Content uploaded by Simone Nunes Brandão
Author content
All content in this area was uploaded by Simone Nunes Brandão
Content may be subject to copyright.
1
Submitted on October 4, 2002. Accepted on November 11, 2003.
2
Museu Nacional/UFRJ, Departamento de Invertebrados. Quinta da Boa Vista, São Cristóvão, 20940-040, Rio de Janeiro, RJ, Brasil.
Current address: Biozentrum Grindel und Zoologisches Museum, Universität Hamburg. Martin-Luther-King Platz 3, 20146, Hamburg, Germany.
E-mail: snbrandao@terra.com.br.
Arquivos do Museu Nacional, Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
ISSN 0365-4508
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912
(CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA)
1
(With 13 figures)
SIMONE N. BRANDÃO
2
ABSTRACT: Ten species of macrocypridids, included in five genera, had been previously recorded from Brazilian
deep-sea. In this study, six samples with Macrocyprididae Müller, 1912 collected from eastern Brazilian continental
slope were studied, and eight species were recorded: Macropyxis adrecta Maddocks, 1990; Macropyxis cf. adunca
Maddocks, 1990; Macropyxis amanda Maddocks, 1990; Macropyxis bathyalensis (Hulings, 1967); Macropyxis
similis (Brady, 1880); Macrosarisa sp.1, Macroscapha aff. inaequata Maddocks, 1990; Macroscapha sp.1. Two
species, Macropyxis adrecta and Macropyxis amanda, are here recorded for the first time from the Western
Atlantic. Macropyxis bathyalensis is recorded for the first time from the Southern Atlantic. Macropyxis similis,
previously recorded from the eastern Brazilian continental slope, has its geographic range extended southwards.
A total of nine named species and six unnamed species of Macrocyprididae are now known from the Brazilian
deep-sea. The known distribution of these species is rather punctual. Additional sampling over the continental
slope and the abyssal plain off Brazil probably will enlarge the number of species recorded from this area
and will clarify their general distribution patterns.
Key words: Macrocyprididae, Ostracoda, Crustacea, deep-sea, Brazil.
RESUMO: Macrocyprididae Müller, 1912 (Crustacea, Ostracoda, Macrocypridoidea) em águas profundas ao
largo do Brasil.
Dez espécies de macrocipridídeos, incluídos em cinco gêneros, foram previamente registrados para águas profundas
ao largo do Brasil. Neste estudo, foram analisadas seis amostras de Macrocyprididae coletadas do talude continental
leste brasileiro, sendo registradas oito espécies: Macropyxis adrecta Maddocks, 1990; Macropyxis cf. adunca
Maddocks, 1990; Macropyxis amanda Maddocks, 1990; Macropyxis bathyalensis (Hulings, 1967); Macropyxis
similis (Brady, 1880); Macrosarisa sp.1, Macroscapha aff. inaequata Maddocks, 1990; Macroscapha sp.1. Duas
espécies, Macropyxis adrecta e Macropyxis amanda, são registradas pela primeira vez para o Atlântico Ocidental.
Macropyxis bathyalensis é registrada pela primeira vez para o Atlântico Sul. Macropyxis similis, previamente
registrada para o talude continental leste brasileiro, tem sua distribuição geográfica estendida para o sul.
Depois deste estudo, um total de nove espécies nomeadas e seis espécies não nomeadas de Macrocyprididae
estão registradas para águas profundas ao largo do Brasil. A distribuição dessas espécies é pontual. Novas
amostragens sobre o talude continental e a planície abissal ao largo do Brasil provavelmente aumentarão o
número de espécies registradas para essas regiões e elucidará os padrões gerais de distribuição.
Palavras-chave: Macrocyprididae, Ostracoda, Crustacea, mar profundo, Brasil.
INTRODUCTION
The family Macrocyprididae includes approximately
140 species in eight genera: Macrocypris Brady,
1868; Macrocyprina Triebel, 1960; Macropyxis
Maddocks, 1990; Macrosarisa Maddocks, 1990;
Macroscapha Maddocks, 1990; Macrocypria Sars,
1923; Macrocyprissa Triebel, 1960; and
Macromckenziea Maddocks, 1990. The first five
genera have been recorded from Brazil.
Three named species of Macrocyprina Triebel, 1960
and two unnamed species were previously recorded
from Brazilian waters (DIAS-BRITO, MOURA &
WÜRDIG, 1988; COIMBRA, RAMOS &
SANGUINETTI, 1992; COIMBRA et al., 1999;
BRANDÃO, in press). All of these species were
recorded from the continental shelf, and one of them,
Macrocyprina sp.1 was also recorded from the shallow
continental slope, deepest record of 223m (Figs.1,
13, Tab.1) (COIMBRA, RAMOS & SANGUINETTI,
1992; COIMBRA, 1995; COIMBRA et al., 1999).
The records of the other four genera known from
Brazil exclusively comprise specimens collected from
the deep-sea (Figs.1-3, 13, Tab.1). These records are
152 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
included in only two studies. BRADY (1880) analysed
the Ostracoda collected by the “H.M.S.Challenger”,
in which two samples originated from the eastern
Brazilian continental slope (stations 120 and 122).
From these two samples, he described two species,
Macrocypris similis and Macrocypris tenuicauda, and
recorded Macrocypris decora (Brady, 1866).
More than one century later, MADDOCKS (1990)
described Macrocypris maioris from the abyssal plan
of Brazil Basin; and Macrosarisa bensoni, which
included the Brazilian specimens cited by BRADY
(1880) as Macrocypris tenuicauda. MADDOCKS (1990)
also transferred Macrocypris tenuicauda to
Macropyxis. Furthermore, MADDOCKS (1990)
recorded on other four species of Macropyxis from off
Brazil: Macropyxis kornickeri Maddocks, 1990, from
the eastern continental slope, and Macropyxis adunca
Maddocks, 1990, from the southern Brazil Basin
(abyssal plan); and two unnamed species, Macropyxis
sp.11 and sp.18, from the eastern continental slope
and from northern Brazil Basin (abyssal plan),
respectively. MADDOCKS (1990) also transferred
Macrocypris similis to Macropyxis, and considered
Macrocypris decora as a nomen dubium. Finally, one
unnamed species of Macrosarisa (as Macrosarisa
sp.23) was recorded from the northern Brazil Basin
(abyssal plan); and one unnamed species of
Macroscapha (as Macroscapha sp.30) was recorded
from the eastern Brazilian continental slope.
Therefore, ten species in five genera, were recorded
from the deep-sea off Brazil.
In the present study, the macrocypridid specimens
collected from the continental slope off Brazil are
identified and figured. The information concerning
species previously recorded from Brazilian deep-
sea is summarised. The geographic distribution of
the deep-sea species of Macrocyprididae occurring
off Brazil is illustrated.
MATERIAL AND METHODS
The six samples analysed were collected from the
eastern Brazilian continental slope (off Espírito
Santo, Rio de Janeiro and São Paulo states), between
21°12’13’’S and 24°06’43’’S, 39°08’00’’W and 41°
52’52’’W, 1092 and 2426m depth. The depths listed
in the items Material examined and Distribution of
each species, are the beginning and end points of
dredgings rather than the ecological depth ranges.
This also happens with some of the bathymetric
ranges transcribed from MADDOCKS (1990:5).
The samples collected were fixed in formalin 4% and
the specimens were transferred to ethanol 70% after
sorting. The specimens were illustrated with the aid
of a camera lucida couplet to a Zeiss microscope.
Identifications of the specimens are based on the
original descriptions and illustrations of the species
(BRADY, 1880; HULINGS, 1967a; MADDOCKS, 1990).
Abbreviations used in this study: (h) height, (l)
length, (LV) left valve(s), (RV) right valve(s), (RLV)
closed right and left valves
; (MNRJ) Museu
Nacional - Rio de Janeiro.
Fig.1- Geographic distribution of Macrocyprididae species
reported exclusively off Brazil: (1) Macropyxis kornickeri, (2)
Macropyxis similis, (3) Macrocyprina sp.1 of COIMBRA (1995)
and COIMBRA et al. (1999), (4) Macrosarisa sp.1, (5)
Macropyxis sp.1 of MADDOCKS (1990), (6) Macropyxis sp.2
of MADDOCKS (1990), (7) Macroscapha aff. inaequata, (8)
Macroscapha sp.1, (9) Macroscapha sp.2. (AL) Alagoas, (AP)
Amapá, (BA) Bahia, (CE) Ceará, (ES), Espírito Santo, (MA)
Maranhão, (PA) Pará, (PB) Paraíba, (PE) Pernambuco, (PI)
Piauí, (PR) Paraná, (SC) Santa Catarina, (SE) Sergipe, (SP)
São Paulo, (RJ) Rio de Janeiro, (RN) Rio Grande do Norte,
(RS) Rio Grande do Sul.
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 153
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Geographic distribution of Brazilian macrocypridid species – fig.2- reported exclusively to the South Atlantic: (1) Macropyxis
adrecta, (2) Macropyxis adunca, (3) Macropyxis amanda, (4) Macrocypris maioris; fig.3- reported from the North and South
Atlantic: (1) Macropyxis bathyalensis, (2) Macrosarisa bensoni.
2
3
154 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Table 1. Records of the Macrocyprididae species reported from the deep-sea off Brazil.
continued...
