Content uploaded by Manuel Cesario
Author content
All content in this area was uploaded by Manuel Cesario on Oct 07, 2017
Content may be subject to copyright.
Short Communication
First Record of Lutzomyia (Lutzomyia)longipalpis
(Diptera: Psychodidae: Phlebotominae) on the Trinational
Frontier (Brazil–Peru–Bolivia) of South-Western Amazonia
Diones Antonio Borges,
1,
* Silvia Maria Guerra Molina,
1
Mara Cristina Pinto,
2
Eunice
Aparecida Bianchi Galati,
3
Manuel Cesario,
4
and Dennys Ghenry Samillan Ortiz
5,6,
*
1
Department of Genetics, Luiz de Queiroz College of Agriculture (ESALQ) – University of S~
ao Paulo (USP), Piracicaba, S~
ao Paulo,
Brazil (dionesborges@hotmail.com; silviamgmolina@usp.br),
2
School of Pharmaceutical Sciences (FCF), State University (UNESP),
Araraquara, S~
ao Paulo, Brazil (marap@fcfar.unesp.br),
3
Department of Epidemiology, School of Public Health, S~
ao Paulo State
University (USP), S~
ao Paulo, S~
ao Paulo, Brazil (egalati@usp.br),
4
Magdala Science and Sustainability, Franca, S~
ao Paulo, Brazil
(manuel.cesario@uol.com.br),
5
Department of Animal Biology, Institute of Biology, State University of Campinas (UNICAMP),
Campinas, S~
ao Paulo, Brazil (d162706@dac.unicamp.br), and
6
Corresponding author, e-mail: d162706@dac.unicamp.br
*These authors contributed equally to this work.
Subject Editor: Richard Johnson
Received 11 January 2017; Editorial decision 28 March 2017
Abstract
In South America, the main sand fly species involved in the transmission of Leishmania infantum chagasi (Cunha
& Chagas, 1937), etiological agent of the visceral leishmaniasis (VL), is Lutzomyia longipalpis (Lutz & Neiva, 1912).
The species has been recorded in Colombia, Venezuela, Bolivia, Argentina, Paraguay, Uruguay, and Brazil, where
it is recorded in 24 of the 27 Brazilian states, except Acre, Amazonas, and Santa Catarina. Collections were carried
out for one year (April 2013 to March 2014) using modified CDC light traps in different environments in Assis
Brasil municipality, state of Acre. Two males of Lu. longipalpis were found in peridomiciliary location in a peri-
urban area. This is the first record of the species in Acre. This finding may be considered by the health agencies
located in the trinational frontier, and new collections are needed to evaluate the real distribution of the species.
Resumo
Na Ame´ rica do Sul, a principal espe´cie de flebotom
ıneo envolvido na transmiss~
ao de Leishmania infantum cha-
gasi, agente etiol
ogico da Leishmaniose visceral (VL), e´ Lutzomyia longipalpis (Lutz & Neiva, 1912). Esta espe´cie
j
a foi registrada na Coloˆ mbia, Venezuela, Bol
ıvia, Argentina, Paraguai, Uruguai e Brasil, onde foram registradas
em 24 dos 27 estados brasileiros, com excec¸~
ao do Acre, Amazonas e Santa Catarina. As coletas foram realiza-
das por um ano (abril de 2013 a marc¸o de 2014) utilizando-se armadilhas de luz tipo de CDC em diferentes ambi-
entes no munic
ıpio de Assis Brasil, estado do Acre. Dois machos de Lu. longipalpis foram encontrados no
peridomic
ılio de uma resid^
encia localizada na
area periurbana, sendo o primeiro registro desta espe´ cie no
estado. Esse achado deve ser considerado pelas ag^
encias de sa
ude localizadas na fronteira trinacional. Novas
capturas ser~
ao necess
arias para avaliar a distribuic¸~
ao real da espe´ cie.
Key words: visceral leishmaniasis, sand fly, spacial distribution, monitoring, Acre
Literature data point out that 528 sand fly species are recorded in
Americas (Shimabukuro et al. 2017); Lutzomyia longipalpis (Lutz
& Neiva, 1912) stands out as the main vector of Leishmania
(Leishmania)infantum chagasi, the etiologic agent of visceral leish-
maniasis (VL). The epidemiological relevance of this disease consists
in its lethality if not treated, and also because there is as yet no avail-
able vaccine (Pan American Health Organization [PAHO] 2016).
