Content uploaded by Luit Slooten
Author content
All content in this area was uploaded by Luit Slooten
Content may be subject to copyright.
Plant
Physiol.
(1995)
107:
737-750
Factors Affecting the Enhancement
of
Oxidative Stress
Tolerance
in
Transgenic Tobacco Overexpressing Manganese
Superoxide Dismutase
in
the Chloroplasts'
Luit Slooten*, Katelijne Capiau, Wim Van Camp, Marc Van Montagu, Chris Sybesma,
and
Dirk
lnzé
Vrije Universiteit Brussel, Laboratorium
voor
Biophysica, Pleinlaan
2,
B-I
050
Brussels, Belgium
(L.S.,
K.C.,
C.S);
and Laboratorium voor Genetika (W.V.C.
M.V.M.)
and Laboratoire associé d'lnstitut National de Ia Recherche
Agronomique
(D.I.),
Universiteit Gent,
K.L.
Ledeganckstraat
35,
B-9000 Gent, Belgium
Two varieties of tobacco (Nicofiana fabacum var PBD6 and var
SR1)
were used to generate transgenic lines overexpressing
Mn-
superoxide dismutase (MnSOD) in the chloroplasts. The overex-
pressed MnSOD suppresses the activity of those
SODs
(endogenous
MnSOD and chloroplastic and cytosolic Cu/ZnSOD) that are prom-
inent in young leaves but disappear largely or completely during
aging of
the
leaves. The transgenic and control plants were grown at
different light intensities and were then assayed for oxygen radical
stress tolerance in leaf disc assays and
for
abundance of antioxidant
enzymes and substrates in leaves. Transgenic plants had an en-
hanced resistance to methylviologen (MV), compared with control
plants, only after growth at high light intensities. In both varieties
the activities of FeSOD, ascorbate peroxidase, dehydroascorbate
reductase, and monodehydroascorbate reductase and the concen-
trations of glutathione and ascorbate (all expressed on a chlorophyll
basis) increased with increasing light intensity during growth. Most
of these components were correlated with MV tolerance.
It
is
argued that SOD overexpression leads to enhancement of the tol-
erance to MV-dependent oxidative stress only if one or more of
these components
is
also present at high levels. Furthermore,
the
results suggest that in var
SR1
the overexpressed MnSOD enhances
primarily the stromal antioxidant system.
The photosynthetic electron transport chain in higher
plant chloroplasts contains, at the acceptor side
of
PSI,
a
number of auto-oxidizable enzymes. For example, Fd in the
reduced state can react with oxygen, yielding the superox-
ide anion radical
(OJ
(Furbank and Badger, 1982; Asada
and Takahashi, 1987), and it has been shown that superox-
ide is also generated in the aprotic membrane interior
(Elstner and Frommeyer, 1978; Takahashi and Asada,
1988). Superoxide does not seem to be particularly toxic,
although it does inactivate a number of metal-containing
enzymes such as Fd-linked nitrate reductase, catalase, and
peroxidases (reviewed by Asada and Takahashi, 1987).
However, superoxide will dismutate nonenzymically and
This
work
was supported
by
grant
No.
2-91-2-21-700-5
(VLAB/067)
from
the
Flemish
Ministry
of
Economic
Affairs
and
grant
No.
3.0104.90
from
the
Belgian
National
Fund
for
Scientific
Research
("0).
D.I.
is
a
Research Director
of
the
Institut
Na-
tional
de
la
Recherche Agronomique
(France).
*
Corresponding author: e-mail
lslooten8vnet3.vub.ac.be;
fax
32-2-6293389.
73
7
enzymically (see below) into hydrogen peroxide and oxy-
gen. The hydrogen peroxide can react in turn with super-
oxide in what
is
known as a metal-catalyzed Haber-Weiss
reaction. This yields the hydroxyl radical
(OH.),
one of the
most reactive species known to chemistry. In chloroplasts,
the Fe atoms of Fd seem to fulfill the role of metal catalysts
(Elstner and Konze, 1974; Elstner et al., 1978). The hydroxyl
radical can also be formed from hydrogen peroxide and
reduced Fd (Hosein and Palmer, 1983; Bowyer and Camil-
leri, 1985). The hydroxyl radical can initiate self-propagat-
ing peroxidative reactions leading to the destruction of
membrane lipids and of DNA, and hence to extensive
tissue damage (see Bowler et al., 1992, for review). In
addition, there is some evidence that part
of
the superoxide
generated in illuminated chloroplasts diffuses toward the
thylakoid lumen. Because
of
the low pH in the lumen
during illumination, the superoxide can be protonated in
that compartment, yielding the perhydroxyl radical (HO,
).
Unlike the superoxide anion, the perhydroxyl radical can
initiate lipid peroxidation directly (reviewed by Asada and
Takahashi, 1987). The conditions leading to this type of
damage will be referred to as oxidative stress.
Two key enzymes for oxygen radical detoxification in the
chloroplast are SOD (EC 1.15.1.1) and APx (EC 1.11.1.11).