SPECIES / OCCURRENCE OFF BRAZIL OTHER LOCALITIES
Macrocyprina sp.1
Eastern continental shelf -
T
amandaré Ba
y
, Pernambuco, 8°44
’
to 8°47’30’’S, 35°05’ to 35°07’W
(COIMBRA, RAMOS &
SANGUINETTI, 1992).
Northern continental shelf and
continental slope - 5°N to 6°S; 35°W
to 51°W, 15 to 223m (COIMBRA,
1995; COIMBRA et al., 1999).
Macrocypris maioris
Brazil Basin - 0°46.0’S to 0°46.5’S,
29°28.0’W to 29°24.0’W, 3459m
(MADDOCKS, 1990).
Sierra Leone Basin - 10°36’N,
17°49’W, 2185m.
Angola Basin - 10°24’S, 9°09’E,
4559 to 4566m; 10°29’S, 9°04’E,
4597 to 4595m; 10°29’S, 9°03’E,
4612 to 4630m (MADDOCKS, 1990).
Macropyxis adrecta
Eastern continental slope -
21°12’13’’S, 39°52’05’’W, 1598 to
1600m; 21°53’11.904’’S,
39°50’44.863’’W, 1240m;
21°53’45.759’’S, 39°50’22.530’’W,
1300m; 24°06’43’’S, 41°52’52’’W,
1556 to 1566m (new records).
Angola Basin - 10°24’S, 9°09’E,
4559 to 4566m; 10°29’S, 9°04’E,
4595 to 4597m; 12°0.5’S, 1°58.5’E,
5631m (MADDOCKS, 1990).
Macropyxis adunca
Eastern continental slope -
21°12’13’’S, 39°52’05’’W, 1598 to
1600m; 24°06’43’’S, 41°52’52’’W,
1556 to 1566m (new records).
Brazil Basin - 30°24.4’S, 39°00’W,
4818m (MADDOCKS, 1990).
Angola Basin - 10°24’S, 9°09’E,
4559 to 4566m; 15°59.9’S, 1°58.5’E,
5550m; 24°0.2’S, 8°28.5’E, 4637m
(MADDOCKS, 1990).
Macropyxis amanda
Eastern continental slope -
21°12’13’’S, 39°52’05’’W, 1598 to
1600m; 21°48’22’’S, 39°49’55’’W;
1092 to 1438m; 21°53’11.904’’S,
39°50’44.863’’W, 1240m;
21°53’45.759’’S, 39°50’22.530’’W,
1300m (new records).
Angola Basin - 9°41’S to 9°43.5’S,
10°55’E to 10°57’E, 2644 to
2754m (MADDOCKS, 1990).
Macropyxis bathyalensis
Eastern continental slope -
21°14’31’’S, 39°08’00’’W; 2302 to
2426m; 24°06’43’’S, 41°52’52’’W,
1556 to 1566m (new records).
European Basin - 49°38’00’’N,
13°28’00’’W, 1955m (TRESSLER,
1941; MADDOCKS, 1990);
49°37’00’’N, 13°34’00’’W, 3230m
(TRESSLER, 1941).
Labrador Basin - 61°44’N, 30°29’W,
2137m; 59°12’N, 51°05’W, 3521m;
57°50’N, 54°06'W, 3369m
(HULINGS, 1967a; HULINGS,
1967b; MADDOCKS, 1990).
North American Basin - 38°46.8’N,
70°6.8’W, 2891m; 37°59.2’N,
69°26.2’W, 3834m; 32°19.4’N to
32°19.0’N, 64°34.9’W to 64°34.8’,
1135 to 1153m (MADDOCKS, 1990).
Continental slope off southeastern
USA - 35°N to 27°N, 79°W to 75°W,
201 to 585m (HULINGS, 1967a).
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 155
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
TAXONOMY
Genus Macropyxis Maddocks, 1990
Type species – Macrocypyxis sapeloensis (Darby,
1965).
Additional species – Macrocypyxis adrecta
Maddocks, 1990; M. adriatica (Breman, 1975); M.
adunca Maddocks, 1990; M. amanda Maddocks,
1990; M. amoena Maddocks, 1990; M.
antonbruunae Maddocks, 1990; M. arta Maddocks,
1990; M. audens Maddocks, 1990; M. bathyalensis
(Hulings, 1967); M. eltaninae Maddocks, 1990; M.
improcera Maddocks, 1990; M. kaesleri Maddocks,
1990; M. kalbi Maddocks, 1990; M. kornickeri
Maddocks, 1990; M. labutisi Maddocks, 1990; M.
... continued
SPECIES / OCCURRENCE OFF BRAZIL OTHER LOCALITIES
Macropyxis kornickeri
Eastern continental slope -
7°50.0’S to 7°58.0’S, 34°17.0’W,
943 to 1007m; 8°2’S to 8°3’S,
34°23’W to 34°25’W, 587m
(MADDOCKS, 1990).
Macropyxis similis
Eastern continental slope -
7°50.0’S to 7°58.0’S, 34°17.0’W,
943 to 1007m; 8°2’S to 8°3’S,
34°23’W to 34°25’W, 587m;
8°37’S, 34°28’W, 1235m;
21°12’13’’S, 39°52’05’’W, 1598 to
1600m; 21°48’22’’S, 39°49’55’’W;
1092 to 1438m; 21°53’11.904’’S,
39°50’44.863’’W, 1240m;
21°53’45.759’’S, 39°50’22.530’’W,
1300m (BRADY, 1880;
MADDOCKS, 1990; new records).
Macropyxis sp.1
Eastern continental slope - 7°58’S,
34°22’W, 834 to 939m
(MADDOCKS, 1990).
Macropyxis sp.2
Brazil Basin - 0°46.0’S to 0°46.5’S,
29°28.0’W to 29°28.0’, 3459m
(MADDOCKS, 1990).
Macrosarisa bensoni
Eastern continental slope - 7°58’S,
34°22’W, 834 to 939m; 9°5’S,
34°49’W, 640m (BRADY, 1880;
MADDOCKS, 1990).
Continental slope off southeastern
USA - 27°20.9’N 95°9.0W, 1079m
(MADDOCKS, 1990).
Macrosarisa sp.1
Brazil Basin - 0°3.0’S, 27°48.0’W,
3730 to 3783m (MADDOCKS, 1990).
Eastern continental slope -
21°14’31”S, 39°08’00”W; 2302-
2426m (new record).
Macroscapha aff. inaequata
Eastern continental slope -
21°53’11.904’’S, 39°50’44.863’’W,
1240m; 24°06’43’’S, 41°52’52’’W,
1556 to 1566m (new records).
Macroscapha sp.1
Eastern continental slope -
21°53’45.759’’S, 39°50’22.530’’W,
1300m (new records).
Macroscapha sp.2
Eastern continental slope -
7°50.0’S to 7°58.0’S, 34°17.0’W,
943 to 1007m; 7°58’S, 34°22’W,
834 to 939m; 8°2’S to 8°3’S,
34°23’W to 34°25’W, 587m
(MADDOCKS, 1990).
156 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
longana (van den Bold, 1960); M. rhodana (van den
Bold, 1960); M. similis (Brady, 1880); M. simulans
Maddocks, 1990; M. steinecki Maddocks, 1990; M.
tenuicauda (Brady, 1880).
Remarks – The genus Macropyxis, with 22 described
species, has a worldwide distribution, with a live depth
range from 49 to 6134m, but recorded dead down to
9m. It has a geologic range from the Eocene to the
Present. Macropyxis and Macrocyprina are the most
diverse genera of Macrocyprididae. Macropyxis is the
characteristic representant of the Macrocyprididae in
psychrospheric fauna, presenting numerous bathyal
and abyssal species in all oceans. The length of the
adult carapace of species varies from 0.8mm in
Macropyxis improcera to 3.0mm in Macropyxis kalbi.
The species of Macropyxis previously recorded from
Brazilian waters are Macropyxis adunca, Macropyxis
kornickeri and Macropyxis similis (BRADY, 1880;
MADDOCKS, 1990).
Macropyxis adrecta Maddocks, 1990
(Figs.4-5)
Macropyxis adrecta Maddocks, 1990:59, figs.8(13-
14), 9(13-14), 19(5), 22(8), 24(7), 26(24-25), 30(6),
34(6), 39(3), 46(62-63), 50(20-21), 51(31-32),
56(3, 21), 58(13), 59(20), 61(8), 63(2), 64(8), 67(9),
80(12), pls. 14(3-6), 15(4-6), 63(1,2), 66(1,2),
78(27-28), 88(1), 102(10, 13), graph 14.
Type-locality – 10°24’S, 9°09’E, 4559 to 4566m,
Atlantis II, cruise 42, station 198.
Material examined – Eastern Brazilian continental
slope. Off Espírito Santo State: 21°12’13’’S, 39°52’05’’W,
1598-1600m, 1 RV, 2 RV, MNRJ 18631. Off Rio
de Janeiro State: 21°53’11.904’’S, 39°50’44.863’’W,
1240m, 4 RV, MNRJ 18633; 21°53’45.759’’S,
9°50’22.530’’W, 1300m 1 RLV, 4 RV, 3 LV, 1
RV MNRJ 18634. Off São Paulo State: 24°06’43’’S,
41°52’52’’W, 1556-1566m, 21 RLV, 131 RV, 39
LV, 3 RLV, 11 RV, 12 LV, MNRJ 18632.
