Content uploaded by Eiji Tanaka
Author content
All content in this area was uploaded by Eiji Tanaka
Content may be subject to copyright.
Mycoscience (2002) 43:87–93 © The Mycological Society of Japan and Springer-Verlag Tokyo 2002
FULL PAPER
Eiji Tanaka · Chihiro Tanaka · Abdul Gafur
Mitsuya Tsuda
Heteroepichloë
, gen. nov. (Clavicipitaceae; Ascomycotina) on bamboo plants
in East Asia
Received: August 8, 2001 / Accepted: November 1, 2001
Abstract The causal agents of witches’ broom of bamboo
plants in East Asia, Epichloë bambusae and E. sasae, were
morphologically and phylogenetically examined. The phy-
logenetic studies were conducted using ITS 1, 2, and 5.8S
rDNA regions. Both Epichloë species produce Ephelis-type
conidia in artificial medium and are phylogenetically situ-
ated in different clades from Epichloë and Parepichloë.
Here, we propose a new genus Heteroepichloë for these two
bambsicolous Epichloë species.
Key words Aciculosporium take · Ephelis-type conidia ·
Epichloë bambusae · Epichloë sasae · Parepichloë
Introduction
Grass endophytes and a related fungal group have received
widespread attention because they have peculiar relation-
ships with their host plants. Among them Epichloë typhina
(Pers.: Fr.) Tul., which causes choke disease of cool season
grasses and related Neotyphodium Glenn et al. orphans, are
extensively studied because of their economical importance
and their ecological significance.
The members of Epichloë (Fr.) Tul. et C. Tul. have been
described on various host species and considered miscella-
neous assemblages to be reexamined (White 1994). The
species on warm season grasses and bamboo plants are the
pertinent quarries. Two Epichloë species on bamboos
have been recorded in East Asia, namely, E. bambusae Pat.
(Patouillard 1897) and E. sasae Hara (Hara 1922; Hino
E. Tanaka · C. Tanaka · M. Tsuda (*)
Graduate School of Agriculture, Kyoto University, Sakyo-ku,
Kitashirakawa, Kyoto 606-8502, Japan
Tel. ⫹81-75-753-6305; Fax ⫹81-75-753-6312
e-mail: tsudam@kais.kyoto-u.ac.jp
A. Gafur
University of Lampung, Bandar, Lampung, Indonesia
1961), respectively; they cause witches’ broom of bamboo
plants. Epichloë sasae was originally described by Hara on
Sasa spp. (Hara 1922), which are small bamboo plants
grown in understories of Japanese beech forests in the
Japanese archipelago. Epichloë bambusae was described by
Patouillard on Gigantochloa spp. (Patouillard 1897), which
are large and tall bamboo plants grown at roadsides or in
cultivated stands in Indonesia and other tropical areas of
Asia. Witches’ broom of bamboo plants caused by
Aciculosporium take I. Miyake (anamorph: Albomyces take
I. Miyake, Clavicipitaceae) is also distributed in Japan and
other East Asia regions. Numerous greatly shortened
shoots emerge at the newly developing branchlets of the
host bamboo plants. The newly developing tillers and leaves
are deformed and dwarfed. Recently, Aciculosporium take
has been rampantly breaking out in bamboo stands of
commercial forests and ornamental and exhibitional gar-
dens in the Japanese archipelago, especially Phyllostachys
bambusoides Siebold et Zucc. It was confirmed that A. take
infected 5 genera, 17 species of bamboos including Sasa spp.
(Sect. Sasa) besides Phyllostachys spp. (Tsuda et al. 1997).
The production of bamboo culms is reduced, and the scen-
ery created by bamboo stands is severely damaged and
disturbed in an unsightly manner.
The symptoms and signs caused by E. bambusae are
considerably different from those caused by A. take
(Gäumann 1927; Tsuda et al. 1997). The signs of A. take can
be easily differentiated those of E. bambusae and E. sasae
by the production of whitish minute anamorphic stroma,
on which pale brown, wartlike teleomorphic stroma de-
velops in summer. The signs of both Epichloë species on
Sasa species (Hino 1961) and Gigantochloa species
(Gäumann 1927), respectively, are almost the same in ap-
pearance. Unfortunately, the identity or taxonomical rela-
tionship between the two Epichloë species has not been
revealed.
