation of the
Ceratobasidium-Rhicotonia complex and Rhizoctonia butinii, a new species attacking spruce.
Mycol Prog 12:763–776
Ogura-Tsujita Y, Yokoyama J, Miyoshi K, Yukawa T (2012) Shifts in mycorrhizal fungi during
the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am J Bot
99:1158–1176
Øien DI, O’Neill JP, Whigham DF, McCormick MK (2008) Germination ecology of the boreal-
alpine terrestrial orchid Dactylorhiza lapponica (Orchidaceae). Ann Bot Fenn 45:161–172
Olive LS (1946) New or rare Heterobasidiomycetes from North Carolina II. J Elisha Mitchell Sci
Soc 62:65–71
Olive LS (1957) Tulasnellaceae of Tahiti. A revision of the family. Mycologia 49:663–679
Ordynets O (2012) New records of corticioid fungi with heterobasidia from Ukraine. Turk J Bot
36:590–602
264 F. Oberwinkler et al.
Ortega-Larrocea MP, Rangel-Villafranco M (2007) Fungus-assisted reintroduction and long-term
survival of two Mexican terrestrial orchids in the natural habitat. Lankesteriana 7:317–321
Otero JT, Ackerman JD, Bayman P (2002) Diversity and host specificity of endophytic Rhizoctonia-
like fungi from tropical orchids. Am J Bot 89:1852–1858
Ouanphanivanh N, Illy
´es Z, Rudno
´y S, Bratek Z (2007) Orchid mycorrhizal fungal diversity of
Orchis militaris habitats. Ta
´j€
okolo
´giai Lapok 5:325–332
Ovando I, Damon A, Bello R, Ambrosio D, Albores V, Adriano L, Salvador M (2005) Isolation of
endophytic fungi and their mycorrhizal potential for the tropical epiphytic orchids Cattleya
skinneri,C. aurantiaca and Brassvola nodosa. Asian J Plant Sci 4:309–315
Pandey M, Sharma J, Taylor D, Yadon VL (2013) A narrowly endemic photosynthetic orchid is
non-specific in its mycorrhizal associations. Mol Ecol 22:2341–2354
Pearson AA (1928) New British Heterobasidiae. Trans Br Mycol Soc 13:69–74
Pecoraro L, Girlanda M, Kull T, Perini C, Perotto S (2012) Analysis of fungal diversity in Orchis
tridentata Scopoli. Dent Eur J Biol 7:850–857
Pecoraro L, Girlanda M, Kull T, Perini C, Perotto S (2013) Fungi from the roots of the terrestrial
photosynthetic orchid Himantoglossum adriaticum. Plant Ecol Evol 146:145–152
Pecoraro L, Girlanda M, Liu Z-J, Huang L, Perotto S (2015) Molecular analysis of fungi associated
with the Mediterranean orchid Ophrys bertolonii Mor. Ann Microbiol 65:2001–2007
Pellegrino G, Luca A, Bellusci F (2014) Relationships between orchid and fungal biodiversity:
mycorrhizal preferences in Mediterranean orchids. Plant Biosyst 3504:1–10
Pereira MC (2009) Diversidade e especificidade micorrı
´zica em orquı
´deas do ge
ˆnera Epidendrum.
Universidade Federal de Vic¸osa, Vic¸osa
Pereira OL, Rollemberg CL, Borges AC, Matsuoka K, Kasuya MCM (2003) Epulorhiza
epiphytica sp. nov. isolated from mycorrhizal roots of epiphytic orchids in Brazil. Mycoscience
44:153–155
Pereira OL, Kasuya MCM, Borges AC, Fernandes de Arau
´jo E (2005a) Morphological and
molecular characterization of mycorrhizal fungi isolated from neotropical orchids in Brazil.