Dimensions – MNRJ 18632: - RV, h: 0.53mm, l:
1.22mm. - RV, h: 0.53mm, l: 1.20mm. - RV, h:
0.55mm, l: 1.21mm. - LV, h: 0.45mm, l: 1.19mm.
- LV, h: 0.46mm, l: 1.19mm. - LV, h: 0.46mm,
l: 1.18mm. - RV, h: 0.48mm, l: 1.25mm. - RV,
h: 0.48mm, l: 1.24mm. - RV, h: 0.49mm, l:
1.28mm. - LV, h: 0.43mm, l: 1.21mm. - LV, h:
0.40mm, l: 1.24mm. - LV, h: 0.43mm, l: 1.26mm.
- LV, h: 0.43mm, l: 1.24mm.
Diagnosis – Female right valve subtrapezoidal in
lateral outline with high-arched dorsal margin and
greatest height located near midlength; ventral
margin gently sinuate, upswung near posterior
margin; posterodorsal margin steeply sloping. Male
right valve elongate in lateral outline, dorsal margin
low-arched and subparallel to ventral margin;
sharp posterior angle of about 65° in female and
45° in male; anterior zone of concrescence broad
to very broad, with elaborately branching radial
pore canals (modified from MADDOCKS, 1990). In
the present study only carapaces were available.
See MADDOCKS (1990:60) for the appendages.
Supplementary description – Carapace small, with
strong sexual dimorphism (Figs.4A-D). Female
distinctively higher, and fairly shorter in length than
male. Female right valve (Fig.4A) subtrapezoidal in
lateral outline with high-arched dorsal margin,
greatest height near midlength; anterior margin
broadly rounded; female left valve (Fig.4B) with
dorsal margin divided in three fairly straight
segments, greatest height anterior to midlength.
Male valves (Figs.4C, D) elongate in lateral outline.
Right valve (Fig.4C) with low-arched dorsal margin,
with anterior part subparallel to ventral margin, and
posterior part steeply sloping; anterior margin
broadly rounded; greatest height near midlength.
Male left valve (Fig.4D) with dorsal margin divided
in three fairly straight segments, anterodorsal angle
more conspicuous than posterodorsal angle; greatest
height anterior to midlength. Male and female right
valves with ventral margin almost straight, slightly
upswung near posterior. Male and female left valves
with ventral margin slightly indented and slightly
upswung near posterior. Posterodorsal margin of
male and female right and left valves steeply sloping,
straight; sharp posterior angle of about 65° in female
and 45° in male. Anterior zone of concrescence broad
to very broad, vestibule fairly constricted, line of
concrescence irregular, elaborately branching radial
pore canals. Posterior zone of concrescence fairly
broad, vestibule deep, line of concrescence fairly
straight; posterior radial pore canals straight.
Carapace ovate in ventral and dorsal views (Figs.4E,
F); with tapering laterals; anterior and posterior ends
more acutely tapering. Ventral margin broadly
sinuous, in ventral view (Fig.4E), with large bow-
shaped process. Hinge margin doubly sinuous in
dorsal view (Fig.4F), with conspicuous stragulum.
Muscle scar pattern (Figs.5A, B) with three dorsal
scars and numerous ventral scars.
Distribution – Holocene; South Atlantic, 1240 to
5631m (Figs.2, 13). Angola Basin, 4559 to 5631m,
live and dead specimens (MADDOCKS, 1990).
Eastern Brazilian continental slope, 1240 to
1600m, dead specimens (new records).
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 157
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Remarks – The specimens of Macropyxis adrecta
recorded by MADDOCKS (1990) are larger
(holotype, - RV, h: 0.76mm, l: 2.17mm; LV, h:
0.71mm, l: 2.08mm) than specimens analysed
in the present study (length approximately
1.2mm). The size variation exhibited by
Macropyxis adrecta (approximately 0.9mm) is the
largest in the genus. In other species, the size
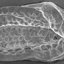
Fig.4- Macropyxis adrecta Maddocks, 1990, MNRJ 18632: (A) right valve of female, (B) left valve of female, (C) right valve
of male, (D) left valve of male, (E) ventral view of carapace, (F) dorsal view of carapace. Scale bar = 1.0mm.
variation of carapace can range from 0.1 to
0.5mm (MADDOCKS, 1990).
Of the three species previously recorded from Brazil,
Macropyxis adrecta is similar to Macropyxis adunca,
while the valves of Macropyxis kornickeri and
Macropyxis similis are lower in relation to length,
with more elongated and acute posterior angles
than Macropyxis adrecta.
A
B
C
D
E
F
158 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
specimens analysed in the present study could not
readily be assessed to any of the two species, because
they present intermediate carapace characters, and
no appendages were available for analysis. In spite
of that, Macropyxis adrecta is recorded from the
samples analysed due to the presence of females with
their distinctive outlines.
MADDOCKS (1990) also described differences in
appendages between Macropyxis adrecta and
Macropyxis adunca. Male fifth appendages and
hemipenis of both species present distinct outlines –
MADDOCKS, 1990: fig.26 (24-25, 30-31), pl.88 (1,
13-14). Macropyxis adunca presents a longer Zenker’s
Organ with the vas deferens irregularly coiled –
MADDOCKS, 1990: pl.100 (10, 13, 15-16) –, while
in Macropyxis adrecta the vas deferens coils more
regularly around it self – MADDOCKS, 1990: pl.102
(10, 13). Finally, Macropyxis adrecta displays the
seventh limb with a longer reflexed seta – MADDOCKS,
1990: fig.61 (5, 8). Unfortunately, the samples did not
present any living specimen for dissection.
Macropyxis adrecta was recorded from the abyssal
region of Angola Basin (MADDOCKS, 1990:59-60).
In the present study, the distribution of this species
is extended to the Western Atlantic.
Macropyxis cf. adunca Maddocks, 1990
(Fig.6)
Macrocypris sp.3 – MADDOCKS, 1977, fig.10.
Macropyxis adunca Maddocks, 1990:61, figs.8(2-3),
9(2-3), 19(6), 22(11), 24(18), 26(30-31), 30(3),
33(2), 41(6), 46(58-59), 50(22), 51(33), 57(8),
58(12), 61(5), 64(7), 66(7), 80(10), pls.14(1-2,
7-8), 15(1-3, 7-8), 58(5), 78(29), 88(13-17),
100(10, 13, 15-19), 111(3), graph 16.
Type-locality – Knorr, cruise 25, station 306,
15°59.5’S, 1°38.5’E, 5550m.
Material examined – Eastern Brazilian continental
slope. Off Espírito Santo State: 21
°12’13’’S,
39°52’05’’W, 1598-1600m, 1 RV, MNRJ 18635. Off
São Paulo State: 24°06’43’’S, 41°52’52’’W, 1556-
1566m, 4 RLV, 11 RV, 8 LV, MNRJ 18747.
Dimensions – MNRJ 18747 - Gender unknown, RV, h:
0.48mm, l: 1.29mm. Gender unknown, RV, h: 0.46mm,
l: 1.23mm. Gender unknown, RV, h: 0.48mm, l:
1.26mm. Gender unknown, RV, h: 0.48mm, l: 1.26mm.
Gender unknown, LV, h: 0.43mm, l: 1.25mm. Gender
unknown, LV, h: 0.41mm, l: 1.24mm. Gender
unknown, LV, h: 0.43mm, l: 1.25mm. Gender
unknown, LV, h: 0.43mm, l: 1.30mm.
Diagnosis – Carapace elongate-ovate in lateral
outline with sinuous contours; dorsal margin
Despite the similarity between Macropyxis adrecta and
Macropyxis adunca, some differences in the carapace
shape can be observed. The valves of Macropyxis
adrecta present strong sexual dimorphism, male and
female valves being well differentiated. On the other
hand, Macropyxis adunca presents a feeble sexual
dimorphism in carapace morphology, and males and
females are only consistently differentiated by the
appendages. The valves of females of Macropyxis
adrecta are easily differentiated from males of the
same species, and also from specimens (males and
females) of Macropyxis adunca, by their more ovate
outline. Furthermore, males of Macropyxis adrecta
present differences when compared to males and
females of Macropyxis adunca. As MADDOCKS (1990)
stated, the carapaces of males of Macropyxis adrecta
have less sinuous outline; more straight ventral
margin; and more broadly rounded anterior margin.
Valves of males and females of Macropyxis adunca
have more sinuous contours; with the mouth region
of the ventral margin being more distinctly indented;
with posterior angle more upswung; and the anterior
margin more narrowly rounded. Otherwise, some
Fig.5- Macropyxis adrecta Maddocks, 1990, MNRJ 18632:
(A) adductor muscle scars of right valve, (B) adductor muscle
scars of left valve. Scale bar = 0.1mm.
A
B
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 159
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
smoothly but gently arched; anterior margin
somewhat narrowly rounded; ventral margin
sinuous, distinctly indented in mouth region and
upswung posteriorly; somewhat flared, truncate,
sinuate posterior angle of about 45° to 55°; zone of
concrescence broad, line of concrescence deeply
scalloped, radial pore canals complexly branching,
arranged in large bundles (MADDOCKS, 1990). In
the present study, only carapaces were available
for analysis. See MADDOCKS (1990:61-62) for
description and illustrations of appendages.