In 1998, White and Reddy (1998) examined the phyloge-
netic relationships of some Epichloë species, including both
species on bamboo plants, and a Balansia species, B.
cynodontis Syd., on grasses and proposed the new genus
of Parepichloë typified by E. cinerea Berk. et Broome on
88
Eragrostis and Sporobolus species. In their treatment, the
two Epichloë species inhabiting bamboo plants are included
in this new genus without considering molecular data but
based only on the superficial morphological resemblance of
herbarium materials.
In the course of our phylogenetic studies on clavici-
pitaceous fungi, we have been aware of the differences of
the Epichloë species recorded on bamboos from those
found on grasses. Fortunately, we were able to collect these
Epichloë species on Sasa and Gigantochloa and E. cinerea
on Sporobolus species. We examined their morphological
characteristics using fresh materials and analyzed their
phylogenetic relationships by comparing DNA sequences.
Here, we present the taxonomic position of these two
bambsicolous Epichloë species.
Materials and methods
Collections
Sources, sample names, and GenBank accession numbers
of the isolates used are indicated as follows.
Epichloë sasae: collected at Botanical Garden of
Hokkaido University, Sapporo-shi, Hokkaido, on Sasa
sp. (Sect. Sasa) (E. sasae-H; AB065432), Ashu Experimen-
tal Forest of Kyoto University, Miyama-cho, Kyoto on
Sasa sp. (Sect. Sasa) (E. sasae-K; AB065430), and Shiga-
Kogen, Yamanouchi-cho, Nagano on Sasa sp. (E. sasae-N;
AB065431).
Epichloë bambusae: collected at Bogor Botanical
Garden, Bogor, on Gigantochloa sp. (E. bambusae Bo-01;
AB065428, Bo-02; AB065429), northern part of Bandung
on Gigantochloa sp. (E. bambusae Ba-01; AB065426),
and Lembang on Gigantochloa sp. (E. bambusae Le-01;
AB065427), Java Island, Indonesia.
Epichloë cinerea: collected at Ilam, Eastern Nepal on
Sporobolus sp. (E. cinerea Ne-01; AB065425).
Aciculosporium take: collected at Uji-shi, Kyoto on
Pleioblastus gramineus (Bean) Nakai (Sect. Nezasa) (A.
take Nezasa; AB065422), Uji-shi, Kyoto on Phyllostachys
bambusoides (A. take Madake; AB065423), Chiyoda-ku,
Tokyo on Phyllostachys bambusoides var. castilloni-inversa
Houz. de Leh. (A. take Ginmeichiku; AB065424), Ashu
Experimental Forest of Kyoto University, Miyama-cho,
Kyoto, on Sasa sp. (Sect. Sasa) (A. take Chimakizasa;
AB066293), and Yoro Bamboo Garden, Gifu on Sasa sp.
(Sect. Lasioderma) (A. take Nambusuzu; AB066292).
The specimens were dehydrated by silica gel drying for
morphological observations and DNA analyses. Cultures
were also obtained from both Epichloë species on bamboo
plants. These species were cultured at 25°C on complete
medium [CM: 0.15% Ca (NO3)2·4H2O, 0.05% KCl, 0.05%
MgSO4·7H2O, 0.04% KH2PO4, 0.003% K2HPO4, 0.1%
yeast extract, 0.1% tryptone, 1% glucose (w/v)] by the
method of Nakada et al. (1994). All voucher specimens or
isolates are deposited at the herbarium of the Kyoto Uni-
versity Museum (KYO).
Morphological observations
Morphological observations were routinely conducted on
dehydrated materials of silica gel-killed materials as well as
on cultured materials.
DNA sequencing and molecular phylogenetic studies
The fungal genomic DNA was extracted from silica gel-
dried materials or liquid cultures on complete medium
according to the method of Nakada et al. (1994). The ex-
tracted DNA were stored in tetraethyl (TE) buffer at
⫺20°C. The ITS 4 and ITS 5 primers amplified the ITS 1,
2, and 5.8 S rDNA regions, as described by White et al.
(1990). Polymerase chain reaction (PCR) was conducted
using Taq polymerase (Takara, Otsu, Japan) on a PCR
Thermal Cycler (TP-3000; Takara). The PCR products
were purified and cloned on plasmid vector pZErOTM-2
(Invitrogen, CA, USA). They were sequenced by the
Sanger method using an ALFred DNA sequencer
(Amersham Pharmacia Biotech, Uppsala, Sweden). The se-
quencing reaction was completed with the Amersham
sequencing kit (Thermo sequenase fluorescent labeled
primer cycle sequencing kit with 7-deaza-dGTP) using Cy-5
fluorescent primers, M13-20 and M13-Rvs (Amersham
Pharmacia Biotech).