SOD catalyzes the dismutation of two molecules of super-
oxide into oxygen and hydrogen peroxide. APx reduces
hydrogen peroxide to water, with ascorbate as electron
donor. SODs are classified, according to their metal cofac-
tor, as FeSOD, MnSOD, or Cu/ZnSOD. Chloroplasts gen-
erally contain Cu/ZnSOD and, in a number of plant spe-
cies, FeSOD (Bowler et al., 1992). Whereas the distribution
of SODs in the chloroplast is not known, APx was recently
found to occur in a stromal and a membrane-bound form
(Miyake and Asada, 1992, 1994). It was proposed that the
re-reduction of the reaction product of APx, monodehy-
droascorbate, proceeds along different pathways depend-
ing on the type of APx involved. The reaction product of
stromal APx would be reduced by NADPH, either directly
Abbreviations: APx,
ascorbate
peroxidase; DHAR, dehy-
droascorbate
reductase; GR, glutathione reductase; MDHAR, mo-
nodehydroascorbate
reductase;
MV,
methylviologen;
aI1,
quantum
efficiency
for
exciton
trapping
by
the
PSII
reaction
center
in
dark-
adapted material;
o,,
standard
deviation
of
the
mean;
SOD,
su-
peroxide dismutase.
738
Slooten et al. Plant Physiol.
Vol.
107,
1995
or via glutathione. The enzymes taking part in this
so-
called ascorbate-glutathione cycle (Foyer and Halliwell,
1976; Nakano and Asada, 1981) are GR (EC 1.6.4.2), DHAR
(EC 1.8.5.11, and MDHAR (EC 1.6.5.4). The latter three are
stromal enzymes (see Asada and Takahashi [1987] and
Halliwell i19871 for reviews). By contrast, the reaction
product of membrane-bound APx would be re-reduced
directly by the photosynthetic electron transport chain
(Miyake and Asada, 1992). In either case the end result is
"pseudo-cyclic electron flow" involving both photosys-
tems, but without net oxygen evolution. Experiments with
"0
indicated that the occurrence of pseudocyclic electron
flow is largely confined to conditions in which assimilatory
electron flow is inhibited (Marsho et al., 1979).
In tobacco, MnSOD occurs in the mitochondria, FeSOD
in the chloroplasts, and Cu/ZnSOD in the chloroplasts as
well as in the cytosol (Bowler et al., 1992).
To
assess the role
of SOD in oxidative stress tolerance we have, as we re-
ported earlier, generated transgenic plants of
Nicotiana
tabacum
var PBD6 that express elevated levels of MnSOD,
derived from
Nicotiana plumbaginifolia,
either in the chloro-
plasts (ChlSOD plants) or in the mitochondria (MitSOD
plants) (Bowler et al., 1991). The influence of the overex-
pressed enzyme on the resistance of the plants against
oxidative stress was investigated in model systems in
which leaf discs were floated on aqueous solutions of MV.
This water- and lipid-soluble compound is reduced in the
light by the photosynthetic electron transport chain, but it
is also (although less rapidly) reduced in the dark by other
as yet unidentified sources. When reduced MV reacts with
oxygen, it yields superoxide. The ensuing oxygen radical
damage was estimated from ion leakage out of the leaf
discs, or from decreases in activity of the reaction center of
PSII. In light-incubation experiments, only ChlSOD plants
were significantly more resistant to MV than control plants.
In dark-incubation experiments, both ChlSOD and MitSOD
plants were significantly more resistant to MV than control
plants (Bowler et al., 1991; Slooten et al., 1992). In addition,
the ChlSOD plants exhibited an up to 3- or 4-fold increase
in tolerance to ozone compared with control plants (Van
Camp et al., 1994). Meanwhile, other groups succeeded in
enhancing the stress tolerance of plants by SOD overex-
pression. Thus, alfalfa
(Medicago sativa)
transformed with
the same construct that we used for the ChlSOD plants was
more tolerant to freezing stress (McKersie et al., 1993).
Potato plants
(Solanum tuberosum)
overexpressing chloro-
plast Cu/ZnSOD from tomato had an increased tolerance
to MV (Perl et al., 1993), and overexpression of chloroplas-
tic Cu/ZnSOD from pea in the chloroplasts of
N.
tabacum
rendered the plants more tolerant to MV as well as to
photoinhibition (Sen Gupta et al., 1993a, 1993b).
The present work grew out of observations suggesting,
first, that plants grown at high light were more tolerant to
MV than plants grown at low light and, second, that over-
expression of MnSOD in the chloroplasts enhanced the
tolerance to MV only when the plants were grown at high-
light intensities. Both observations could be explained by
considering that in the chloroplast antioxidant system,
SODs are one link in a chain of enzymes. We hypothesized
that overexpression
of
SOD would enhance thc oxidative
stress tolerance only when other antioxidant enzymes or
substrates did not limit the oxygen-radical-scat enging ca-
pacity. Growth at high-light intensities would enhance ex-
pression of these other enzymes or substrates, thus increas-
ing the tolernnce to MV and, in addition, a1 owing the
overexpressed SOD to cause a further enhancement
of
the
oxidative-stress tolerance.