Brazil is of fundamental importance in the epidemiology of VL
because, along with Argentina and Paraguay, the area in it of the
transmission of the disease is spreading, and also because from 2001
to 2014, of the 48,720 cases of VL registered in the Americas,
46,976 of them (96.42%) were concentrated in Brazil (PAHO
2016).
The spread of Lutzomyia longipalpis in Brazil, from rural to
urban areas, has been well documented over time (Lainson and
Rangel 2005,Brazil 2013). This species has already been found in
27 Brazilian states (Rangel and Vilela 2008,Souza et al. 2009,
Santos et al. 2012,Galardo et al. 2013).
V
CThe Authors 2017. Published by Oxford University Press on behalf of Entomological Society of America.
All rights reserved. For Permissions, please email: journals.permissions@oup.com 1
Journal of Medical Entomology, 2017, 1–5
doi: 10.1093/jme/tjx086
Short Communication
Acre state presents a high coefficient of detection of American
tegumentary leishmaniasis (ATL) whose etiology has been attributed
to various species of Leishmania (Secretaria de Vigil^
ancia em Saude
[SVS] 2007). Such epidemiological scenario has motivated several
researchers to undertake projects to investigate the phlebotomine
fauna with a view to identifying possible vectors. Data from the lit-
erature indicate that Acre presents a great diversity of species, by the
end of 2008, 52 species had been reported (Azevedo et al. 2008);
later 16 new records (Teles et al. 2013a, 2016) and three new species
(Teles et al. 2013a,De Oliveira et al. 2015,Brilhante et al. 2017)
were reported, with a total of 71 species of sand fly. However,
Lu. longipalpis had not previously been found in this state, even in
captures in rural areas of Assis Brasil (Teles et al. 2016).
Materials and Methods
Study Area
The Brazilian municipality of Assis Brasil lies on the triple Brazil–
Peru–Bolivia border in the South-Western Amazon, in the southwest
of the State of Acre (105602700 S and 69340400 W), being located
on the left bank of the Acre River (Fig. 1). It occupies a total area of
4,974.175 km
2
that represents 3.03% of the territory of the Acre
State. It has a population of 6,072 inhabitants, of which whom
39.1% live in the rural area, and has a Municipal Human
Development Index (MHDI) of 0.601 (Secretaria de Estado de
Planejamento do Acre [SEPLAN] 2013, Instituto Brasileiro de
Geografia e Estat
ıstica [IBGE] 2016). The vegetation is of the open
forest type with bamboo and palm trees and dense ombrophilous
forest (Secretaria de Estado de Meio Ambiente do Acre [SEMA]
2010); the average annual temperatures in the State range from
24.5 Cto32
C, the relative humidity is around 90%, and the
annual rainfall varies from 1,600 mm to 2,750 mm (Acre 2016).
The entomological collections were carried out in the municipal-
ity of Assis Brasil from April 2013 to March 2014, with modified
CDC light traps (Pugedo et al. 2005) in five consecutive nights. The
traps were set up in the intradomiciliary and peridomiciliary envi-
ronment and in the forest adjacent to four houses, one of which
located in the urban area, two in a peri-urban environment, and one
in a rural area. The traps were installed at 1.50 m above from the
ground from 6 p.m. to 6 a.m. for five consecutive nights, in the
phase of the moon known as the New Moon. Altogether, 60 catches
were carried out in each of the environments. All traps were new
and used for the first time in this study.
The specimens were sexed and preserved in plastic flasks con-
taining naphthalene, until their maceration in accordance with the
technique described by Forattini (1973). Then they were mounted in
Balsam of Canada and identified, as proposed by Galati (2016).
Results
In the areas sampled, a total of 22,334 sand flies were collected—
10,733 males (48.1%) and 11,601 females (51.9%). In the peri-urban
areas 14,805 specimens were captured, giving an average of 41.1
Fig 1. Distribution of Lu. longipalpis in Acre and neighboring places. Map A. Sites with the presence of Lu. longipalpis in Bolivia and Rondonia, Brazil. Map B. Site
(1056012.54100 S/6933029.99900 W) with the presence of Lu. longipalpis in Assis Brasil, Brazil.
2Journal of Medical Entomology, 2017, Vol. 00, No. 0
insects per trap; in the rural area 7,424 (41.2 insects per trap) and in
the urban area 105 (0.6 insects per trap). Of the total number of sand
flies caught, 18,033 insects (80.7%) were captured in the forested envi-
ronment, 3,495 (15.5%) in the peridomiciles, and 806 (3.6%) indoors.