Can J Bot 83:54–65
Pereira OL, Kasuya MCM, Rollemberg CL, Chaer GM (2005b) Isolamento e identificac¸~
ao de
fungos micorrı
´zicos rizoctonio
´ides associados a tre
ˆs espe
´cies de orquı
´deas epı
´fitas neotropicais
no Brasil. R Bras Ci Solo 29:191–197
Pereira OL, Kasuya MCM, Rollemberg CL, Borges AC (2005c) Induc¸~
ao in vitro da germinac¸~
ade
sementes de Oncidium flexuosum (Orchidaceae) por fungos micorrı
´zicos rizoctonio
´ides. R
Bras Ci Solo 29:199–206
Pereira MC, Pereira OL, Costa MD, Rocha RB, Kasuya MCM (2009) Diversidade de fungos
micorruı
´zicos Epulorhiza spp. isolados de Epidendrum secundum (Orchidaceae). Rev Bras
Cienc Solo 33:1187–1197
Pereira MC, Torres DP, Rodrigues Guimaraes FA, Pereira OL, Kasuya MCM (2011a) Seed
germination and protocorm development of Epidendrum secundum Jacq. (Orchidaceae) in
association with Epulorhiza mycorrhizal fungi. Acta Bot Brasilica 25:534–541
Pereira MC, Moreira Vieira N, To
´tala MR, Kasuya MCM (2011b) Total fatty acid composition in
the characterization and identification of orchid mycorrhizal fungi Epulorhiza spp. Rev Bras
Cienc Solo 35:1159–1165
Pereira G, Romero C, Suz LM, Atala C (2014a) Essential mycorrhizal partners of the endemic
Chilean orchids Chloraea collicensis and C. gavilu. Flora 209:95–99
Pereira MC, da Silva Coelho I, da Silva Valadares RB, Oliveira SF, Bocayuva M, Pereira OL,
Ferandes Arau
´jo E, Kasuya MCM (2014b) Morphological and molecular characterization of
Tulasnella spp. fungi isolated from the roots of Epidendrum secundum, a widespread Brazilian
orchid. Symbiosis 62:111–121
Pereira MC, Rocha DI, Veloso TGR, Pereira OL, Francino DMT, Strozi Alves Meira RM, Kasuya
MCM (2015) Characterization of seed germination and protocorm development of
Cyrtopodium glutiniferum (Orchidaceae) promoted by mycorrhizal fungi Epulorhiza spp.
Acta Bot Brasilica 29:567–574
12 Biogeography and Ecology of Tulasnellaceae 265
Perkins AJ, Masuhara G, McGee PA (1995) Specificity of the associations between Microtis
parviflora (Orchidaceae) and its mycorrhizal fungi. Aust J Bot 43:85–91
Peterson RL, Currah RS (1990) Synthesis of mycorrhizae between protocorms of Goodyera repens
(Orchidaceae) and Ceratobasidium cereale. Can J Bot 68:1117–1125
Phillips RD, Barrett MD, Dixon KW, Hopper SD (2011) Do mycorrhizal symbioses cause rarity in
orchids? J Ecol 99:858–869
Phillips RD, Peakall R, Hutchinson MF, Linde CC, Xu T, Dixon KW, Hopper SD (2014)
Specialized ecological interactions and plant species rarity: the role of pollinators and mycor-
rhizal fungi across multiple spatial scales. Biol Conserv 169:285–295
Polemis E, Roberts P, Dimou DM, Zervakis GI (2016) Heterobasidiomcetous fungi form Aegean
Islands (Greece): new annotated records for a neglected group. Plant Biosyst 150:295–303
Porras-Alfaro A, Bayman P (2007) Mycorrhizal fungi of Vanilla: diversity, specificity and effects
on seed germination and plant growth. Mycologia 99:510–5225
Pressel S, Bidartondo M, Ligrone R, Duckett J (2010) Fungal symbioses in bryophytes: new
insights in the twenty first century. Phytotaxa 9:238–253
Preußing M, Nebel M, Oberwinkler F, Weiß M (2010) Diverging diversity patterns in the
Tulasnella (Basidiomycota, Tulasnellales) mycobionts of Aneura pinguis (Marchantiophyta,
Metzgeriales) from Europe and Ecuador. Mycorrhiza 20:147–159
Rafter M, Yokoya K, Shofield EJ, Zettler LW, Sarasan V (2016) Non-specific symbiotic germi-
nation of Cynorkis purpurea (Thouars) Kraezl., a habitat-specific terrestrial orchid from the
Central Highlands of Madagascar. Mycorrhiza 26:541–552
Rasmussen HN (2002) Recent developments in the study of orchid mycorrhiza. Plant Soil