Supplementary description – Carapace small
(Figs.6A, B), with sexual dimorphism weakly
developed, females and males clearly distinguishable
by appendages only. Right valve (Fig.6A) elongate
in lateral outline with low-arched dorsal margin,
greatest height located anterior to midlength;
anterior margin somewhat narrowly rounded. Left
valve (Fig.6B) with dorsal margin with conspicuous
anterodorsal angle; margin segment anterior to this
angle fairly straight; margin segment posterior to
anterodorsal angle unevenly curved, posterodorsal
angle fairly indistinct; greatest height anterior to
midlength. Right and left valves with sinuous ventral
margin; upswung posterior angle of about 45° to
55°. Anterior and posterior zone of concrescence
broad, vestibule constricted to fairly constricted,
anterior line of concrescence deeply scalloped,
posterior line of concrescence fairly straight; anterior
radial pore canals complexly branching, arranged
in large bundles, posterior radial pore canals
straight. Carapace elongate in ventral and dorsal
views (Figs.6E, F); with subparallel laterals; anterior
and posterior ends slightly tapering. Ventral margin
sinuous, in ventral view (Fig.6E), with large bow-
shaped process. Hinge margin sinuous in dorsal view
Fig.6- Macropyxis cf. adunca Maddocks, 1990, MNRJ 18747: (A) right valve, (B) left valve, (C) adductor muscle scars of
right valve, (D) adductor muscle scars of left valve, (E) ventral view of carapace, (F) dorsal view of carapace. Scale bars (A,
B, E, F) = 1.0mm, (C, D) = 0.1mm.
A
B
E
F
C
D
160 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
(Fig.6F), with conspicuous stragulum. Muscle scar
pattern (Figs.6C, D) with three dorsal scars and
numerous ventral scars.
Distribution – Holocene; South Atlantic, 1556 (?)
to 5550m (Figs.2, 13). Angola Basin, 4559 to
5550m, live specimens (MADDOCKS, 1977; 1990).
Brazil Basin, 4818m, live specimen (MADDOCKS,
1977; 1990); Eastern Brazilian continental slope
(?), 1556 to 1600m, dead specimen (new record).
Remarks – The specimens herein identified as
Macropyxis cf. adunca present a more sinuous
outline, and a more upswung posterior angle than
the specimens identified as Macropyxis adrecta.
These differences in outline, and also other
differences in the appendages, were used by
Maddocks (1990:60) to distinguish the two species.
But, as the differences in outline are rather subtle
and no appendages were analysed, it is not possible
to fully ensure the occurrence of Macropyxis adunca
in the samples analysed.
Specimens of Macropyxis cf. adunca analysed in
the present study are smaller (length approximately
1.3mm) than the specimens of Macropyxis adunca
analysed by MADDOCKS (1990) (holotype , RV,
h: 0.80mm, l: 2.14mm; LV, h: 0.75mm, l: 2.09mm).
The zone of concrescence of the specimens analysed
in the present study are slightly wider than the zone
of concrescence of specimens figured by MADDOCKS
(1990: figs.8.2, 3, 9.2, 3, 19.6). Consequently,
vestibules of the former specimens are more
constricted than vestibules of the last specimens,
but this difference can be age dependent.
Macropyxis adunca is differentiated from Macropyxis
kornickeri and Macropyxis similis, which also occur
in the Brazilian waters, by its less elongated and
acute posterior angle. Furthermore, Macropyxis
kornickeri is somewhat larger, with a more sinuous
outline, and Macropyxis similis has a more straight
dorsal margin than Macropyxis adunca.
Macropyxis adunca had previously been recorded
from the abyssal waters of Angola and Brazil Basins
(MADDOCKS, 1990:61-62). In the present, the
bathymetric distribution of this species might be
extended to the continental slope, and the
geographic distribution in the Brazilian waters
might be extended northwards.
Macropyxis amanda Maddocks, 1990
(Figs.7-8)
Macrocypris sp.5 – MADDOCKS, 1977:155, fig.10.
Macropyxis amanda Maddocks, 1990:62, figs.8.6, 7,
9.6, 7, 19.1, 22.7, 24.9, 26.26, 27, 30.10, 33.8,
41.5, 46.54, 55, 67.10, 80.4, pls. 12.1-6, 13.1-
6, 79.1, 2, 83.1, 88.5-7, 102.1-4, graph 17.
Type-locality – Atlantis II, cruise 42, station 200,
9°41’ to 9°43.5’S, 10°55’ to 10°57’E, 2644 to 2754m.
Material examined – Eastern Brazilian continental
slope. Off Espírito Santo State: 21
°12’13’’S,
39°52’05’’W, 1598-1600m, 3 RLV, 8 RV, 5 LV,
MNRJ 18748. Off Rio de Janeiro State: 21°48’22’’S,
39°49’55’’W, 1092-1438m, 1 RLV, 8 RV, 9 LV,
MNRJ 18749; 21°53’11.904’’S, 39°50’44.863’’W,
1240m, 6 RLV, 19 RV, 14 LV, MNRJ 18750;
21°53’45.759’’S, 39°50’22.530’’W, 1300m, 1 RLV,
18 RV, 10 LV, MNRJ 18751.
Dimensions – MNRJ 18748 - Gender unknown,
RV, h: 0.85mm, l: 2.20mm. Apparent , LV, h:
0.78mm, l: 2.25mm. Gender unknown, LV, h:
0.78mm, l: 2.30mm. MNRJ 18749 - Apparent ,
RV, h: 0.83mm, l: 2.20mm. MNRJ 18751 5728 -
Apparent , RV, h: 0.88mm, l: 2.20mm. Apparent
, LV, h: 0.80mm, l: 2.19mm. Apparent , LV,
h: 0.75mm, l: 2.19mm. Apparent , LV, h:
0.80mm, l: 2.26mm.
Diagnosis – Carapace fairly large, elongate-oblong
in lateral outline with pointed posterior angle;
anterior margin slightly obliquely rounded;
ventral margin slightly sinuous to nearly straight,
slightly upswung posteriorly; dorsal margin
broadly arched in a high, sweeping curve,
greatest height located anterior to midlength;
posterior angle sharp, about 45° to 55°; anterior
zone of concrescence fairly wide; radial pore
canals complexly and somewhat irregularly
branching (MADDOCKS, 1990). In the present
study, only carapaces were available for analysis.
See MADDOCKS (1990:61) for description and
illustrations of appendages.
Supplementary description – Carapace large, with
feeble sexual dimorphism, females and males
clearly distinguishable by appendages only. Right
valve (Figs.7A, B) elongate-oblong, somewhat
subtriangular in lateral outline; dorsal margin
broadly arched in a high, sweeping curve, greatest
height near midlength; anterior margin slightly
obliquely rounded. Left valve (Figs.7C, D) with
dorsal margin with slightly conspicuous
anterodorsal angle; margin segment anterior to
this angle fairly straight to slightly concave;
margin segment posterior to anterodorsal angle
evenly curved, posterodorsal angle indistinct;
greatest height anterior to midlength. Right and
left valves with ventral margin slightly indented
to fairly straight; slightly upswung posterior angle,
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 161
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
about 45° to 55°. Anterior zone of concrescence
fairly broad, vestibule constricted to fairly
constricted, line of concrescence irregular, radial
pore canals complexly and somewhat irregularly
branching. Posterior zone of concrescence narrow,
vestibule deep, line of concrescence fairly straight;
posterior radial pore canals straight. Carapace
lozenge-shaped in ventral and dorsal views
(Figs.7E, F); with tapering laterals; anterior end
rounded; posterior end acutely tapering. Ventral
margin sinuous, in ventral view (Fig.7E), with large
bow-shaped process. Hinge margin sinuous in
dorsal view (Fig.7F), with conspicuous stragulum.
Muscle scar pattern (Figs.8A, B) with three dorsal
scars and numerous ventral scars.
Distribution – Holocene; South Atlantic, 1092 to
2754m (Figs.2, 13). Angola Basin, 2644 to 2754m,
live and dead specimens (MADDOCKS, 1977;
1990). Eastern Brazilian continental slope, 1092
to 1600m, dead specimens (new records).
Fig.7- Macropyxis amanda Maddocks, 1990, MNRJ 18750: (A) right valve, (B) left valve, (C) right valve, (D) left valve, (E)
ventral view of carapace, (F) dorsal view of carapace. Scale bar = 1.0mm.
A
B
C
D
E
F
162 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Remarks – In the synonymy of the description of
Macropyxis amanda, MADDOCKS (1990:62)
included “Macrocypris sp.5 MADDOCKS, 1979,
fig.10” [sic], but she probably wanted to refer to
MADDOCKS, 1977. In the study of MADDOCKS
(1979) there are two plates, the plate I figure 10
refers to the dentiform corner of a new species
tentatively assigned to the genus Macrocypris. While
the plate II figure 10 shows the female fifth
appendage of a new species of Macrocyprina.
Otherwise, the figure 10 of MADDOCKS (1977)
illustrates the external view of right valves of seven
species assigned to Macrocypris, and one of these
species is Macrocypris sp.5. Furthermore, the same
specimen illustrated in the earlier study
(MADDOCKS, 1977, fig.10) was also figured in
the description of Macropyxis amanda
(MADDOCKS, 1990: pls.12.1, 13.1). This
specimen appears in both works (MADDOCKS,
1977; 1990) with the same collection number
(1457). A list containing MADDOCKS’ numbers,
the identification, the collecting locality, the
repository and some notes of each specimen is
given by MADDOCKS (1990: appendix IV).