Only two Lu. longipalpis males were captured (on the same night)
in a peridomicile located in the peri-urban area of Assis Brasil - AC.
(Fig. 2). The total of sand fly fauna will be published in a future paper.
Discussion
The two specimens of Lu. longipalpis were collected in May 2013, in
the peri-urban region, in the peridomiciliary environment (Fig. 3),
characterized by the cultivation of vegetables, the presence of trees
such as the banana plant (Musa spp.), acerola (Malpighia emarginata
DC), cashew (Anacardium occidentale L), and cupuac¸u (Theobroma
grandiflorum Schum). In addition, there are domestic animals such as
dogs and fowl (chickens and ducks). In the environment, there is no
structured chicken coop and the chickens use the trees for shelter.
This environment is consistent with several studies which report the
presence of Lu. longipalpis in peridomiciles due to this sand fly’s
attraction to hens and dogs as food source, with the possibility of the
circulation of Leishmania spp. between insect vectors and mammal
hosts (Alexander et al. 2002,Sant’Anna et al. 2010).
The opening of new areas of human occupation through Land
Use and Cover Change, especially in south-western Amazonia
(which embraces the Brazilian states of Acre, Rondoˆ nia, western
part of Amazonas; Madre de Dios Department—Peru; Pando
Department—Bolivia) has led to the expansion of the transmission
area of ATL (Luna 2002,Cesario and Andrade-Morraye 2008,
Mooney et al. 2009,Cesario et al 2011). Acre has been reported to
have 20 times higher incidence rates (coefficient of detection) of
ATL than the level considered as “very high transmission” by the
Brazilian Ministry of Health (SVS 2007, Secretaria de Ci^
encia e
Tecnologia e Insumos Estrate´ gicos [SCTIE] 2010).
There are probably two possible routes of vector dispersion:
from Bol
ıvia (in the Yungas region and the municipality of Toro
Toro in Potosi; Le Pont and Desjeux 1985,Le Pont et al. 1989,
Dujardin et al. 1997,Garcia et al. 2009) or from Rondoˆ nia
Brazilian state (in the municipalities of Cacaul^
andia, Monte Negro,
Buritis, and Campo Novo; Gil et al. 2003,Teles et al. 2013b;Fig.
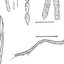
Fig 2. Abdomen and male genitalia of Lu. longipalpis.(A) Pale patches containing tergal papillae in the fourth abdominal tergite (arrow). (B) Fourth abdominal ter-
gite amplified (arrow). (C) Bristles with curved, hooked apex on the dorsal margin of the paramere (arrow).
Journal of Medical Entomology, 2017, Vol. 00, No. 0 3
1). The distances from Assis Brasil and the nearest places where Lu.
longipalpis was collected in Bol
ıvia and Brazil is almost the same,
roughly 600 Km, but Bolivia presents the highest altitudes (950–
2,800 AMSL) compared to Brazil (190 AMSL). New entomological
captures will be necessary to arrive at a better understanding of the
dispersion of this species in the region.
In addition, other studies, such as the biological cycle (Souza
et al. 2009), sexual pheromone analysis (Hamilton et al. 2005), and
males’ mating sound (De Souza et al. 2002) would be opportune to
clarify the relation of the Lu. longipalpis population from Assis
Brasil with other allopatric populations.
According to the classification of the areas of Visceral Leishmaniasis
Control Program of the Brazilian Ministry of Health, the municipality
of Assis Brasil could be classified as a silent area (where no autochtho-
nous cases of VL have been reported), receptive (with the confirmed
presence of Lu. longipalpis), and vulnerable (with intense migratory
flow, especially as it is a border area with Peru and Bolivia; SVS 2006).
The relevance of this register is justified as an alert for govern-
ment health agents, since the Brazilian Ministry of Health estab-
lishes the following recommendations for silent and receptive areas:
environmental sanitation, control of the wandering canine popula-
tion, and survey of canine serological sampling (SVS 2006).
Due to the proximity of the Brazilian municipality of Assis Brasil
to Peru and Bolivia, a greater evaluation of the risk of the dispersion
of Lu. longipalpis in these bordering countries as the possibility of
the appearance of the disease in the future is to be recommended.