244:149–163
Rasmussen H, Rasmussen FN (1991) Climatic and seasonal regulation of seed plant establishment
in Dactylorhiza majalis inferred from symbiotic experiments in vitro. Lindleyana 6:221–227
Rasmussen H, Rasmussen FN (2007) Trophic relationships in orchid mycorrhiza – diversity and
implications for conservation. Lankesteriana 7:334–341
Rasmussen HN, Dixon KW, Jersa
´kova
´J, Te
ˇs
ˇitelova
´T (2015) Germination and seedling estab-
lishment in orchids: a complex of requirements. Ann Bot 116:391–402
Richardson KA, Peterson RL, Currah RS (1992) Seed reserves and early symbiotic protocorm
development of Platanthera hyperborea (Orchidaceae). Can J Bot 70:291–300
Richardson KA, Currah RS, Hambleton S (1993) Basidiomycetous endophytes from the roots of
neotropical epiphytic Orchidaceae. Lindleyana 8:127–137
Riofrı
´o M, Cruz DJ, Torres E, De La Cruz M, Iriondo J-M, Sua
´rez JP (2013) Mycorrhizal
preferences and fine spatial structure of the epiphytic orchid Epidendrum rhopalostele.AmJ
Bot 100:1–10
Roberts P (1992) Spiral-spored Tulasnella species from Devon and the New Forest. Mycol Res
96:233–236
Roberts P (1993a) The genus Tulasnella in Norway. Windahlia 20:67–74
Roberts P (1993b) Allantoid-spored Tulasnella species from Devon. Mycol Res 97:213–220
Roberts P (1994a) Long-spored Tulasnella species from Devon, with additional notes on allantoid-
spored species. Mycol Res 98:1235–1244
Roberts P (1994b) Globose and ellipsoid-spored Tulasnella species from Devon and Surrey, with a
key to the genus in Europe. Mycol Res 98:1431–1452
Roberts P (1996) Heterobasidiomycetes from Majorca & Cabrera (Balearic Islands). Mycotaxon
60:111–123
Roberts P (1999) Rhizoctonia-forming fungi: a taxonomic guide. Royal Botanic Gardens, Kew
Roberts P (2003) Tulasnella echinospora: an unusual new species from Great Britain and Sweden.
Cryptogam Mycol 25:23–27
Roberts P (2006) Caribbean heterobasidiomycetes: 2. Jamaica. Mycotaxon 96:83–107
Roberts P, Pia˛tek M (2004) Heterobasidiomycetes of the families Oliveoniaceae and
Tulasnellaceae from Poland. Polish Bot J 49:45–54
266 F. Oberwinkler et al.
Roche SA, Carter RJ, Peakall R, Smith LM, Whitehead MR, Linde CC (2010) A narrow group of
monophyletic Tulasnella (Tulasnellaceae) symbiont lineages are associated with multiple
species of Chiloglottis (Orchidaceae): implications for orchid diversity. Am J Bot
97:1313–1327
Rogers DP (1933) A taxonomic review of the Tulasnellaceae. Ann Mycol 31:181–203
Ruibal MP, Peakall R, Smith LM, Linde CC (2013) Phylogenetic and microsatellite markers for
Tulasnella (Tulasnellaceae) mycorrhizal fungi associated with Australian orchids. Appl Plant
Sci 1(3):1200394
Salifah HAB, Muskhazli M, Rusea G, Nithiyaa P (2011) Variation in mycorrhizla specificity for
in vitro symbiotic seed germination of Grammatophyllum speciosum Blume. Sains Malays
40:45–455
Salman R, Prendergast G, Roberts P (2001) Germination in Dactylorhiza fuchsii seeds using fungi
from non-orchid sources. In: Kindlmann P, Willems JH, Whigham DF (eds) Conference on
trends and fluctuations and underlying mechanisms in terrestrial orchid populations location.
Ceske Budejovice, pp 133–153
Sathiyadash K, Muthukumar T, Uma E, Pandey RR (2012) Mycorrhizal association and morphol-
ogy in orchids. J Plant Interact 7:238–247
Sathiyadash K, Muthukumar T, Murugan SB, Sathishkumar R, Pandey RR (2014) In vitro
symbiotic seed germination of South Indian endemic orchid Coelogyne nervosa. Mycoscience
55:183–189
Schatz B, Geoffroy A, Dainat B, Bessie
`re J-M, Buatois B, Hossaert-McKey M, Selosse M-A
(2010) A case study of modified interactions with symbionts in a hybrid mediterranean orchid.