To test this hypothesis we grew transgenic and nontrans-
genic plants at different light intensities and measured the
tolerance to MV as well as the abundance of enzymes and
substrates known to be involved in oxygen re dica1 scav-
enging. In addition, we correlated the observetl MV toler-
ante
with the abundance of a11 antioxidant enzymes and
substrates investigated. Such correlations were 2xpected to
yield clues as to which enzymes or substrates w 2re limiting
the
oxygen-radical-scavenging
capacity under 3ur growth
conditions. This may serve as a guideline for selecting
enzymes that should be overexpressed in order to enhance
the oxidative-stress tolerance of the plants.
MATERIALS AND METHODS
Plant Material and
Growth
Conditions
Two varieties of
Nicotiana tabacum
were Ujed for the
genetic transformations: var PBD6 and var Petit Havana
SR1. Transgenic PBD6 plants overexpressing MnSOD in
the chloroplasts were described by Bowler et al. (1991), and
the
SR1
transgenics were obtained in a similar way. Briefly,
the coding sequence of the mature, mitochondiial MnSOD
of
Nicotiana plumbaginifolia
was coupled in-fríime behind
the chloroplast transit sequence of the pea Rubisco (for
details, see Bowler et al., 1991). Under the control
of
the
cauliflower mosaic virus 355 promoter, this construct al-
lows overproduction of MnSOD in the chloroplasts. Pri-
mary transformants, selected on kanamycin,
w
ere assayed
for MnSOD activity. Transformants overexpressing Mn-
SOD to high levels and containing a single lacus T-DNA
insertion were made homozygous. One homozygous line
was used for future research. The transgenics overproduc-
ing MnSOD in chloroplasts will be referred to as ChlSOD-
PBD6 and ChlSOD-SR1.
The plants were grown on peat-based compcst and were
given fertilizer once
a
week. Most
of
the plants were grown
at light intensities (between 400 and 700 nm) of approxi-
mately 40, 90, or 135 pmol m-*
s-'
in a 12-11 light/l2-h
dark cycle, with day and night temperatures of about 22
and 16"C, respectively. Illumination was provided by halo-
gen-metal vapor discharge lamps with a daylight spectrum
(HQI/T-D, Osram, München, Germany), except at the low-
est light intensity, for which cool-white and red (Fluora,
Osram) fluorescent tubes were used. Unless indicated oth-
erwise, the experiments were carried out with the seventh
to ninth leaf from the top at 10 to 13 weeks after sowing.
One series of experiments was carried out with plants
grown at
800
pmol m-'
s-';
for these expuiments the
seedlings were transferred to a growth chamber at 2 weeks
after sowing. In the growth chamber they rectzived a 16-h
light/8-h dark cycle with day and night temperatures of 25
Mn
Superoxide Dismutase-Overproducing Tobacco 739
and 22"C, respectively. During the first 6 d the light inten-
sity was gradually raised from 360 to
800
kmol
mP2
s-I.
The assays were carried out 15 to 18 d after transfer to the
growth chamber.
Assessment
of
Oxidative-Stress Tolerance
The oxidative-stress tolerance of the plants was assessed
as
described previously (Bowler et al., 1991). Briefly, leaf
discs were incubated overnight in the dark at room tem-
perature with water or an aqueous solution of MV. They
were then illuminated for 2 h at 30 kmol m-'
s-'
provided
by cool-white fluorescent tubes and were subsequently
incubated for another 20 h at 28°C. The MV-dependent
oxygen radical damage was estimated first from ion leak-
age out of the leaf discs, due to destruction of membrane
lipids. Ion leakage was measured as an increase in the
conductance of the floating solution. In addition, we mea-
sured the MV-dependent decrease in activity of the reac-
tion center of
PSII.
As a measure of this activity we used
a,,.
The fluorescence measurements were made with a
PAM fluorometer (Walz, Effeltrich, Germany). The average
values of three to four leaf discs at each MV concentration
(including zero) were used to calculate the MV-induced
increase in conductance of the floating solution or the
MV-induced decrease in
aI1.
The decrease in
aI1
was ex-
pressed
as
a percentage of the
QII
value in leaf discs with-
out MV.
Alternatively, leaf discs floating on water at 4°C were
either kept in the dark or illuminated with white light at
360 pmol m-'
s-'
for 4 h. Following this treatment the leaf
discs were dark-incubated at room temperature for 1 h, and
thereafter the light-induced damage was estimated from
the decrease in the activity of
PSII,
as above.
Biochemical Assays
All biochemical assays were done with leaf disc extracts
prepared by homogenization in ice-cold mortars. Debris
was pelleted by centrifugation at 40,OOOg for 30 min. The
activity of APx was determined according to Chen and
Asada (1989), that of GR according to Schaedle and
Bassham (1977), that of DHAR according to Asada (1984),
and that
of
MDHAR according to Polle et al. (19901, with
minor modifications. For these enzymes, 1 unit of activity
equals
1
pmol/min. The extraction buffer for these assays
was as described by Chen and Asada (1989). Total ascor-
bate was determined according to Okamura (1980), and
total glutathione was determined according to Griffith
(1980). For these assays the leaf discs were extracted as
described by Tanaka et al. (1985).