MADDOCKS (1990:62) stated that the valves of
males of Macropyxis amanda are slightly shorter and
distinctly less high than females, with more elongate
outline. Otherwise, through the observation of
MADDOCKS’ (1990) illustrations (pls.12.1-6, 13.1-
6), it is possible to observe that some of the males
and females present similar outlines. Therefore, due
to the absence of appendages, the gender of the
specimens analysed in the present study were not
determined, except by some of the measured
specimens, which were separated in “apparent
females” and “apparent males”.
The specimens analysed in the present study have
shorter carapaces (approximately 2.1mm) than the
African specimens (approximately 2.7mm).
Macropyxis amanda is easily differentiated from
other Brazilian species of this genus. Macropyxis
amanda is larger, with a more subtriangular
outline, a more arched dorsal margin, and more
numerous radial pore canals than Macropyxis
adrecta and Macropyxis adunca. Macropyxis
kornickeri has a more inequilateral outline, with a
more elongated and more acute posterior angle
than Macropyxis amanda. Despite the similar size,
the valves of Macropyxis amanda are easily
separated from Macropyxis similis, which are lower,
with a wider zone of concrescence and more
elongate posterior angle.
The geographic range of Macropyxis amanda,
previously recorded from the Angola Basin, is here
extended to the Southwestern Atlantic. Its depth
range is also extended to shallower waters, from
2644m to 1092m.
Macropyxis bathyalensis (Hulings, 1967)
(Fig.9)
Macrocypris bathyalensis Hulings, 1967a:638,
figs.3f (part), 4r-u; HULINGS, 1967b:314, figs.1
(part), 8a-d, pl.4.
not Macrocypris bathyalensis – MADDOCKS, 1977,
figs.1, 5, 10; CRONIN, 1983:107, figs.A, C, D, pl.4.
Macrocypris minna – TRESSLER, 1941:98, pl.19,
fig.26.
Fig.8- Macropyxis amanda Maddocks, 1990, MNRJ 18750:
(A) adductor muscle scars of right valve, (B) adductor muscle
scars of left valve. Scale bar = 0.2mm.
A
B
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 163
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
? Macrocypris sp. cf. Macrocypris minna – WHATLEY
& COLES, 1987:1, fig.7.
Macropyxis bathyalensis – MADDOCKS, 1990:66,
figs.110.2, 4, 11.2, 4, 18.8, 19.4, 22.2, 10, 24.5,
26.16, 17, 30.7, 12, 33.5, 34.5, 39.5, 46.41-43,
50.24, 51.19, 20, 67.3, 80.7, pls. 12.7-12, 13.7-
12, 22.7, 62.1, 63.7-10, 12, 66.5, 79.11-13, 90.5-
8, 11, 13, 102.11, 12, 103.4-9, graph 22.
Type-locality – Vema, cruise 17, station 118,
55
°50’N, 56°06’W, 3369m.
Material examined – Eastern Brazilian continental
slope. Off Espírito Santo: 21
°14’31’’S, 39°08’00’’W,
2302-2426m, 2 RV, 1 LV, MNRJ 18753. Off São
Paulo State: 24
°06’43’’S, 41°52’52’’W, 1556-1566m,
1 RLV, 5 RV, 9 LV, MNRJ 18752.
Dimensions – MNRJ 18752 - Gender unknown, RV,
h: 0.80mm, l: 1.94mm. Gender unknown, RV, h:
0.70mm, l: 1.94mm. Gender unknown, LV, h: 0.65mm,
l: 1.84mm. Gender unknown, LV, h: 0.60mm, l:
1.81mm. Gender unknown, LV, h: 0.58mm, l: 1.90mm.
Diagnosis – Carapace medium-sized to large,
elongate-oblong in lateral outline, tapered
posteriorly; dorsal margin arching smoothly to
greatest height near midlength in females or slightly
anterior to midlength in males; anterior margin
broadly and fairly evenly rounded; ventral margin
nearly straight to slightly sinuate, with weak
indentation in mouth region, weakly upswung
posteriorly in females, nearly straight in males;
posterodorsal margin sloping steeply and
continuously to sharp posterior angle of 50° to 60°;
anterior zone of concrescence very wide, with
complexly branching radial pore canals arranged
in elaborate bundles, anterior vestibule
correspondingly shallow; posterior zone of
concrescence of moderate width, vestibule deep,
especially in males (MADDOCKS, 1990). In the
present study, only carapaces were available for
analysis. See MADDOCKS (1990:64) for description
and illustrations of appendages.
Supplementary description – Carapace medium-
sized, with sexual dimorphism present but weak,
females and males clearly distinguished only by
appendages. Right valve (Figs.9A, B) elongate-oblong
in lateral outline, tapered posteriorly; dorsal margin
arching smoothly to greatest height near midlength
in females or slightly anterior to midlength in males;
anterior margin broadly and fairly evenly rounded;
ventral margin nearly straight to slightly sinuate.
Dorsal margin of left valve (Figs.9C, D) with slightly
conspicuous to inconspicuous anterodorsal angle,
segment anterior anterodorsal angle concave;
segment posterior to anterodorsal angle evenly
curved, posterodorsal angle fairly indistinct;
greatest height anterior near midlength. Left and
right valves with weak indentation in mouth
region, weakly upswung posteriorly in females,
nearly straight in males; posterodorsal margin
sloping steeply and continuously to sharp
posterior angle of 50° in males and 60° in females.
Anterior zone of concrescence wide (Fig.9A), with
complexly branching radial pore canals arranged
in elaborate bundles (Fig.9B), anterior vestibule
correspondingly shallow; posterior zone of
concrescence (Fig.9B) of moderate width, vestibule
deep, especially in males; line of concrescence
irregular. Carapace narrowly elongated in ventral
and dorsal views (Figs.9E, F); with tapering
laterals. Ventral margin sinuous, in ventral view
(Fig.9E), with large bow-shaped process. Hinge
margin sinuous in dorsal view (Fig.9F).
Distribution – Quaternary; Atlantic Ocean, 1092 to
3834m (Figs.3, 13). Continental slope off
southeastern USA, 201 to 667m, dead specimens
(HULINGS, 1967a). North American Basin, 1135 to
3834m, live and dead specimens (MADDOCKS,
1990). Labrador Basin, 2137 to 3521m, live and
dead specimens (HULINGS, 1967a, 1967b;
MADDOCKS, 1990). European Basin, 1955 to
3230m, dead specimens (TRESSLER, 1941;
MADDOCKS, 1990). Eastern Brazilian continental
slope, 1556 to 2426m, dead specimens (new record).
Remarks – Macropyxis bathyalensis is distinguished
from most other Atlantic species of this genus by its
nearly straight ventral margin and tapering posterior
end (MADDOCKS, 1990). Among the Brazilian
species, Macropyxis adrecta and Macropyxis adunca
are smaller, with more sinuous outlines, and less
arched dorsal margin than Macropyxis bathyalensis.
Macropyxis amanda is slightly larger, proportionately
more elongate, with a less arched dorsal margin and
more elaborately branching radial pore canals when
compared to Macropyxis bathyalensis, and also
presents different sexual dimorphism (MADDOCKS,
1990). Compared with Macropyxis kornickeri,
Macropyxis bathyalensis is similar in shape but
higher in proportion to length, with less prolonged
posterior angle; and greatest height located near
middle length, while in Macropyxis kornickeri
greatest height is anterior to middle length.
The geographic range of Macropyxis bathyalensis,
previously recorded to North Atlantic, is here
extended to the Southwestern Atlantic.
164 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Macropyxis similis (Brady, 1880)
(Figs.10-11)
Macrocypris similis Brady, 1880:42, figs.2a-d;
MÜLLER, 1912:122; PURI & HULINGS,
1976:260, pl.1, figs.13,14.
not Macrocypris similis – SCOTT, 1903:370;
CHAPMAN, 1919:20; figs.2a-d, pl.2; TRESSLER,
1954:433; MADDOCKS, 1977, figs.3, 6, 7; HANAI,
IKEYA & YAHIMA, 1980:121.
Fig.9- Macropyxis bathyalensis (Hulings, 1967), MNRJ 18752 (A, C, E, F), MNRJ 18753 (B, D): (A) right valve of probable
female, (B) left valve of probable female, (C) right valve of probable male, (D) left valve of probable male, (E) ventral view of
carapace, (F) dorsal view of carapace. Scale bar = 1.0mm.
not Macrocypris sp. aff. similis – HERRIG, 1977:1156.
not Macrocypris sp. cf. Macrocypris similis – NEALE,
1967:7, figs.s, s’, t, pl.1.
not Macrocyprina similis – BHATIA, GUHA &
MCKENZIE, 1972:37.
Macropyxis similis – MADDOCKS, 1990:73, figs.10.6,
7, 11.5, 6, 18.6, 22.13, 26.28, 29, 30.5, 34.16, 38.4,
46.49, 50, 67.6, 80.6, pls. 24.2, 3, 11, 12, 25.2, 3,
79.26, 27, 90.9, 10, 14, 103.10-12, graph 29.