The sequence data were edited with the software
package DNAsis-Mac (version 3.0; Hitachi Software
Engineering, Tokyo, Japan). We used the newly analyzed
sequences of Epichloë bambusae, E. sasae, A. take, and E.
cinerea (⫽ P. cinerea), together with the sequences of other
clavicipitaceous fungi used in our previous study (Tanaka
et al. 2001). The sequences were aligned with CLUSTAL W
(Thompson et al. 1994).
Phylogenetic analyses were performed with the soft-
ware PAUP 3.12 (Swofford 1993) and PHYLIP (version
3.72; Felsenstein 1993), using DNADIT, NEIGHBOR,
SEQBOOT, DNAML, and CONSENSE. Phylogenetic
trees were constructed using parsimony with a heuristic
search and maximum likelihood. The bootstrap analysis was
implemented using 100 replicates of heuristic searches to
determine the confidence levels of the inferred phylogenies
(Felsenstein 1985).
Results
Morphological study
Some different characteristics were found between the two
species on bamboo plants and Parepichloë species proposed
by White and Reddy (1998). Those Parepichloë species
have not been documented for the anamorph. However,
E. bambusae and E. sasae produced anamorphic conidia
on CM agar medium. They were morphologically similar
to Ephelis-type conidia accommodated in some clavicipi-
taceous fungi. The conidia were unicellular, filiform, and
hyaline, 5.8 ⫾ 0.5 ⫻ ca. 1.5 µm for E. bambusae and 8.5 ⫾ 0.8
89
⫻ ca. 1.6µm for E. sasae (Fig. 1A,B). They were holoblasti-
cally born on hyaline and simple conidiophores.
The ascostromata of the two Epichloë species on bam-
boo plants develop on the leaf sheath of host plants (Fig.
2A,B). At the young stage, the surface is yellowish in E.
bambusae and purplish in E. sasae. The ascostromata are
guided by the growth of the inrolled young leaves and pro-
trude from the apex of the sheath or burst up from the
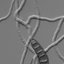
Fig. 1. Morphological character-
istics of Heteroepichloë sasae
and H. bambusae. A Conidia of
Heteroepichloë sasae produced
on CM agar medium. B Conidia
of Heteroepichloë bambusae
produced on CM agar medium.
C Ascus of Heteroepichloë sasae.
D Ascus of Heteroepichloë
bambusae. E Ascus tip of
Heteroepichloë sasae in an
ascostromata. F Ascus tip of
Heteroepichloë bambusae in an
ascostromata. G Asci and part-
spores of Heteroepichloë sasae.
H Ascus arrangement of
Heteroepichloë bambusae.
Bars A,B,G 20µm, C,D 25 µm;
E,F 10µm; H 50 µm
90
middle part of the sheath. They grow loosely twisted up to
10 cm long. They are soft and fleshlike in the sheath, and
later become hard and glutinous. The perithecia are embed-
ded and arranged regularly in the ascostromata (Fig. 2C,D).
The asci are abundantly produced in the perithecium
and spread when the perithecium is crushed (Fig. 1C,D,H).
Their ascal tips are round and somewhat thickened (Fig.
1E,F), but their capitation is not so prominent as E. typhina
nor so flattened as E. cinerea, as depicted by Sharma and
Tewari (1969) and White and Reddy (1998). Ascospores are
hyaline, filiform, and multiseptated and easily fragmented
to dumbbell shaped part-spores in the ascus (Fig. 1G).
These figures concur with those given by Gäumann (1927)
for E. bambusae and by Hino (1961) for E. sasae.
The type species of Parepichloë J.F. White et P.V.
Reddy, P. cinerea (Berk. et Broome) J.F. White et P.V.
Reddy (⫽ E. cinerea), grows on the inflorescences of warm
season grasses, Eragrostis and Sporobolus spp., and en-
circles the whole of the inflorescence. The ascostromata are
brittle and perithecia are randomly and sparsely arranged
in the ascostromata, as pointed out by White and Reddy
(1998) and previous authors (e.g., Sharma and Tewari
1969). E. cinerea (⫽ P. cinerea) materials from Nepal used
in this work morphologically fit the previous descriptions
(Mhaskar and Rao 1976; White 1994).