SOD activity was determined first in a solution assay,
and second after an activity staining following electro-
phoresis on nondenaturing polyacrylamide gels (Bowler et
al., 1991). The gels were stained by illuminating them for
exactly 10 min in the assay mixture described by
Beauchamp and Fridovich (1973) under weak light (30
pmol m-'s-') provided by two fluorescent tubes. After
staining, the gels were scanned with a one-dimensional
scanning densitometer. The relative contribution of each of
the SOD isoforms to the overall activity was determined
from the contribution of the area under the corresponding
peak of the densitogram to the total area. The overall SOD
activity in leaf disc extracts was determined in the solution
assay with the same assay mixture. In the solution assay,
1
unit of SOD activity causes a half-maximal inhibition of the
rate of light-induced, riboflavin-mediated reduction of ni-
troblue tetrazolium. In both assays the activity was mea-
sured with and without 2 mM KCN, which causes a virtu-
ally complete inhibition
of
Cu/ZnSOD (Geller and Winge,
1984).
Chl was determined according to Arnon (19491, as mod-
ified by Lichtenthaler (1987). All assays were done within
5
d with the same leaf. Results are usually presented as mean
?
a,. a,
equals the population
SD
divided by
Jn,
where
n
is the number of experiments. The significance of the dif-
ference between means was assessed with a one-sided
t
test.
RESULTS
Control Experiments on MV-lnduced Oxygen Radical
Damage
As reported earlier, prolonged dark incubation of leaf
discs on aqueous solutions of MV can cause significant
oxygen radical damage (Bowler et al., 1991). However,
under the conditions used in the present experiments the
damage was dependent mainly on light-induced electron
transport. This is demonstrated in Figure 1, which shows
the effect of DCMU, an inhibitor of photosynthetic electron
transport acting at the leve1 of
QA
(a plastoquinone mole-
cule bound to the reaction center of
PSII).
Without DCMU,
illumination in the presence of MV caused
a
strong de-
crease in PSII activity (as estimated from
@,,)
and a pro-
nounced increase in the conductance of the floating solu-
tion. Both effects were almost completely prevented by the
inclusion of DCMU in the incubation mixture. This indi-
cates that under the present assay conditions the damage
arose within the chloroplast and then spread outward,
causing damage to the cell membranes and ion leakage. As
expected, DCMU had no protective effect when it was
added immediately after illumination of the MV-treated
leaf discs (not shown).
Oxidative Stress Tolerance in Transgenic and
Nontransgenic Plants in Relation to Light lntensity
Received during Growth
In most of the remaining experiments, the resistance
against MV was tested using a MV concentration of 2
PM.
At this concentration large differences in MV sensitivity
were observed between plants grown at light intensities of
40 and 135 pmol m-'s-' (see below). However, leaf discs
from plants grown at
800
pmol m-'
s-'
were virtually
insensitive to
2
p~
MV.
These leaf discs were accordingly
treated with 5 to
7
p~
MV. Figure 2 shows that the damage
induced by 2
p~
MV was less extensive in plants grown at
higher light intensities. This was the case in var PBD6
(Fig.
2,
A and
B),
as
well as in vai SR1 with plants grown at light
intensities ranging from 40 to 135 pmol m-'s-' (Fig. 2, C
740
Slooten et al. Plant Physiol.
Vol.
107,
1995
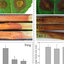
Figure
1. DCMU prevents MV-mediated oxy-
gen radical damage. Data were obtained with
nontransgenic PBD6. The experiment was car-
ried out as described in "Material5 and Meth-
ods," except that the MV concentration was
varied.
N.A.,
No
further additions. Where indi-
cated, DCMU (20
PM)
was added along with
o
DCMU
MV. Error bars indicating population
SD
for four
replicas are shown only if their magnitude
ex-
O123456 23456
N.A.
5
-
:
100
-5
I-
DCMU
__.
O
ceeds that of the symbols.
and D). ChlSOD-PBD6 plants were on average more toler-
ant of MV than control plants, independent of the light
intensity received during growth. However, the differences
between transgenic and control plants were more signifi-
cant after growth at higher light intensities. In addition, the
attenuation of MV-induced ion leakage in the transgenic
plants (Fig. 2A) was more extensive and more significant
than the attenuation of MV-induced decrease in
mI1
(Fig.
2B).
In ChlSOD-SR1 plants, the transgenic MnSOD had no
positive effect at a11 on the average MV-induced decrease in
mI1
(Fig. 2D). However, it did attenuate the MV-induced
ion leakage (Fig. 2C), but only when the plants were grown
at relatively high light intensities; in fact, the differences
between transgenic and control plants were significant
only when the plants were grown at
800
pmol m-'s-'.
Taken together, these data indicate that the protection pro-
vided by overexpressed MnSOD against MV-dependent
oxidative stress is not a static characteristic, but is depen-
dent on (i.e. is positively correlated with) light intensity
received during growth. This extends our earlier finding
that the protection provided by overexpressed MnSOD
against MV-dependent oxidative stress increased with in-
creasing age of the leaf and the plant (Bowler et al., 1991).
With var PBD6 grown at 135 pmol m-*
s-',
as well as
with var SR1 grown at 135 or
800
pmol m-'
s-',
we found
that the transgenic MnSOD had no detectable effect on the
Figure
2.
MV-dependent conductance increase
A
extent of photoinhibition induced by illuminalion at 4°C
(not shown).