Am J Bot 97:1278–1288
Schr€
oter J (1888) Die Pilze Schlesiens. In: Cohn JV (ed) Kryptogamenflora von Schlesien, vol
3. Kern JV Verlag, Breslau
Selosse M-A, Weiss M, Jany J, Tillier A (2002) Communities and populations of sebacinoid
basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) LCM Rich.
and neighbouring tree ectomycorrhizae. Mol Ecol 11:1831–1844
Shan XC, Liewe EC, Weatherhead MA, Hodgkiss IJ (2002) Characterization and taxonomic
placement of Rhizoctonia-like endophytes from orchid roots. Mycologia 94:230–239
Sharma J, Zettler LW, van Sambeek JW (2003a) A survey of mycobionts of federally threatened
Platanthera praeclara (Orchidaceae). Symbiosis 34:145–155
Sharma J, Zettler LW, van Sambeek JW, Ellersieck MR, Starbuck CJ (2003b) Symbiotic seed
germination and mycorrhizae of federally threatened Platanthera praeclara (Orchidaceae).
Am Midl Nat 149:104–120
Shefferson RP, Weiß M, Kull T, Taylor DL (2005) High specificity generally characterizes
mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Mol Ecol
14:613–626
Shefferson RP, Taylor DL, Weiß M, Garnica S, McCormick MK, Adams S, Gray HM, McFarland
JW, Kull T, Tali K, Yukawa T, Kawahara T, Miyoshi K, Lee Y-I (2007) The evolutionary
history of mycorrhizal specificity among lady’s slipper orchids. Evolution 61:1380–1390
Shefferson RP, Kull T, Tali K (2008) Mycorrhizal interactions of orchids colonizing Estonian
mine tailings hills. Am J Bot 95:156–164
Shefferson RP, Cowden CC, McCormick MK, Yukawa T, Ogura-Tsujita Y, Hashimoto T (2010)
Evolution of host breadth in broad interactions: mycorrhizal specificity in East Asian and North
American rattlesnake plantains (Goodyera spp.) and their fungal hosts. Mol Ecol
19:3008–3017
Shimura H, Sadamoto M, Matsuura M, Kawahara T, Naito S, Koda Y (2009) Characterization of
mycorrhizal fungi isolated from the threatened Cypripedium macranthos in a northern island of
Japan: two phylogenetically distinct fungi associated with the orchid. Mycorrhiza 19:525–534
Smith ZF, James EA, McLean CB (2007) Experimental reintroduction of the threatened terrestrial
orchid Diuris fragrantissima. Lankesteriana 7:377–380
12 Biogeography and Ecology of Tulasnellaceae 267
Smith ZF, James EA, McLean CB (2010) Mycorrhizal specificity of Diuris fragrantissima
(Orchidaceae) and persistence in a reintroduced population. Aust J Bot 58:97–106
Smreciu EA, Currah RS (1989) Symbiotic germination of seeds of terrestrial orchids of North
America and Europe. Lindleyana 4:6–15
Stark C, Babik W, Durka W (2009) Fungi from the roots of the common terrestrial orchid
Gymnadenia conopsea. Mycol Res 113:952–959
Steinfort U, Verdugo G, Besoain X, Cisterna MA (2010) Mycorrhizal association and symbiotic
germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst. (Orchidaceae). Flora
205:811–817
Stewart SL, Kane ME (2006) Symbiotic seed germination of Habenaria macroceratitis
(Orchidaceae), a rare Florida terrestrial orchid. Plant Cell Tissue Organ Cult 86:159–167
Stewart SL, Zettler LW (2002) Symbiotic germination of three semi-aquatic rein orchids
(Habenaria macroceratitis,H. quinqueseta,H. repens) from Florida. Aquatic Bot 72:25–35
Stewart SL, Zettler LW, Minso J, Brown PM (2003) Symbiotic germination and reintroduction of
Spiranthes brevilabris Lindley, and endangered orchid native to Florida. Selbyana 24:64–70
St€
ockel M, Te
ˇs
ˇitelova
´T, Jersa
´kova
´J, Bidartondo MI, Gebauer G (2014) Carbon and nitrogen gain
during the growth of orchid seedlings in nature. New Phytol 202:606–615