A
B
C
D
E
F
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 165
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
margin slightly concave; remaining dorsal
margin evenly curved, posterodorsal angle
indistinct; greatest height located near
midlength. Right valve with ventral margin
slightly indented; left valve with ventral margin
with conspicuous indentation; both valves with
slightly upswung posterior angle, about 30° to
40°. Anterior zone of concrescence broad,
vestibule constricted, line of concrescence
irregular, anterior radial pore canals complexly
and irregularly branching. Posterior zone of
concrescence narrow, vestibule deep, line of
concrescence fairly straight; most of posterior
radial pore canals straight, few slightly
branching. Carapace elongate-ovate in ventral
and dorsal views (Figs.10E, F); with tapering
laterals; anterior end rounded; posterior end
acutely tapering. Ventral margin sinuous, in
ventral view (Fig.10E), with large bow-shaped
process. Hinge margin sinuous in dorsal view
(Fig.10F), with conspicuous stragulum. Muscle
scar pattern (Figs.11A, B) with two dorsal scars
and numerous ventral scars.
Distribution – Holocene; Southwestern Atlantic,
587 to 1600m (Figs.1, 13). Eastern Brazilian
continental slope, 587 to 1600m, live and dead
specimens (BRADY, 1880; MADDOCKS, 1990;
present study).
Remarks – MADDOCKS (1990:74) stated that the
valves of males of Macropyxis similis are as long
as the valves of females but not as high. Because
of the uncertainty in determining the gender of
specimens based solely in caparace outline, and
once the appendages were not available, the
gender of the specimens analysed in the present
study were not determined.
The supposition of MADDOCKS (1990:74) that
“size appears to decrease with depth” might
be confirmed in the present study, as
specimens from southeastern Brazilian waters
are shorter (1600m, approximately 2.1mm)
than specimens collected in northeastern
waters (1234m, approximately 2.3mm; 587m,
approximately 2.5mm).
Macropyxis amanda and Macropyxis similis
occurred in the same samples of eastern
Brazilian continental slope, both species being
the largest Brazilian Macrocyprididae.
The geographic range of Macropyxis similis is here
extended southwards, and its bathymetric range
is also extended to slightly deeper waters (from
1234m to 1600m).
Type-locality – H.M.S.Challenger, station 120,
8°37’S, 34°28W, 675 fathoms (1234m), off
Pernambuco, Brazil, trawled, red mud, surface
temperature 78
° F, September 9, 1873.
Material examined – Eastern Brazilian
continental slope. Off Espírito Santo State:
21
°12’13’’S, 39°52’05’’W, 1598-1600m, 4 RLV, 6
RV, 8 LV, MNRJ 18754. Off Rio de Janeiro State:
21
°48’22’’S, 39°49’55’’W, 1092-1438m, 12 RV, 10
LV, MNRJ 18755 5732; 21°53’11.904’’S,
39°50’44.863’’W, 1240m, 2 RLV, 22 RV, 17 LV,
MNRJ 18756; 21°53’45.759’’S, 9°50’22.530’’W,
1300m, 3 RLV, 16 RV, 10 LV, MNRJ 18757.
Dimensions – MNRJ 18754 – Apparent – RV, h:
0.79mm, l: 2,23mm. Apparent - RV, h: 0.78mm,
l: 2.20mm. Apparent - LV, h: 0.68mm, l:
2.16mm. Apparent - LV, h: 0.73mm, l: 2.16mm.
MNRJ 158755 - Gender unknown - RV, h: 0.75mm, l:
2.18mm. Gender unknown - LV, h: 0.65mm, l: 2.15mm.
MNRJ 18756 - Gender unknown - RV, h: 0.73mm, l:
2.20mm. Gender unknown - RV, h: 0.75mm, l: 2.23mm.
Gender unknown - LV, h: 0.68mm, l: 2.20mm. Gender
unknown - LV, h: 0.68mm, l: 2.18mm.
Diagnosis – Carapace elongate-siliquose in
lateral outline with sinuous contours; dorsal
margin low arched, somewhat irregular, with
greatest height located at midlength; anterior
margin broadly and evenly rounded; ventral
indentation shallow but distinct; posteroventral
margin nearly straight in female, slightly upturned
in male; prolonged, truncate posterior angle of
about 30° to 40°, located a little above the venter,
flared in male; anterior zone of concrescence very
broad, vestibules correspondingly small, anterior
radial pore canals elaborately branching
(MADDOCKS, 1990). In the present study, only
carapaces were available for analysis. See
MADDOCKS (1990:73) for description and
illustrations of appendages.
Supplementary description – Carapace fairly
large to large, with sexual dimorphism present
but weak (Figs.10A-D). Female right valve
(Fig.10A) elongate-oblong, with sinuous
contours; dorsal margin fairly high-arched.
Male right valve (Fig.10B) elongate, with
sinuous contours; dorsal margin low-arched.
Female and male right valves with greatest
height located near midlength; anterior margin
broad and evenly rounded. Left valve very
elongated (Figs.10C, D), dorsal margin with
anterodorsal angle slightly conspicuous in
female and inconspicuous in male; anterodorsal
166 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Genus Macrosarisa Maddocks, 1990
Type species – Macrosarisa bensoni Maddocks, 1990.
Additional species – Macrosarisa capacis Maddocks,
1990; M. elegantula (Whatley & Downing, 1983); M.
exquisita (Kaye, 1964); M. graysonensis (Alexander,
1929); M. hiulca Maddocks, 1990; M. muensteriana
(Jones & Hinde, 1890); M. siliqua (Jones, 1849); M.
simplex (Chapman, 1898); M. texana Maddocks,
1990; M. wrightii (Jones & Hinde, 1890).
Remarks – The genus Macrosarisa, with 11
described species, occurs in the Atlantic and
southwestern Indic, with a live depth range from
40 to 3783m, and with a geologic range from the
Lower Cretaceous (Albian) to Recent. The length
of adult carapace of species of Macrosarisa varies
from 0.8mm in Macrosarisa graysonensis to
2.5mm in Macrosarisa wrightii.
Only one species of Macrosarisa was this far
recorded from Brazil. MADDOCKS (1990:94)
reported Macrosarisa sp.23, which is the same
species as the one described below.
Fig.10- Macropyxis similis (Brady, 1880), MNRJ 18756 (A-D), MNRJ 18754 (E-F): (A) right valve of probable female, (B) left
valve of probable female, (C) right valve of probable male, (D) left valve of probable male, (E) ventral view of carapace, (F)
dorsal view of carapace. Scale bar = 1.0mm.
A
B
C
D
E
F
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 167
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Macrosarisa sp.1
(Fig.12A)
Macrosarisa sp.23 – MADDOCKS, 1990:94,
figs.12.16, 28.44, 45, 35.18, 44.1, 47.9, 68.3,
80.21, pls.28.1-3, 29.1-3, 74.2, 4, 83.12, 84.1,
91.7, 8, 104.8, 9, graph 53.
Material examined – Eastern Brazilian continental
slope. Off Rio de Janeiro: 21
°14’31’’S, 39°08’00’’W,
2302-2426m, 1 LV, MNRJ 18758.
Dimensions – Gender unknown – LV, h:
0.49mm, l: 1.54mm.
Description – Carapace thin, medium-sized, left
valve (Fig.12A) elongate in lateral outline; dorsal
margin unevenly arched; greatest height located
near midlength; anterior margin narrowly truncate;
anteroventral dentiform corner conspicuous;
ventral margin nearly straight, slightly upswung
posteriorly; posteroventral angle truncate, of about
45°; anterior and posterior zone of concrescence
very narrow, with straight radial pore canals;
anterior and posterior vestibules deep.
Fig.11- Macropyxis similis (Brady, 1880), MNRJ 18756: (A) adductor muscle scars of right valve, (B) adductor muscle
scars of left valve. Scale bar = 0.2mm.
A
B
168 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Distribution – Holocene; Southwestern Atlantic,
2302 to 3783m (Figs.1, 13). Brazil Basin, 3730 to
3783, live specimen (MADDOCKS, 1990). Eastern
Brazilian continental slope, 2302 to 2426m, dead
specimen (new record).
Remarks – The outline of the specimen analysed in
the present study (Macrosarisa sp.1) is very similar
to the outline of specimen of Macrosarisa sp.23 of
MADDOCKS (1990:94, fig.12.16, pls.28.1-3, 29.2,
3), what indicates that they are a single species.
The specimen analysed by MADDOCKS (1990) is
larger (1.94mm) than specimen analysed in the
present study (1.54mm). This difference could
indicate a size variation related to depth or
geographic locality. Otherwise, the smallest
specimen could also be a juvenile; with the largest
specimen being an adult (MADDOCKS, 1990). The
posterior region of the valves, which can be
shortened in juveniles (MADDOCKS, 1990), is
similar in both specimens. The calcified inner
lamella and zone of concrescence, which are
narrower in juveniles, were not figured by
MADDOCKS (1990). And, finally, the absence of
the appendages in the specimen analysed herein
makes it impossible to know its age.
This is the only species of Macrosarisa this far
recorded from Brazil, being conspicuously
distinct from other species of the genus, with “the
more elongate and the less angulate, sinuous
outline than any other living species” of
Macrosarisa, as stated by MADDOCKS (1990:94).