Abundance
of
SODs
in Transgenic and
Nontransgenic Plants in Relation to Light lntensity
Received during Crowth
In leaf extracts from nontransgenic plants of var SR1 and
var PBD6, the total SOD activity was on tlie average
around
50
units/g fresh weight, fairly independent of the
light intensity received during growth. The activity in
transgenic plants of var SRl and var PBD6 was on the
average approximately
40
and
100%
higher, rctspectively,
than in the nontransgenic plants (data not shown). The
difference between the two varieties was not dite to differ-
ent levels of transgenic MnSOD activity, but rather to a
differential suppression of endogenous activities in the
transgenic plants of var SR1 and var PBD6, as will be
detailed below.
Figure
3
shows some densitometer scans of SOD activity
gels
obtained with
leaf
extracts from var
PB136.
Similar
patterns were obtained with extracts from v2.r
SR1
(not
shown). In the nontransgenic plants, four peaEa were ob-
served after staining without
KCN
(left, dashed line). These
peaks represent mitochondrial MnSOD (band
1);
a nonre-
solved band representing chloroplastic FeSOD ,and cytoso-
PBD6
B
PBD6
(A-and C) and MV-dependent decrease in
@,,
(B
and D) in transgenic (black bars) and control
plants (gray bars) of var PBD6
(A
and
B)
and var
SR1
(C
and D) grown at different light intensi-
ties, as indicated along the x-axes. The conduc-
tance increase is expressed in microsiemens
cm-'
(g
fresh weight)-' (mean and
a,,
n
=
7-9). The numbers in the figure indicate the
probability (in percent) that the difference be-
o
8
Zo0
2
i
ls0
807-
tween the means of transgenic and control
plants
is
significant (one-sided
t
test).
C
SRI D
SRI
80
3
60
c
s
.-
e40
57
%
-
20
O0
t
40
90
135
800
Light intensity
(pmioI,m-Z.s-~)
Light intensily
(pmo1.m-2.s-I)
Mn
Superoxide Dismutase-Overproducing Tobacco
741
I
12
345 12
3
I
Figure
3.
Banding pattern of
SOD
activity gels obtained with whole-
leaf extracts from transgenic (solid lines) and nontransgenic (dashed
lines) var PBD6. The gels were stained
in
the absence (left) or
presence (right) of
KCN.
The bands migrate to the right. For more
details, see text.
lic Cu/ZnSOD (band
3);
and two forms of chloroplastic
Cu/ZnSOD (bands
4
and 5; Bowler et al.,
1991).
The trans-
genic plants contain a strong additional band representing
the overexpressed MnSOD (band 2). Preincubation with
KCN prior to staining caused a complete inactivation of
Cu/ZnSOD (right),
so
that band
3
now represents only
FeSOD. In each experiment the total area under the peaks
of the densitogram was plotted against the activity in the
solution assay. This yielded a straight line passing through
the origin, indicating that the total area under the peaks
was proportional to the total activity (not shown). This
allowed
us
to estimate the activity
of
the individual SOD
species as outlined in ”Materials and Methods.”
Below, the activities
of
cytosolic Cu/ZnSOD and endo-
genous, mitochondrial MnSOD are expressed in units/g
fresh weight. However, unless indicated otherwise, the
activities of the SOD species known to be located in the
chloroplasts (transgenic MnSOD, FeSOD, and chloroplastic
Cu/ZnSOD) were expressed in units/mg Chl. This was
more relevant
to
our interest in the chloroplastic defense
mechanisms against oxidative stress.
On a Chl basis, the activity level of the overexpressed
MnSOD in ChlSOD-PBD6 plants was about the same as in
ChlSOD-SR1 plants, fairly independent of the light inten-
sity received during growth (Fig.
4A).
The total activity
of
the chloroplastic SOD species was around 2.5 to
3.5
times
higher in the transgenic plants than ip the control plants
(Fig. 4B).
In nontransgenic plants the average activity
of
FeSOD
increased with increasing light intensity received during
growth, at least above a threshold value
of
90
pmol m-’
s-*
(Fig.
5,
A
and C). We did not observe consistent dif-
ferences in FeSOD activity between transgenic and control
plants.
In
var SR1 (but not in var PBDG) the activity level of
chloroplastic Cu/ZnSOD tended to decrease with increas-
ing light intensity received during growth (Fig. 5,
B
and D).
In both varieties the activity
of
chloroplastic Cu/ZnSOD
tended to be lower in transgenic plants than in nontrans-
genic plants. The largest and most significant differences
between transgenic and control plants with respect
to
ac-
tivities of chloroplastic Cu/ZnSOD were observed in var
SR1. However, the results were somewhat variable in this
respect and depended on the light intensity received dur-
ing growth, although not in a consistent manner when the
two varieties are compared.
The activity of cytosolic Cu/ZnSOD (in units/g fresh
weight) in nontransgenic plants was not significantly de-
pendent on the light intensity received during growth, as
shown in Figure
6,
A
and C. However, in var SR1 this
activity was consistently and considerably lower in the
transgenic plants than in the control plants. With var PBD6
the same trend was observed, but in general to a lesser
extent.
The activity
of
endogenous, mitochondrial MnSOD was
usually low, and it was often absent,
so
that the errors on
the average values were relatively large. Nevertheless, it
can be seen from Figure
6,
B
and D, that its activity tended
to be lower in transgenic than in nontransgenic plants,
especially in var SRI. In nontransgenic plants the activity
of
mitochondrial MnSOD varied rather irregularly with
light intensity received during growth.