Phylogenetic studies
We found two base differences in the ITS 1 sequences
between our collection and P. cinerea sequences obtained
from GenBank. This variation is considered to occur at the
intraspecific level, compared to the variation in P. sclerotica
(Pat.) J.F. White et P.V. Reddy in which four base differ-
ences have been recognized. We could not find mature asci
in our materials. The ascus tip of P. cinerea is clearly differ-
ent from our bambusicolous species (cf. fig. 8 in White and
Reddy 1998; fig. 6 in Sharma and Tewari 1969). Ascospores
of P. cinerea are also easy to separate to part-spores; they
are not dumbbell shaped but cylindrical.
The phylogenetic analysis using ITS 1, 2, and 5.8S
rDNA sequences was also combined with our previous
study (Tanaka et al. 2001). Epichloë bambusae and E. sasae
formed a different independent group from Parepichloë
or Epichloë group in a parsimonious and a maximum-
likelihood tree (Figs. 3, 4). They showed some similarities
to Myriogenospora species on Andropogon virginicus L. or
Erianthus contortus Baldw. ex Elliot in the parsimonious
tree. The E. bambusae-E. sasae group was clearly different
from P. cinerea, which was located within the Balansia
group. It was suggested that Parepichloë species were more
closely related to Balansia than to Epichloë species (Tanaka
Fig. 2. Gross morphology of
Heteroepichloë sasae and H.
bambusae. A Ascostromata of
Heteroepichloë sasae produced
on Sasa sp. B Ascostromata of
Heteroepichloë bambusae
produced on Gigantochloa sp.
C Perithecial arrangement of
Heteroepichloë sasae in an
ascostromata. D Perithecial
arrangement of Heteroepichloë
bambusae in an ascostromata.
Bars A,B 1 cm; C,D 100µm
91
et al. 2001). The results did not support the conclusions of
White and Reddy (1998) that the bambusicolous Epichloë
species belong to the genus Parepichloë. Aciculosporium
take were included in an independent group related to
Claviceps africana Freder., Mantle et De Miliano and C.
sorghicola Tsukib., Shimam. et T. Uematsu.
Discussion and taxonomy
Morphological characteristics of E. bambusae and E. sasae
clearly bear little structural similarity to those of the genus
Epichloë, typified by E. typhina. The recent treatment of
Fig. 3. One of 13 trees of bambusicolous Epichloë (Epichloë sasae and
Epichloë bambusae) and other related clavicipitaceous fungi inferred
from a parsimony analysis of ITS 1,-2, and 5.8S regions of rDNA.
Heuristic search found 13 equally parsimonious trees. Topological dif-
ferences among the 13 trees were intraspecific changes of Ephelis or
Epichloë isolates. The values shown at the nodes are the confidence
levels from 100 replicate bootstrap samplings. The tree length was 789,
the consistency index (CI) was 0.615, the homoplasy index (HI) was
0.385, the retention index (RI) was 0.833, and the rescaled consistency
index (RC) was 0.512. Newly analyzed sequences are shown in bold-
face. The data obtained from GenBank are indicated with their acces-
sion numbers. Bar is branch length
92
White and Reddy (1998) for both fungi as members of
Parepichloë is also considered to be incorrect. The genus
Parepichloë was established mainly on the basis of the phy-
logenetic topology calculated from nucleotide sequences
of the ITS 1 region of E. sclerotica Pat., E. schumanniana
Henn. (treated as E. sclerotica), E. cinerea, and Balansia
cynodontis by comparing to other warm season grass-
inhabiting epibiont-related species. Unfortunately, the
members included in the genus, such as E. oplismeni Henn.
and E. volkensii Henn., together with E. sasae and E.
bambusae, were only considered by comparing morphologi-
cal characteristics of herbarium materials.
The morphology of hypothallus (or stromata) is believed
to be important in these epibiotic clavicipitaceous fungi
(Diehl 1950; White 1994). If so, whether the species en-
circles entire tillers or inflorescences or part of foliage of
host plants or not might be one of the morphological char-
acteristics that have some expression in the evolution
process. The stroma of P. cinerea clearly encircle the
whole inflorescence of host plants. The ascostroma of P.
cynodontis (⫽ B. cynodontis) also entirely encircles tillers
of Cynodon dactylon (L.) Pers. Another species, P. scle-
rotica (⫽ E. sclerotica), also has hornshaped ascostromata
half-encircling the tillers or inflorescences of gramineous
grasses. Their ascostromata are somewhat brittle. In
contrast, the ascostromata of Epichloë species on the
Bambusoides do not encircle the whole of the new leaves
and are hard and glutinous. Thus, the difference among
Epichloë species typified by E. typhina, Parepichloë, and
Epichloë species on the Bambusoides is clear.