Its outline resembles the outline of the
Cretaceous (Cenomanian) species Macrosarisa
graysonensis (Alexander, 1929), but some
differences are observed: Macrosarisa
graysonensis has the carapace higher in
proportion to length; and has a more sinuous
ventral margin, with anterior and posterior angles
projected ventrally, while ventral margin of
Macrosarisa sp.1 is nearly straight. Furthermore,
appendages of this unnamed species also present
distinctive features when compared to other
species of Macrosarisa: hemipenis outline; vas
deferens very thick and stiff, not coiled or looped,
shaped like an elongate question mark, and more
than twice as long as the muscularized part of
the Zenker’s organ (MADDOCKS, 1990:74,
pls.91.7, 8, 104.8, 9). Therefore, this unnamed
species probably constitute a new taxon. It will
not be named in the present study due to the
insufficient material available.
Macrosarisa sp.23 was recorded by MADDOCKS
(1990) for the equatorial Atlantic, Brazil Basin, from
3730 to 3783m. In the present study, the
geographic range of this species is extended
southwards, and its bathymetric range is extended
to the continental slope (2302m).
Genus Macroscapha Maddocks, 1990
Type species – Macroscapha atlantica Maddocks,
1990.
Additional species – Macroscapha gyreae
Maddocks, 1990; M. heroica Maddocks, 1990; M.
inaequalis (Müller, 1908); M. inaequata Maddocks,
1990; M. jiangi Maddocks, 1990; M. marchilensis
(Hartmann, 1961); M. opaca Maddocks, 1990; M.
sinuata Maddocks, 1990; M. tensa (Müller, 1908);
M. turbida (Müller, 1908).
Remarks – The genus Macroscapha, with 11
described species, occurs worldwide, with a live
depth range from 16 to 3694m, but recorded dead
up to 9m, and known only from the Holocene. The
genus Macroscapha is widely distributed in
sublittoral and bathyal depths, being best
represented in the Antarctic and southern regions
of Southern Hemisphere. The length of adult
carapace of species of Macroscapha varies from
1.3mm in Macroscapha gyreae to 2.5mm in
Macroscapha tensa.
There was only one record of Macroscapha from
Brazil. MADDOCKS (1990:108) recorded one
unnamed specimen as Macroscapha sp.30.
Macroscapha aff. inaequata Maddocks, 1990
(Figs.12B-D)
Type-locality – Eltanin, cruise 6, station 350, 55°03’
- 55
°00’S, 58°57' - 58°51’W, 2452m.
Material examined – Eastern Brazilian continental
slope. Off Rio de Janeiro State: 21°53’11.904’’ S,
39°50’44.863’’W, 1240m, 1 RV, 1 LV, MNRJ 18760.
Off São Paulo State: 24
°06’43’’S, 41°52’52’’W, 1556-
1566m, 1 RV, MNRJ 18759.
Dimensions – MNRJ 18760 – RV, h: 0.64mm, l:
1.60mm. LV, h: 0.61mm, l: 1.55mm.
Description – Carapace fairly small, elongate-
subtriangular to oblong in lateral outline
(Figs.12B, C); posterodorsal margin of right valve
sloping more acutely than anterodorsal margin;
dorsal margin with indistinct dorsal angle;
greatest height at midlength; anterior margin
evenly rounded, without distinct angles; ventral
indentation conspicuous, remainder of ventral
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 169
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
margin nearly straight, posterior end slightly
upswung; posterior angle very broadly rounded,
about 40°, located at or slightly above the venter;
zone of concrescence fairly broad, especially in
posterodorsal and anteroventral portions; most
of radial pore canals straight, few slightly
branching. Muscle scar pattern (Figs.12D) with
three dorsal scars and numerous ventral scars
arranged in three groups.
Distribution – Holocene; Southwestern Atlantic
(Figs.1, 13). Eastern Brazilian continental slope,
1240 to 1566m, dead specimens (new record).
Remarks – The specimens analysed here are
considered Macroscapha aff. inaequata because,
despite their a great similarity to Macroscapha
inaequata, some differences could be observed
between them and the typical representatives of
the latter species. The specimens of Macroscapha
aff. inaequata present evenly rounded anterior
margin, while specimens of Macroscapha
inaequata present angulate anterior margin –
MADDOCKS, 1990: fig.14 (15-16); Macroscapha
aff. inaequata has an unevenly sloping dorsal
margin, while in Macroscapha inaequata the
dorsal margin slopes evenly to anterior and
posterior ends. Furthermore, Macroscapha aff.
inaequata is slightly smaller (1.5-1.6mm) than
specimens analysed by MADDOCKS (1990: graph
47) (1.8-2.2mm). In this way, the specimens
analysed in the present study can represent a
new species, but more material with soft parts is
necessary to solve this question. Only 2 right and
1 left valves were examined, and the lack of
appendages prevents a better description.
There was only one previous record of
Macroscapha from Brazil, Macroscapha sp.30
(MADDOCKS, 1990:108) collected from the
eastern Brazilian continental slope (834-839m).
The juvenile female (last instar) figured by
MADDOCKS (1990: figs.52.41, 53.40, 64.19) is
smaller (length 1.17mm), but similar to
specimens analysed in the present study. In this
way, the specimens analysed in the present study
and that analysed by MADDOCKS (1990) may be
included in a single species.
Macroscapha inaequata was previously recorded
in 7 samples from the Antarctica and southeastern
tip of South America (MADDOCKS, 1990), from
55° to 75°S. If the specimens analysed in the
present study are considered Macroscapha
inaequata, the geographic range of this species
should be extended northwards.
Macroscapha sp.1
(Fig.12E)
Material examined – continental slope off
Rio de Janeiro State, Brazil. 21°53’45.759’’S,
39°50’22.530’’W, 1300m, 1 juvenile damaged
RV, MNRJ 18761.
Dimension – MNRJ 18761 - RV, h: 0.70mm, l:
1.78mm.
Description – Carapace medium-sized, oblong-
elongate (Fig.12E) in lateral outline; dorsal
margin low arched; greatest height slightly
anterior to midlength; anterior margin evenly,
broadly rounded; ventral margin nearly straight;
anterior and posterior zone of concrescence very
narrow, with straight radial pore canals; anterior
and posterior vestibules shallow.
Distribution – Holocene; Southwestern Atlantic
(Figs.1, 13). Eastern Brazilian continental slope,
1300m, dead specimen (new record).
Remarks – The unnamed female juvenile
( Macroscapha sp.30) recorded from
northeastern Brazilian continental slope by
MADDOCKS (1990:108, figs.52.41, 53.40,
64.19), is considerably smaller (length
1.17mm), with a wider vestibule, more sinuous
ventral margin, and more arched dorsal
margin, than specimen examined herein.
Therefore, these two specimens are considered
different species.
Although the specimen analysed in the present
study (Macroscapha sp.1, Fig.12E) resembles
adults of Macroscapha marchilensis (Hartmann,
1965) or juveniles of Macroscapha atlantica
Maddocks, 1990, Macroscapha inaequata
Maddocks, 1990, Macroscapha opaca
Maddocks, 1990, and Macroscapha sinuata
Maddocks, 1990 (MADDOCKS, 1990:
figs.52.19-21, 29, 30, 53.13, 24, 25, 26), some
differences could be noted. These five species
have a more arched dorsal margin, and a more
sinuate ventral margin than Macroscapha sp.1.
Furthermore, Macroscapha atlantica and
Macroscapha sinuata have posterodorsal
concavity, while in Macroscapha sp.1 the
posterodorsal margin is straight; and
Macroscapha opaca and Macroscapha sinuata
have a more truncate anterior margin, while in
Macroscapha sp.1 the anterior margin is
broadly rounded. Therefore, Macroscapha sp.1
might be a new species, but more material is
necessary for analysis.
170 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
Fig.12- Macrosarisa sp.1, MNRJ 18758: (A) left valve; Macroscapha aff. inaequata Maddocks, 1990, MNRJ 15737: (B) right
valve, (C) left valve, (D) adductor muscle scars of right valve; Macroscapha sp.1, MNRJ 15738: (E) right valve. Scale bars
(A-C, E) = 1.0mm, (D) = 0.1mm.
Fig.13- Bathymetric distribution of the macrocypridid species reported from the deep-sea off Brazil: (1) Macrocyprina sp.1
of COIMBRA (1995) and COIMBRA et al. (1999), (2) Macropyxis bathyalensis, (3) Macropyxis kornickeri, (4) Macropyxis
similis, (5) Macroscapha sp.2, (6) Macrosarisa bensoni, (7) Macropyxis sp.1 of MADDOCKS (1990), (8) Macropyxis amanda,
(9) Macropyxis adrecta, (10) Macroscapha aff. inaequata, (11) Macroscapha sp.1, (12) Macropyxis adunca, (13) Macrocypris
maioris, (14) Macrosarisa sp.1, (15) Macropyxis sp.2 of MADDOCKS (1990).
A
C
D
B
E
0
1000
2000
3000
4000
5000
6000
123456789101112131415
Sp ecies
Depth
BRAZILIAN DEEP-SEA MACROCYPRIDIDAE MÜLLER, 1912 (CRUSTACEA, OSTRACODA, MACROCYPRIDOIDEA) 171
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
DISCUSSION
Eight species were observed in the six samples
analysed: Macropyxis adrecta, Macropyxis adunca,
Macropyxis amanda, Macropyxis bathyalensis,
Macropyxis similis, Macrosarisa sp.1, Macroscapha
aff. inaequata and Macroscapha sp.1. Macropyxis
adrecta and Macropyxis amanda, previously
collected in the Eastern Atlantic, are recorded for
the first time from the Western Atlantic. Macropyxis
bathyalensis, previously recorded from the North
Atlantic, is recorded for the first time from the South
Atlantic. Macropyxis similis has its geographic range
extended southwards.