In summary, the activities of the endogenous MnSOD
and Cu/ZnSODs were generally lower in transgenic plants
than in nontransgenic plants. There were no indications of
a positive correlation between the average abundances of
the various endogenous SOD species and tolerance to MV-
dependent oxidative stress (Fig.
2).
40
90
135
40
90
135
800
40
90
135
40
90
135
800
Light
intensity
(pmo1.m-2,s-1)
Light
lntensity
(pmol.m-?s-I)
Figure
4.
A, Activity
of
transgenic MnSOD in plants grown at different light intensities. The error bars indicate
a,
(n
=
8-9,
except for SR1 grown at
800
pmol
m-*
s-’,
where
n
=
3).
The data were obtained with the leaves used for the experiments
shown in Figure
2.
The data shown in Figures
4
to
6
were obtained with the same extracts.
B,
Relative increase in total
chloroplastic
SOD
activity in plants grown at different light intensities. The ordinate shows the ratio between transgenic and
control plants with respect to total chloroplastic
SOD
activity, as measured in leaf extracts.
742
Slooten et al. Plant Physiol.
Vol.
107,
1995
Figure
5.
Activity of chloroplastic FeSOD
(A
A
PBD6
and C) and chloroplastic Cu/ZnSOD (B and D)
in control and transgenic plants of var PBD6 (A
and E) and var
SR1
(C and
D)
Rrown at different
T
-
light intensities, as indicated along the x-axes.
Activities are expressed in units (mg Chl)-’. For
further details see legend
to
Figure
4A.
C
SR1
Abundance
of
SODs
in Relation
to
Age
of
the Leaves
The above data indicate that transgenic MnSOD tends to
suppress the expression of the endogenous MnSOD and
Cu/ZnSODs. These are precisely the SODs for which the
activity is high in young leaves but disappears or declines
upon maturation of the leaf. An example is shown in
Figure
7.
At 10 or
11
weeks after sowing, chloroplastic
Cu/ZnSOD and endogenous, mitochondrial MnSOD activ-
ity were present only in the immature top leaves. These
enzymes declined to very low or undetectable levels upon
maturing of the leaves. Cytosolic Cu/ZnSOD activity like
Light intensity &mol.m-z.s-l)
B PBD6
D SRl
I
7RANSGENIC
m
CONTROL
Light intensity (pmcll.m-?s-l)
wise decreased strongly with increasing leaf a?,e, although
it did not disappear completely. In 13-week-old plants,
chloroplastic Cu/ZnSOD activity was very low even in the
top leaves, which had reached approximatelj, their final
size by that time. By contrast, chloroplastic FeSOD re-
mained present at a relatively constant level, independent
of leaf age
or
position. The data presented in the previous
and following sections were obtained with the seventh to
ninth leaf from the top at
10
to
13
weeks after sowing (see
”Materials and Methods”). This would correspond, for ex-
ample, with leaves
9
to
11
(counted from the base) of the
plant represented in the middle pane1 of Figure
7.
A
PBD6
Figure
6.
Activity of cytosolic Cu/ZnSOD (A
and C) and endogenous MnSOD (B and
D)
in
control and transgenic plants of var PBD6 (A
and B) and var
SRI
(C and
D)
grown at different
light intensities, as indicated along the x-axes.
Activities are expressed in units
(g
fresh
weight)-‘. For further details see legend
to
Fig-
ure
4A.
C SRI
LigM intensity (pmo1.m-2,s-1)
B PBD6
T
68
I
D
SRl
Wd
CONTROL
Light intensity (pmo1.m-2,s-I)
Mn
Superoxide Dismutase-Overproducing Tobacco
743
30
P
g 20
4
10
o
4
6
7
9
11 13 15 16
40,
30]
llweeks
.-
b
2
20
I
10
o
4
6
7
9
11
13
15 16
2
3
10
a
o
4
6
7
9
11 13 15 16
Leaf
number
Figure
7.
SOD activities in whole-leaf extracts from a nontransgenic
plant of var SR1 grown at 135
pmol
m-*
s-’.
The age of the plant,
in weeks after sowing,
is
indicated within each panel. The leaves
were numbered starting from the base of the stem. Activities are
expressed in units
(mg
Chl)-’ for FeSOD and chloroplastic (chl)
Cu/ZnSOD and in units
(g
fresh weight)-’ for MnSOD and cytosolic
(cyt) Cu/ZnSOD.
Abundance
of
Other Antioxidant Enzymes and Substrates
in Relation to Light lntensity Received during Growth
The data concerning other enzymes and substrates in-
volved in oxygen-radical scavenging have been summa-
rized in Tables
I
and
11.
We did not find any difference
between transgenic and control plants with respect to
abundances of APx, DHAR, GR, MDHAR, ascorbate, or
glutathione. Therefore, Tables I and I1 show the pooled
data from both transgenic and control plants.
In var PBD6 the average abundance of most of the en-
zymes and substrates was, on a Chl basis, about the same
in plants grown at a light intensity of 45 or
90
pmol m-’
s-l
(Table I). The only exception was DHAR, which exhib-
ited, on a Chl basis, an activity about
70%
higher after
growth at
90
pmol
m-’
s-’.