Judging from these results, it is wise to treat Epichloë
species of the Bambusoides as belonging to a separate ge-
nus from both Epichloë and Parepichloë. Here we propose
a new genus for these species hitherto treated as a member
of Epichloë or Parepichloë.
Fig. 4. Phylogenetic relationships of bambusicolous Epichloë (E. sasae
and E. bambusae) and other related clavicipitaceous fungi inferred
from a maximum-likelihood analysis of ITS 1,-2, and 5.8 S regions of
rDNA. The Ln likelihood was ⫺2967.07132 and the estimated transi-
tion/transversion ratio was 1.553007. The values shown at the nodes are
the confidence levels from 100 replicate bootstrap samplings. Newly
analyzed sequences are shown in boldface. The data obtained from
GenBank are indicated with their accession numbers
Heteroepichloë E. Tanaka, C. Tanaka, Abdul Gafur et
Tsuda, gen. nov.
Stromatibus primum folium inevolutum in vagina folii
circumdatis, postremo relaxtus spiraliter emergentibus,
atris, solitariis, primo carnosis, tandem coriaceis ad maturi-
tatum vel in sicco; peritheciis in stromate immersis, oblongis
vel ovato-oblongis, apice ostiolatis, aparaphysatis; ascis
unitunicatis, cylindraceis, apice rotundatis et incrassatis;
ascosporis fasciculatis, filiformibus vel linearibus, in maturi-
tate separatis. Forma anamorpha sporis filiformibus
praedita Ephelidis similis.
Stromata primarily on leaves within a sheath, half encir-
cling the leaves, later emerging from the sheath by loosely
spiraled development, black, fleshy when young, becom-
ing hard and glutinous when mature or on desiccation;
perithecia embedded in stroma, ovate with nonemerged
to slightly emerged ostioles; asci cylindrical, with thickened
rounded apex; ascospores filiform, septate, hyaline.
Anamorph, filiform, Ephelis-type spores present.
This genus is similar to Epichloë and Parepichloë, but
differs most notably in possession of an epibiotic habit and
stromata that have black surfaces with glutinous texture
when mature.
Type species: Heteroepichloë bambusae (Pat.) E.
Tanaka, C. Tanaka, Abdul Gafur et Tsuda, comb. nov. ⫽
Epichloë bambusae Pat., Ann. Jard. Buitenz. I (suppl):125–
126, 1897.
Basionym: ⫽ Parepichloë bambusae (Pat.) J.F. White et
P.V. Reddy, Mycologia 92:231, 1998.
Other species included in the genus: Heteroepichloë
sasae (Hara) E. Tanaka, C. Tanaka, Abdul Gafur et Tsuda,
comb. nov. ⫽ Epichloë sasae Hara, Shizuokaken-nokaiho
300:163, 1922 (basionym) ⫽ Parepichloë sasae (Hara) J.F.
White et P.V. Reddy, Mycologia 92:231, 1998.
The two Heteroepichloë species on bamboo plants have
very similar morphology but the phylogenetic comparison
clearly separates them into different species (see Fig. 3).
They share common characteristics such as scleroid stro-
mata developing on the leaf sheath. When at the young
93
stage, the stomata are yellowish in H. bambusae and pur-
plish in H. sasae.
Other species included in Parepichloë by White and
Reddy (1998), such as P. volkensii and E. oplismeni without
determining the molecular phylogenetic relationships,
should await future examination. Thus, here we retained
our comments for taxonomic treatments. More precise evi-
dence on both a phylogenetic and morphological basis is
needed.
The epibiont species of Balansia and its allied genera
have been recorded on some bambusoid plants (Diehl
1950). Balansia linearis (Rhem) Diehl and Balansiopsis
gaduae (Rhem) Höhn., both of which have several syn-
onyms, are recorded from South America. They are clearly
different from fungal species of the Asian bamboo in
their morphology by lacking scleroid ascostromata. Some
other species, such as Epichloë warbulgiana Magnus and
Echinodothis tuberiformis (Berk. et Ravenel) G.F. Atk., are
reported as an epibiont of bambusoid grasses by White
(1994). However, they may be different species judging
from his descriptions. In the meantime, a fungus looking
like Heteroepichloë on Ochlandra tranvancoria (Bedd.)