Acknowledgments
We thank Dr. Marco Tulio Antonio Garcia-Zapata for all helpful suggestion
and discussions. We also thank the support received in Assis Brasil, provided
by the Municipal Health Secretariat, by the Chico Mendes Institute for
Biodiversity Conservation (ICMBio), and the various citizens that allowed
and permitted our work in their households, in Assis Brasil, Acre, Brazil, for
the support received. We are grateful to CNPq (National Council for
Scientific and Technological Development Fellowship #140363/2015-7) and
CAPES (Coordination for the Improvement of Higher Education Personnel),
by the scholarships for the doctorate in the respective programs, Animal
Biology (UNICAMP) and Apllied Ecology (inter-unit USP/ESALQ and
CENA). The entomological collections were made possible through the
FAPESP (S~
ao Paulo Research Foundation) Thematic Grant 08/58.156-8 and
the SNIS trinational “Andes-Amazon” grant.
ReferencesCited
Acre 2016. O Estado do Acre. Governo do Estado do Acre, Rio Branco.(http://
www.ac.gov.br/wps/portal/acre/Acre/estado-acre) (accessed12 October
2016).
Fig 3. Sand flies sampling sites. (A) Typical wood-built domicile located in the peri-urban region of the Assis Brasil. (B) Presence of holes on the walls and the floor.
(C) Peridomicile with forest adjacent (trees) and domestic animals (collect site of Lu. longipalpis). (D) Secondary forest adjacent to thesampling site in Assis Brasil.
4Journal of Medical Entomology, 2017, Vol. 00, No. 0
Alexander, B., R. L. de Carvalho, H. McCallum, and M. H. Pereira. 2002.
Role of the domestic chicken (Gallus gallus) in the epidemiology of urban
visceral leishmaniasis in Brazil. Emerg. Infect. Dis. 8: 1480–1485.
Azevedo, A. C., S. M. Costa, M. C. Pinto, J. L. Souza, H. C. Cruz, J. Vidal,
and E. F. Rangel. 2008. Studies on the sandfly fauna (Diptera: Psychodidae:
Phlebotominae) from transmission areas of American Cutaneous
Leishmaniasis in state of Acre, Brazil. Mem. Inst. Oswaldo Cruz. 103:
760–767.
Brazil, R. P. 2013. The dispersion of Lutzomyia longipalpis in urban areas.
Rev. Soc. Bras. Med. Trop. 46: 263–264.
Brilhante, A. F.,. B. S
abio, and E.A.B. Galati. 2017. A New Species of Sand
Fly, Psathyromyia elizabethdorvalae sp. n. (Diptera: Psychodidae:
Phlebotominae), From Brazil. J. Med. Entomol. 54: 76–81.
Cesario, M., and M. Andrade-Morraye. 2008. Land-use and land-cover
changes and the (re) emergence of diseases in Brazil. Source 11: 61–68.
Cesario, M., R. R. Cesario, and M. Andrade-Morraye. 2011. Environmental
change and health impacts in Amazonia. IHDP Update 1: 26–33.
De Oliveira, A.F.J., C.B.G. Teles, J. F. Medeiros, L.M.A. Camargo, and
F.A.C. Pessoa. 2015. Description of Trichophoromyia ruifreitasi, a new
phlebotomine species (Diptera, Psychodidae) from Acre State, Brazilian
Amazon. ZooKeys 526: 65–73.
De Souza, N. A., R. D. Ward, J. G. Hamilton, C. P. Kyriacou, and A. A.
Peixoto. 2002. Copulation songs in three siblings of Lutzomyia longipalpis
(Diptera: Psychodidae). Trans. R. Soc. Trop. Med. Hyg. 96: 102–103.
Dujardin, J.-P., E. M. Torrez, F. L. Pont, D. Hervas, and D. Sossa. 1997.
Isozymic and metric variation in the Lutzomyia longipalpis complex. Med.
Vet. Entomol. 11: 394–400.
Forattini, O. P.1973. Entomologia Me´dica. vol. 4: Psychodidae.
Phlebotominae. Leishmanioses. Bartonelose. Ed. Edgard Blucher & Ed.
USP, S~
ao Paulo, SP.
Galardo, A.K.R., C. D. Galardo, A. A. Santana, J.C.C. Mendes, F.R.A.D.
Souza, J. P. Duarte, J. F. Saraiva, L.C.L. Pinna, R. W. Carvalho, A. P. Sales
de Andrade Correa, et al. 2013. Primeira ocorr^
encia de Lutzomyia
(Lutzomyia) longipalpis Lutz & Neiva, 1912 (Diptera: Psychodidae:
Phlebotominae) no Estado do Amap
a, Brasil. AGRIS 3: 179–183.