However, plants grown at 135
Fmol
m-*
s-’
exhibited an increase in a11 components,
compared with plants grown at
90
pmol
m-’
s-’.
Ascor-
bate exhibited the strongest increase
(90-100%),
followed
by APx and glutathione
(40-50%),
and DHAR,
GR,
and
MDHAR (20-30% increase).
In var SR1, the average abundance (on a Chl basis) of
DHAR, MDHAR, and ascorbate was higher in plants
grown at
90
pmol m-’s-’ than in plants grown at
40
pmol
m-2
s-l
(Table
11).
The increases amounted to approxi-
mately
80%
for DHAR and 25% for total ascorbate and
MDHAR. The abundance of APx, GR, and glutathione was,
on a Chl basis, about the same in plants grown at these two
light intensities. Plants grown at 135 pmol m-‘
s-’
exhib-
ited a further increase in a11 components except DHAR,
compared with plants grown at
90
pmol m-’
s-’.
The
increases amounted to around
60%
for glutathione, GR,
and MDHAR, and 40 to 50% for APx and ascorbate. Addi-
tional increases in the concentration of ascorbate and the
activity of APx, DHAR, and MDHAR were observed after
growth at
800
pmol m-’s-’. Thus, in general, there was a
fair correspondence between the increase in abundance
of
components involved in oxygen-radical scavenging and
the decrease in sensitivity to MV when the plants were
grown at increasing light intensities.
Correlation
of
MV
Tolerance with Abundance
of
Antioxidant Enzymes and Substrates
More direct evidence for correlations between enzyme or
substrate concentrations and tolerance to MV was sought
from scattergrams in which the abundance of a given en-
zyme or substrate was plotted against MV-induced dam-
age in the same leaf. This was done with a data base
comprising a11 experiments in which oxygen radical dam-
Table
1.
Abundances of enzymes and substrates
in
whole leaf extracts from nontransgenic and transgenic plants of var
PBD6,
in
relation to
light intensity received
during
growth
used for the experiments shown in Figure 2, mean
t
o,
(n).
Enzyme activities are expressed in units
(mg
Chl)-’ and substrate concentrations in
pmol
(mg
Chl)-’. The data were obtained with the leaves
Abundance
Enzvme
or
Substrate
At
40
pmol
m-‘
s-‘
At
90
pmol
m-2
5-l
At
135
pnol
m-’
5-l
Enzymes
APx 7.01
t
0.57
(12)
6.89
t
0.39 (18) 10.5
t
1.2 (12)
DHAR 0.57
t
0.05 (12) 0.99
t
0.06 (18) 1.19
2
0.13
(12)
CR 0.14
t
0.01
(12)
0.15
t
0.01
(18) 0.20
t
0.03
(12)
MDHAR 0.23
+-
0.02 (12) 0.18
t
0.02 (18) 0.23
t
0.03 (12)
Ascorbate 0.41
2
0.05 (16) 0.43
t
0.04 (18) 0.80
t
0.04 (16)
G
I
utath ione
0.11
2
0.02 (14)
0.10
+-
0.01
(18) 0.14
+-
0.01
(14)
Substrates
744
Slooten
et ai.
Plant
Physiol.
Vol.
107,
1995
Table
II.
Abundances of enzymes and substrates
in
whole leaf extracts from nontransgenic and transgenic plants of var
SRI,
in
rolation to
light
intensity received
during
growth
used for the experiments shown in Figure
2,
mean
?
uhil
(n).
Enzyme activities are expressed in units
(mg
Chl)-' and substrate conceritrations in
pmol
(mg
Chl)-'. The data were obtained with the leaves
Enzyme
or
Substrate
Enzyme
APx
DHAR
GR
MDHAR
Su bstrate
Ascorbate
G
I
utath ione
Abundance
At 90
pmol
m-'
sC1
At
135
pmol
m-*
5-l
At 800
pmol
m-*
s-l
_____
At
40
pmol
m-*
s-'
7.86
2
1.31 (13) 7.80
t
0.54 (15) 11.1
t
0.8 (18) 18.5
t
:!.O
(6)
0.78
2
0.05 (13) 1.38
t
0.22 (15) 1.25
t
0.07
(18)
2.72
t
0.39 (6)
0.19
t
0.03 (13) 0.19
t
0.02 (15) 0.31
t
0.02 (18) 0.26
t
0.04 (6)
0.34
t
0.03 (13) 0.43
t
0.05 (15) 0.68
2
0.1
1
(18)
0.99
rt
0.27 (6)
0.37
t
0.04 (15) 0.47
rt
0.02 (17) 0.67
2
0.04
(18)
1.09
t
0.08 (4)
0.11
t
0.02 (13) 0.10
2
0.01 (15) 0.16
2
0.02 (17)
nda
a
nd, Not determined.
age had been elicited with
2
p~
MV.
To
avoid ambiguities
we restricted our analysis to groups of data in which the
range of both the Chl concentrations and the fresh weights
were limited in such a way that neither of these exhibited
a significant correlation with MV-induced damage.