Strid A (1975) Lignicolous and corticolous fungi in Alder vegetation in Central Norway with
special reference to Aphyllophorales Basidiomycetes. Kong Norske Vidensk Selskab Skrif
4:1–52
Strullu DG, Gourret JP (1974) Ultrastructure et e
´volution du champignon symbiotique des racines
de Dactylorchis maculata. J Microsc 20:285–294
Sua
´rez JP, Kottke I (2016) Main fungal partners and different levels of specificity of orchid
mycorrhizae in the tropical mountain forests of Ecuador. Lankesteriana 16:299–305
Sua
´rez JP, Weiss M, Abele A, Garnica S, Oberwinkler F, Kottke I (2006) Diverse tulasnelloid
fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycol Res
110:1257–1270
Sua
´rez JP, Eguiguren JS, Herrera P, Jost L (2016) Do mycorrhizal fungi drive speciation in
Teagueia (Orchidaceae) in the upper Pastaza watershed of Ecuador? Symbiosis 69:161–168
Sufaati S, Agustini V, Suharno (2012) Isolation and phylogenetic relationship of orchid-
mycorrhiza of Spathoglottis plicata of Papua using mitochondrial ribosomal large subunit
(mt-Ls) DNA. Biodiversitas 13:59–64
Sun Y, He X, Glenny D (2014) Transantarctic disjunctions in Schistochilaceae (Marchantiophyta)
explained by early extinction events, post-Gondwanan radiations and palaeoclimatic changes.
Mol Phylogenet Evol 76:189–201
Suryantini R, Wulandari RS, Kasiamandri RS (2015) Orchid mycorrhizal fungi: identification of
Rhizoctonia from West Kalimantan. Microbiol Indones 9:157–162
Swangmaneecharern P, Serivichyaswat P, Nontachaiyapoom S (2012) Promoting effect of orchid
mycorrhizal fungi Epulorhiza isolates on seed germination of Dendrobium orchids. Sci Hortic
148:55–58
Talbot PHB (1973) Holobasidiomycetidae: Tulasnellales. In: Ainsworth GC, Sparrow FK,
Sussman AS (eds) The fungi, vol IV, Sect. B. Academic Press, New York, pp 322–325
Tan X-M, Chen X-M, Wang C-L, Jin X-H, Cui J-L, Chen J, Guo S-X, Zhao L-F (2012) Isolation
and identification of endophytic fungi in roots of nine Holcoglossum plants (Orchidaceae)
collected from Yunnan, Guangxi, and Hainan provinces of China. Curr Microbiol 64:140–147
Tan XM, Wang CL, Chen XM, Zhou YQ, Wang YQ, Luo AX, Liu ZH, Guo SX (2014) In vitro
seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium
officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.) Sci Hortic 165:62–68
Tao G, Liu ZY, Hyde KD, Liu XZ, Yu ZN (2008) Whole rDNA analysis reveals novel and
endophytic fungi in Bletilla ochracea (Orchidaceae). Fungal Divers 33:101–122
Taylor JW, Berbee ML (2006) Dating divergences in the Fungal Tree of Life: review and new
analyses. Mycologia 98:838–849
268 F. Oberwinkler et al.
Taylor DL, McCormick MK (2008) Internal transcribed spacer primers and sequences for
improved characterization of basidiomycetous orchid mycorrhizas. New Phytol
177:1020–1033
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Ko
˜ljalg U (2008a) Strong host
preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA
barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, Suvi T, Jairus T, Ko
˜ljalg U (2008b) Forest microsite effects on community compo-
sition of ectomycorrhizal fungi on seedlings of Picea abies and Betula pendula. Environ
Microbiol 10:1189–1201
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity,
distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Teixeira AFS, Pessoa HP, Miranda L, Resende PH, Pereira MC (2015) Effect of mycorrhizal fungi
and abiotic factors on the development and distribution of Oeceoclades maculata (Lindl.)