Galati, E. A. 2016. Phlebotominae (Diptera, Psychodidae) classificac¸~
ao, mor-
fologia, terminologia e identificac¸~
ao de adultos. Departamento de
Epidemiologia Faculdade de Sa
ude P
ublica. Apostila Disciplina HEP 5752
Bioecologia e Identificac¸~
ao de Phlebotominae 2016.(http://www.fsp.usp.br/
egalati/) (accessed 10 November 2016).
Garcia, A. L., R. Parrado, E. Rojas, R. Delgado, J. C. Dujardin, and R.
Reithinger. 2009. Leishmaniases in Bolivia: Comprehensive review and cur-
rent status. Am. J. Trop. Med. Hyg. 80: 704–711.
Gil, L. H., S. A. Basano, A. A. Souza, M. G. Silva, I. Barata, E. A. Ishikawa, L.
M. Camargo, and J. J. Shaw. 2003. Recent observations on the sand fly
(Diptera: Psychodidae) fauna of the State of Rondoˆ nia, Western Amazoˆ nia,
Brazil: the importance of Psychdopygus davisi as a vector of zoonotic cuta-
neous leishmaniasis. Mem. Inst. Oswaldo Cruz. 98: 751–755.
Hamilton, J. G., R. D. Maingon, B. Alexander, R. D. Ward, and R. P. Brazil.
2005. Analysis of the sex pheromone extract of individual male Lutzomyia
longipalpis sandflies from six regions in Brazil. Med. Vet. Entomol. 19:
480–488.
(IBGE) Instituto Brasileiro de Geografia e Estat
ıstica 2016. Cidades@ - Assis
Brasil. (http://cidades.ibge.gov.br/v3/cidades/municipio/1200054) (accessed
01 December 2016)
Lainson, R., and E. F. Rangel. 2005.Lutzomyia longipalpis and the eco-
epidemiology of American visceral leishmaniasis, with particular reference
to Brazil: A Review. Mem. Inst. Oswaldo Cruz. 100: 811–827.
Le Pont, F., and P. Desjeux. 1985. Leishmaniasis in Bolivia. I. Lutzomyia long-
ipalpis (Lutz & Neiva, 1912) as the vector of visceral leishmaniasis in Los
Yungas. Trans. R. Soc. Trop. Med. Hyg. 79: 227–231.
Le Pont, F., J. Mouchet, and P. Desjeux. 1989. Phle´ botomes de Bolivie: VII.
Re´partition des deux morphotypes du phle´botome Lutzomyia longipalpis
(Lutz et Neiva, 1912) (Diptera: Psychodidae) dans le pie´ mont andin de
Bolivie. Mem. Inst. Oswaldo Cruz. 84: 423–426.
Luna, E.J.A. 2002. A emerg^
encia das doenc¸as emergentes e as doenc¸as infec-
ciosas emergentes e reemergentes no Brasil. Rev. Bras. Epidemiol. 5:
229–243.
Mooney, H., A. Larigauderie, M. Cesario, T. Elmquist, O. Hoegh-Guldberg,
S. Lavorel, G. M. Mace, M. Palmer, R. Scholes, and T. Yahara. 2009.
Biodiversity, climate change, and ecosystem services. Curr. Opin. Environ.
Sustain. 1: 46–54.
(PAHO) Pan American Health Organization/World Health Organization
2016. Leishmaniases: Epidemiological Report in the Americas. PAHO/
WHO, 313 Washington, DC. (http://www2.paho.org/hq/index.php?
option=com_docman&task=doc_download&Itemi d=&gid=35859&lang=
en) (accessed 12 October 2016).
Pugedo, H., R. A. Barata, J. C. Franc¸a-Silva, J. C. Silva, and E. S. Dias. 2005.
HP: um modelo aprimorado de armadilha luminosa de succ¸~
ao para a cap-
tura de pequenos insetos. Revista da Sociedade Brasileira de Medicina
Tropical 38: 70–72.
Rangel, E. F., and M. L. Vilela. 2008.Lutzomyia longipalpis (Diptera,
Psychodidae, Phlebotominae) and urbanization of visceral leishmaniasis in
Brazil. Cad. Saude Publica. 24: 2948–2952.
Sant’Anna, M. R., A. Nascimento, B. Alexander, E. Dilger, R. R. Cavalcante,
H. M. Diaz-Albiter, P. A. Bates, and R. J. Dillon. 2010. Chicken blood pro-
vides a suitable meal for the sand fly Lutzomyia longipalpis and does not
inhibit Leishmania development in the gut. Parasit. Vectors 3: 3.