After the present study, a total of nine named species
and six unnamed species of Macrocyprididae have
been recorded from Brazilian deep-sea. The known
distribution of these species is rather punctual due
to the low number of samples analysed (Figs.1-3).
Additional sampling over Brazilian continental slope
and abyssal plain will probably increase the number
of species recorded from these regions.
All 15 species of deep-sea Macrocyprididae recorded
from Brazil are endemic to the Atlantic Ocean. Four
of them, Macropyxis kornickeri, Macropyxis similis,
Macrocyprina sp.1, Macrosarisa sp.1, have been
recorded exclusively from Brazilian waters, being
endemic to Brazil Basin and the adjacent continental
slope (Fig.1). Macropyxis adrecta, Macropyxis adunca,
Macropyxis amanda, and Macrocypris maioris occur
in the South Atlantic (Fig.2), these four species occur
in the Brazil Basin and adjacent continental slope,
and in the Angola Basin; and Macrocypris maioris
occurs also in the Sierra Leone Basin. Macropyxis
bathyalensis and Macrosarisa bensoni occur in the
South and North Atlantic (Fig.3). Macropyxis
bathyalensis occurs on the eastern Brazilian
continental slope, in the North American, Labrador,
and European Basins. Macrosarisa bensoni occurs
on the continental slopes off northeastern Brazil and
southeastern USA. The remaining five species,
Macropyxis sp.1, Macropyxis sp.2, Macroscapha aff.
inaequata, Macroscapha sp.1 and Macroscapha sp.2,
which were left in open nomenclature, have doubtful
geographic distribution (Fig.1). The records of the
species previously recorded from off Brazil are listed
in table 1.
ACKNOWLEDGEMENTS
I thank Paulo S. Young (Museu Nacional - Rio de
Janeiro – MNRJ), for taxonomic advise and his
prompt availability for discussions; João C.
Coimbra (Universidade Federal do Rio Grande do
Sul – UFRGS), for teaching me in the beginning of
my studies on Ostracoda, and for providing
bibliography; Renner Baptista (MNRJ) for revising
the English; Dietmar Keyser (Universität
Hamburg), for providing bibliography; Cristiana
S. Serejo (MNRJ), for several comments; Koen
Martens (Royal Belgian Institute of Natural
Sciences); Louis C. Kornicker (Smithsonian
Institution, and Anne C. Cohen (University of
California), for kindly stimulating me in the
beginning of my study on Ostracoda; Gustavo
A. Carvalho (Universidade do Estado do Rio de
Janeiro), for inking some of the drawings. This
study was supported by Fundação Universitária
José Bonifácio (FUJB), Fundação de Amparo à
Pesquisa do Estado do Rio de Janeiro (FAPERJ),
Conselho Nacional de Desenvolvimento
Científico e Tecnológico (CNPq), and
Coordenação de Aperfeiçoamento de Pessoal de
Nível Superior (CAPES).
LITERATURE CITED
BHATIA, S.B.; GUHA, D.K. & MCKENZIE, K.G., 1972.
Check list of Ostracoda recorded from the Indian
Continent and Ceylon (1841-1971). In: MCKENZIE,
K.G. (Ed.) Shallow marine and fresh-water
Ostracoda of Thetys IPU Study Group. Cheltenham:
Standard Commercial Printers. 55p.
BRADY, G.S., 1880. Report on the Ostracoda dredged
by H.M.S.Challenger during the years 1873-1876.
Report on the Scientific Results of the Voyage of
H.M.S.Challenger, Zoology, Edinburgh, 1(3):1-184.
BRANDÃO, S.N., in press. Three New species of
Macrocyprina Triebel, 1960 (Crustacea, Ostracoda,
Macrocyprididae) from Brazilian shallow waters.
Zoosystema, Paris.
CHAPMAN, F., 1919. Ostracoda. Australasian Antarctic
Expedition 1911-14, Scientific Reports, Series C.
Zoology and Botany, Sydney, 5(7):1-45.
COIMBRA, J.C., 1995. Ostracodes Recentes e Sub-
recentes da Plataforma Continental Equatorial do
Brasil – Taxonomia, Zoogeografia e Ecologia. Porto
Alegre, 204p. Tese (Doutorado em Geociências),
Curso de Pós-Graduação em Geociências, Instituto
de Geociências, Universidade Federal do Rio
Grande do Sul.
COIMBRA, J.C.; PINTO, I.D.; WÜRDIG, N.L. & CARMO,
D.A., 1999. Zoogeography of Holocene Podocopina
(Ostracoda) from the Brazilian Equatorial shelf.
Marine Micropaleontology, New York, 37:365-379.
COIMBRA, J.C.; RAMOS, M.I.F. & SANGUINETTI, Y.T.,
1992. Sub-Recent Ostracodes of the Tamandaré Bay,
Northeastern Brazil – A Preliminary Report on
Biofacies. Pesquisas, Porto Alegre, 19(1):94-105.
172 S.N.BRANDÃO
Arq. Mus. Nac., Rio de Janeiro, v.62, n.2, p.151-172, abr./jun.2004
CRONIN, T.M., 1983. Bathyal ostracodes from the
Florida-Hatteras slope, the straits of Florida, and the
Blake Plateau. Marine Micropaleontology, New
York, 8:89-119.
DIAS-BRITO, D.; MOURA, J.A. & WÜRDIG, N., 1988.
Relationships between Ecological Models Based on
Ostracods and Foraminifers from Sepetiba Bay (Rio
de Janeiro – Brazil). In: HANAI, T., IKEYA, N. &
Ishizaki, K. (Eds.) Evolutionary Biology of
Ostracoda, its Fundamentals and Applications.
Proceedings of the Ninth Internation Symposium
on Ostracoda, Shizuoka, Japan. Tokyo: Kodansha/
Elsevier. p.467-484.
HANAI, T.; IKEYA, N., & YAHIMA, M., 1980. Checklist of
Ostracoda from Southeast Asia. The University of
Tokyo Museum Bulletim, Tokyo, 17:1-236.
HERRIG, E., 1977. Ostracoden aus dem Plio-?Pleistozän
der Socialistischen Republik Vietnam, Teil 1. Zeitschrift
für Geologische Wissenschaften, Berlin, 5:1153-1167.
HULINGS, N.C., 1967a. Marine Ostracoda from the
western North Atlantic Ocean between Cape Hatteras,
North Carolina, and Jupiter Inlet, Florida. Bulletin
of Marine Science, Coral Gables, 17:629-659.
HULINGS, N.C., 1967b. Marine Ostracoda from western
North Atlantic Ocean: Labrador Sea, Gulf of St.
Lawrence, and off Nova Scotia. Crustaceana, Leiden,
13(3):310-328.
MADDOCKS, R.F., 1977. Zoogeography of
Macrocyprididae (Ostracoda). In: LÖFFLER, H. &
DANIELOPOL, D. (Eds.) Aspects of Ecology and
Zoogography of Recent and Fossil Ostracoda. The
Hague: Junk Publishers ). p.147-157.
MADDOCKS, R.F., 1979. The “dentiform corner” of
Macrocypria, and some other peculiar and
pathological structures observed in Macrocyprididae.
In: KRSTIC, N. (Ed.) Taxonomy, Biostratigraphy and
Distribution of Ostracodes, Proceedings of the VII
International Symposium on Ostracoda. Belgrade:
Serbian Geological Society. p.247-250.
MADDOCKS, R.F., 1990. Living and fossil Macrocyprididae
(Ostracoda). University of Kansas Paleontological
Contributions, Monograph, Texas, 2:1-404.
MÜLLER, G.H., 1912. Ostracoda. Das Tierreich, Berlin,
31:1-434.
NEALE, J.W., 1967. An ostracod fauna from Halley Bay,
Coasts Land, British Antarctic Territory. British
Antarctic Survey Scientific Reports, London,
58:1-50.
PURI, H.S. & HULINGS, N.C., 1976. Designation of
lectotypes of some Ostracoda from the Challenger
Expedition. Bulletin of British Museum (Natural
History), Zoology, London, 29:249-315.
SCOTT, A., 1903. Report on Ostracoda collected by Prof.
Herdman, at Ceylon, in 1902. In: HERDMAN, W.A.
(Ed.) Report to the Government of Ceylon on the
Pearl Oyster Fisheries of the Golf of Manaar, with
supplementary reports upon the marine biology
of Ceylon by other naturalists (part 3). Manaar:
Royal Society of London. p.365-384.
TRESSLER, W.L., 1941. Geology and Biology of North
Atlantic deep-sea cores. Part 4. Ostracoda.
Geological Survey, Professional Paper, Ipoh, 196-
C:95-106.
TRESSLER, W.L., 1954. Marine Ostracoda. In:
GALTSOFF, P.S. (Ed.) The Gulf of Mexico, its Origin,
Waters, and Marine Life. Fishery Bulletin 89 of
the Fish and Wild life Service, Washington,
55:429-437.
WHATLEY, R. & COLES, G., 1987. The Late Miocene to
Quaternary Ostracoda of Leg 94, Deep Sea Drilling
Project. Revista Española de Micropaleontologia,
Madrid, 19:33-97.