Lindl. in understory of Avocado. Evol Conserv Biodivers 6:23–32
Te
ˇs
ˇitelova
´T, Jersa
´kova
´J, Roy M, Kuba
´tova
´B, Te
ˇs
ˇitel J, Urfus T, Tra
´vnı
´c
ˇek P, Suda J (2013)
Ploidy-specific symbiotic interactions: divergence of mycorrhizal fungi between cytotypes of
the Gymnadenia conopsea group (Orchidaceae). New Phytol 199:1022–1033
Torkelsen A-E (1977) Jelly fungi in western Norway. Blyttia 35:179–192
Uetaka Y, Ogoshi A, Hayakawa S (1999) Observations of teleomorphs of rhizoctonias
(Thanatephorus orchidicola and Tulasnella) isolated from orchids. Hokkaido Univ Coll
Scholar Acad Pap 22:121–125
Van de Put K, Antonissen I (1996) Tulasnella’s uit Vlaanderen. Sterbeeckia 17:44–69
Veldre V, Abarenkov K, Bahram M, Florent Martos F, Selosse M-A, Tamm H et al (2013)
Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as
revealed from publicly available ITS sequences. Fungal Ecol c6:256–268
Voyron S, Ercole E, Ghignone S, Perotto S, Girlanda M (2016) Fine-scale spatial distribution of
orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytol. doi:10.1111/nph.
14286
Wang X, Yam TW, Meng Q, Zhu J, Zhang P, Wu H, Wang J, Zhao Y, Song X (2016) The dual
inoculation of endophytic fungi and bacteria promotes seedlings growth in Dendrobium
catenatum (Orchidaceae) und in vitro culture conditions. Plant Cell Tissue Organ Cult
126:523–531
Warcup JH (1971) Specificity of mycorrhizal association in some Australian terrestrial orchids.
New Phytol 70:41–46
Warcup JH (1973) Symbiotic germination of some Australian terrestrial orchids. New Phytol
72:387–392
Warcup JH (1981) The mycorrhizal relationships of Australian orchids. New Phytol 87:371–381
Warcup JH (1985) Rhizanthella gardneri (Orchidaceae), its Rhizocotonia endophyte and close
association with Melaleuca uncinata (Myrtaceae) in western Australia. New Phytol
99:273–280
Warcup JH, Talbot PHB (1967) Perfect states of Rhizoctonias associated with orchids I. New Phytol
66:631–641
Warcup JH, Talbot PHB (1971) Perfect states of Rhizoctonias associated with orchids II. New
Phytol 76:35–40
Warcup JH, Talbot PHB (1980) Perfect states of Rhizoctonias associated with orchids III. New
Phytol 86:267–272
Waterman RJ, Bidartondo MI, Stofberg J, Combs JK, Gebauer G, Savolainen V, Barraclaugh TG,
Pauw A (2011) The effects of above- and belowground mutualisms on orchid speciation and
coexistence. Am Nat 177:E54–E68
Waud M, Busschaert P, Lievens B, Jacquemyn H (2016a) Specificity and localised distribution of
mycorrhizal fungi in the soil may contribute to co-existence of orchid species. Fungal Ecol
20:155–165
12 Biogeography and Ecology of Tulasnellaceae 269
Waud M, Wiegand T, Brys R, Lievens B, Jacquemyn H (2016b) Nonrandom seedling establish-
ment corresponds with distance-dependent decline in mycorrhizal abundance in two terrestrial
orchids. New Phytol 211:255–264
Weiß M, Bauer R, Begerow D (2004) Spotlights on heterobasidiomycetes. In: Agerer R,
Piepenbring M, Blanz P (eds) Frontiers in Basidiomycote mycology. Eching, IHW-Verlag,
pp 7–48
Whitridge and Southworth (2005) Mycorrhizal symbionts of the terrestrial orchid Cypripedium
fasciculatum. Selbyana 26:328–334
Wickett NJ, Goffinet B (2008) Origin and relationship of the myco-heterotrophic liverwort
Cryptothallus mirabilis Malmb. (Metzgeriales, Marchantiophyta). Bot J Linn Soc 156:1–12
Wojewoda W (1978) Polish Tulasnellales part 1. Tulasnella inclusa new record. Acta Mycol
14:109–112
Wojewoda W (1983) Polish Tulasnellales 2. Tulasnella hyalina new record. Acta Mycol 19:41–46
Wojewoda W (1986) Polish Tulasnellales III. Tulasnella violacea (Johan-Olsen ap. Bref.) Juel.