Santos, D. R.D., A. C. Ferreira, and A. Bisetto Junior. 2012. The first record of
Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae:
Phlebotominae) in the State of Paran
a, Brazil. Rev. Soc. Bras. Med. Trop.
45: 643–645.
(SCTIE) Secretaria de Ci^
encia e Tecnologia e Insumos Estrate´gicos. Ministe´ rio
da Sa
ude. 2010. Doenc¸as negligenciadas: estrate´ gias do Ministe´ rio da
Sa
ude. Rev. Sa
ude P
ublica. 44: 200–202.
(SEMA) Secretaria de Estado de Meio Ambiente do Acre 2010. Resumo educa-
tivo do Zoneamento Ecol
ogico-Econoˆ mico do Acre: fase II. Secretar
ıa de
Estado de Meio Ambiente do Acre, R
ıo Branco. (http://www.agencia.ac.
gov.br/wp-content/uploads/2011/10/downloads_zee_resumo_educativo.
pdf) (accessed 10 November 2016).
(SEPLAN) Secretaria de Estado de Planejamento do Acre 2013. Acre em
numeros 2013. Secretaria de Estado de Planejamento, Departamento de
Estudos e Pesquisas. (http://www.ac.gov.br/wps/portal/acre/Acre/estado-
acre/sobre-o-acre/) (accessed 12 October 2016).
Shimabukuro, P.H.F., A. J. de Andrade, and E.A.B. Galati. 2017. Checklist of
American sand flies (Diptera, Psychodidae, Phlebotominae): genera, species,
and their distribution. ZooKeys 660: 67–106.
Souza, G. D., E. Santos, and J. D. Andrade Filho. 2009. The first report of the
main vector of visceral leishmaniasis in America, Lutzomyia longipalpis
(Lutz &Neiva) (Diptera: Psychodidae: Phlebotominae), in the state of Rio
Grande do Sul, Brazil. Mem. Inst. Oswaldo Cruz 104: 1181–1182.
(SVS) Secretaria de Vigil^
ancia em Sa
ude. Ministe´rio da Sa
ude 2006. Manual
de vigil^
ancia e controle da leishmaniose visceral. Ministe´ rio da Sa
ude:
Secretaria de Vigil^
ancia em Sa
ude, Bras
ılia. (http://bvsms.saude.gov.br/bvs/
publicacoes/manual_vigilancia_controle_leishmaniose_visceral.pdf)
(accessed 10 November 2016)
(SVS) Secretaria de Vigil^
ancia em Sa
ude.Ministe´rio da Sa
ude 2007.Manualde
Vigil^
ancia da Leishmaniose Tegumentar Americana. Ministe´rio da Sa
ude:
Secretaria de Vigil^
ancia em Sa
ude, Bras
ılia. (http://bvsms.saude.gov.br/bvs/publi
cacoes/manual_vigilancia_leishmaniose_2ed.pdf) (accessed 10 November 2016).
Teles, C. B., R. A. Freitas, A.F.D. Oliveira, G. M. Ogawa, E.A.D. Araujo, J. F.
Medeiros, F. A. Pessoa, and L. M. Camargo. 2013a. Description of a new
phlebotomine species (Diptera: Psychodidae, Phlebotominae) and new records
of sand flies from theState of Acre, northern Brazil. Zootaxa 3609: 85–90.
Teles, C.B.G., S. A. Basano, M. Zagonel-Oliveira, J. J. Campos, A.F.J.D.
Oliveira, R.A.D. Freitas, J. F. Medeiros, F.A.C. Pessoa, A. Barral, and
L.M.A. Camargo. 2013b. Epidemiological aspects of American cutaneous
leishmaniasis and phlebotomine sandfly population, in the municipality of
Monte Negro, State of Rondoˆ nia, Brazil. Rev. Soc. Bras. Med. Trop. 46:
60–66.
Teles, C.B.G.,. P.D.A.D. Santos, R. A. Freitas, A.F.J.D. Oliveira, G. M.
Ogawa, M. S. Rodrigues, F. A. C. Pessoa, J. F. Medeiros, and L.M.A.
Camargo. 2016. Phlebotomine sandfly (Diptera: Psychodidae) diversity and
their Leishmania DNA in a hot spot of American Cutaneous Leishmaniasis
human cases along the Brazilian border with Peru and Bolivia. Mem
orias do
Instituto Oswaldo Cruz 111: 423–432.
Journal of Medical Entomology, 2017, Vol. 00, No. 0 5