Benth. was identified as B. linearis in India. The morpho-
logical characteristic of B. linearis cited by Diehl based on
original descriptions and materials from South America is
thin filmy stromata (Diehl 1950). Judging from the figures
provided by Mohanan (Mohanan 1997; figs. 50, 52), the
fungus on O. tranvancoria might not be B. linearis but
rather a new member of Heteroepichloë or H. bambusae
itself.
The host plants of H. bambusae are mainly distributed in
tropical Asia and those of H. sasae are distributed in cool
regions of the Japanese archipelago, where the host plants
are covered with snow for several months. The hosts of the
former are big bamboos and the hosts of the latter are Sasa
species, which are considered to be indigenous to the north-
ern part of northern Asia. Investigation on the origin of
both species is therefore very interesting. However, the
evolutionary differentiation of bamboo plants is not fully
understood (Suzuki 1978). In the Japanese archipelago,
Aciculosporium take seems to have an identical ecological
niche as H. bambusae in large bamboo plants in tropical
areas. The fungus sometimes has been wrongly cited as
Balansia take (I. Miyake) Hara, and it frequently shares
host species such as Sasa species with H. sasae in cool
regions.
Acknowledgments We thank Dr. Y. Doi, National Science Museum,
Tokyo, and Dr. M.K. Ajikari, National Herbarium and Plant Labora-
tory, Ministry of Forest and Soil Conservation, Nepal, for collecting E.
cinerea specimens. We also thank to two anonymous reviewers for their
kind suggestions on the revision of the manuscript.
References
Diehl WW (1950) Balansia and the Balansiae in America. Agricul-
ture Monograph No. 4. U.S. Department of Agriculture, (USDA),
Washington, DC
Felsenstein J (1985) Confidence limits on phylogenies: an approach
using the bootstrap. Evolution 39:783–791
Felsenstein J (1993) PHYLIP (phylogeny inference package),
version 3.572c. Department of Genetics, University of Washington,
Seattle
Gäumann E (1927) Mycologische Mitteilungen III. Ann Mycol 25:167–
177
Hara K (1922) Nihon Gaikingaku, Shizuokaken-nokaiho (in
Japanese). 300:163
Hino I (1961) Icones Fungorum Bambusicolorum Japonicorum. The
Fuji Bamboo Garden, Gotenba
Mhaskar DN, Rao VG (1976) Development of the ascocarp in
Epichloë cinerea (Clavicipitaceae). Mycologia 68:994–1001
Mohanan C (1997) Diseases of bamboos in Asia. International Devel-
opment Research Centre, New Delhi
Nakada M, Tanaka C, Tsunewaki K, Tsuda M (1994) RFLP analysis
for species separation in genera Bipolaris and Curvularia.
Mycoscience 35:271–278
Patouillard N (1897) Enumerations des champinions recoltes a Java
par M. Massart. Ann Jard Bot Buitenzorg I (suppl):107–127
Sharma BB, Tewari JP (1969) Epichloë cinerea on Sporobolus indicus
from India. Mycopathol Mycol Appl 37:221–224
Suzuki S (1978) Index to Japanese Bambusaceae. Gakken, Tokyo
Swofford DL (1993) PAUP: phylogenetic analysis using parsimony,
version 3.11. Illinois Natural History Survey, Champaign, IL
Tanaka E, Kawasaki S, Matsumura K, Kusuda R, Tanaka C, Peng Y,
Tsukiboshi T, Tsuda M (2001) Phylogenetic studies of Ephelis
species from various locations and hosts in Asia. Mycol Res 105:811–
817
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving
the sensitivity of progressive multiple sequence alignment through
sequence weighting, position specific gap penalties and weight
matrix choice. Nucleic Acids Res 22:4673–4680
Tsuda M, Shimizu K, Matsumura K, Tanaka E, Tanaka C, Doi Y
(1997) Host range of Aciculosporium take, the causal agent of
witches’ broom of bamboo plants. Bull Natl Sci Mus Tokyo Ser B
23:25–34
White JF Jr (1994) Taxonomic relationships among the members of
the Balansiae (Clavicipitales). In: Bacon CW, White JF Jr (eds)
Biotechnology of endophytic fungi of grasses. CRC Press, Boca
Raton, pp 3–20
White JF Jr, Reddy PV (1998) Examination of structure and molecular
phylogenetic relationships of some graminicolous symbionts in gen-
era Epichloë and Parepichloë. Mycologia 90:226–234
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct
sequencing of fungal ribosomal DNA for phylogenetics. In: Innis
MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a
guide to methods and applications. Academic Press, New York, pp
315–322