Acta Mycol 22:99–102
Xing X, Ma X, Deng Z, Chen J, Wu F, Guo S (2013) Specificity and preference of mycorrhizal
associations in two species of the genus Dendrobium (Orchidaceae). Mycorrhiza 23:317–324
Yang G, Li C (2012) General description of Rhizoctonia species complex. In: Cumagun CJ
(ed) Plant pathology. InTech, Rijeka. isbn: 978–953–51-0489-6
Yokoya K, Zettler LW, Kendon JP, Bidartondo MI, Stice AL, Skarha S, Corey LL, Knight AC,
Sarasan V (2015) Preliminary findings on identification of mycorrhizal fungi from diverse
orchids in the central highlands of Madagascar. Mycorrhiza 25:611–625
Youm J-Y, Han H-K, Chung J-M, Cho Y-C, Lee B-C, Eom A-H (2012) Identification of orchid
mycorrhizal fungi isolated from five species of terrestrial orchids in Korea. Kor J Mycol
40:132–135
Yu Y, Cui Y-H, Hsiang T, Zeng Z-Q, Yu Z-H (2015) Isolation and identification of endophytes
from roots of Cymbidium goeringii and Cymbidium faberi (Orchidaceae). Nova Hedwigia
101:57–64
Yuan L, Yang ZL, Li S-Y, Hu H, Huang J-L (2010) Mycorrhizal specificity, preference, and
plasticity of six slipper orchids from south western China. Mycorrhiza 20:559–568
Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J (2009) Mycorrhizal diversity in
Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. Am J Bot
96:1997–2009
Zelmer CD, Currah RS (1995) Ceratorhiza pernacatena and Epulorhiza calendulina ssp. nov.:
Mycorrhizal fungi of terrestrial orchids. Can J Bot 73:1981–1985
Zelmer CD, Currah RS (1997) Symbiotic germination of Spiranthes lacera (Orchidaceae) with a
naturally occurring endophyte. Lindleyana 12:142–148
Zettler LW, Hofer CJ (1998) Propagation of the little club-spur orchid (Platanthera clavellata)by
symbiotic seed germination and its ecological implications. Environ Exp Bot 39:189–195
Zettler LW, Perlman S, Dennis DJ, Hopkins SE, Poulter SB (2005) Symbiotic germination of the
federally endangered Hawaiian endemic, Platanthera holochila (Orchidaceae) using a
mycobiont from Florida: a conservation dilemma. Selbyana 26:269–276
Zettler LW, Poulter SB, McDonald KI, Stewart L (2007) Conservation-driven propagation of an
epiphytic orchid (Epidendrum nocturnum) with a mycorrhizal fungus. HortScience
42:135–139
Zettler WW, Corey AL, Jacks AL, Gruender LT, Lopez AM (2013) Tulasnella irregularis
(Basidiomycota: Tulasnellaceae) from roots of Encyclia tampensis in South Florida, and
confirmation of its mycorrhizal significance through symbiotic seed germination.
Lankesteriana 13:119–128
Zhang F-S, Lv Y-L, Zhao Y, Guo S-X (2013) Promoting role of an endophyte on the growth and
contents of kinsenosides and flavonoids of Anoectochilus formosanus Hayata, a rare and
threatened medicinal orchidaceae plant. J Zheijang Univ Sci B 14:785–792
270 F. Oberwinkler et al.
Zhao X, Zhang J, Chen C, Yang J, Zhu H, Liu M, Lv F (2014a) Deep-sequencing-based
comparative transcriptional profiles of Cymbidium hybridum roots in response to mycorrhizal
and non-mycorrhizal beneficial fungi. BMC Genomics 15:747
Zhao X-L, Yang J-Z, Liu S, Chen C-L, Zhu H-Y, Cao J-X (2014b) The colonization patterns of
different fungi on roots of Cymbidium hybridum plantlets and their respective inoculation
effects on growth and nutrient uptake of orchid plantlets. World J Microbiol Biotechnol
30:1993–2003
Zhou X, Gao JY (2016) Highly compatible Epa-01 strain promotes seed germination and
protocorm development of Papilionanthe teres (Orchidaceae). Plant Cell Tissue Organ Cult
125:479–493
Zi X-M, Sheng C-L, Goodale UM, Shao S-C, Gao J-Y (2014) In situ seed baiting to isolate
germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae).
Mycorrhiza 24:487–499
12 Biogeography and Ecology of Tulasnellaceae 271