For the calculation of the correlation coefficients it was
assumed that the MV-induced damage (conductance in-
crease or decrease in
QII)
was linearly dependent on the
abundance of the enzyme or substrate under consideration.
The correlation coefficient can be thought of as a measure
of the goodness of fit of the data with this model. Its value
ranges from
O
(no correlation) to
+1
or
-1
(perfect corre-
lation), the sign depending on the slope of the regression
line. In our case, protective effects give rise to regression
lines with negative slopes, and hence to negative correla-
tion coefficients. We also calculated the probability that the
regression coefficient (the slope of the regression line) has
the indicated sign, i.e. that it differs from zero (in
a
one-
sided
t
test). For the sake of clarity, entries in the tables are
confined to enzymes or substrates for which the absolute
value of the correlation coefficient was at least 0.35, and for
which, in addition, the correlation was significant at the
95% leve1
(P
>
0.95).
The results obtained with nontransgenic ancl transgenic
plants are shown in Tables
I11
and
IV,
respectively. We
arrived at these results by using pools of data obtained
with plants grown at two "adjacent" light intensities: either
40
and
90,
or
90
and 135 pmol
m-'
s-'.
Furthermore,
enzyme and substrate levels were correlated either with
MV-induced increase in conductance or with FdV-induced
decrease in
aI1.
This yielded four types of correlations,
which will be denoted as correlation types
1
to
4
for ease of
Table
111.
Correlation coefficients for linear correlations of enryme activities and substrate concentrations with MV-induced coriductance
increase and MV-induced decrease
in
The data were obtained with leaves from plants grown at the indicated light intensity (expressed in
pmol
m-'
s-'). From the available data,
selections were made in which the range of fresh weights and Chl contents of the leaf discs was limited such that there were no significant
correlation between Chl content or fresh weight with sensitivity to MV. The number of experiments used for the regression analys
s
is indicated.
In the regression analysis, enzyme activities were expressed in units
(mg
Chl)-' and substrate concentrations in pmol
(mg
Chl)-'. Only cytosolic
CuRnSOD was expressed in units
(g
fresh weight)-' . Only those correlation coefficients are shown that were significantly diffe,.ent from zero
in a one-sided
t
test (P
>
0.95;
with four exceptions, P
>
0.97).
Correlations that are illustrated in Figure
8
are indicated. Cond. Incr.,
Conductance increase.
in
nontransgenic plants
Correlation Type
1
7
7
4
Parameter Cond. Incr. Cond. Incr.
A@,,
li@,l
Light intensity
40
+
90 90
+
135 40
+
90
90
+
135
Var PBD
SR1
PBD SR1 PBD
SR1
PBD
SR1
n
13 8
12
14 13 8 12 14
FeSOD
-0.52 -0.68a
Chloroplastic Cu/ZnSOD
0.76
0.80
APx
-0.66a -0.57 -0.60
DHAR
-0.52
MDHAR
-0.55
Ascorbate
-0.60 -0.70 -0.66" -0.60 -0.63 -0.55
C
I
utathione
-0.83 -0.64 -0.47 -0.79 -0.57
Chl
-0.23 -0.33 -0.33 -0.11 -0.29 0.1 7 -0.24 -0.07
Fresh weight
-0.14 -0.24 -0.25 -0.31 0.21 -0.28 -0.26 -0.31
a
See Figure
8.
Mn
Superoxide Dismutase-Overproducing Tobacco
745
90
and
135
3
100
U
V
6
Ascorbate
(pmoUmg
chlorophyll)
90
and
135
E
a,
3
100
a
P
7 9
11
APx
(Ulmg
chlorophyll)
B
120
SRI
90
and
135
,.
I
40
0';
23456789'
FeSOD
(Uhg
chlorophyll)
D
IOOr
tr.
SRI
40
and 90
e
2
t
O'"''
I2
16
tr.
MnSOD
(Ulrng
chlorophyll)
reference. The two bottom lines of Tables I11 and IV are
controls indicating that within each group
of
experiments,
the Chl content and the fresh weight of the leaf discs were
at most only weakly correlated with MV-induced damage.
These correlations were a11 insignificant.
In nontransgenic plants (Table
III)
ascorbate and gluta-
thione were negatively correlated with MV-induced
damage in a11 types of correlations. In other words, these
substrates seemed to exert control on the extent
of
MV-
induced decrease in
QI1
and/or MV-induced increase in
conductance in plants grown at light intensities ranging
from
40
to 135 pmol m-'
s-'.
Such correlations were
observed in var SR1 as well as in var PBDG. An example is
shown in Figure 8A. Furthermore, FeSOD and APx were
negatively correlated with MV-induced damage in var SRl
as well as in var PBD6. Examples are shown in Figure 8,
B
and C, respectively. DHAR and MDHAR were negatively
correlated with MV-induced damage in var SR1 only. In
MDHAR this extends the observation that its activity in var
PBD6 was almost independent of the light intensity re-
ceived during growth (Table
I).
By contrast, the sensitivity
to MV decreased strongly with increasing light intensity
received during growth (Fig.
2).
FeSOD, DHAR, and
MDHAR yielded significant results only in type
2
correla-
tions; APx yielded significant results only in type
2
and
type
4
correlations. In other words, the activities
of
FeSOD,
DHAR, and MDHAR seemed to exert control only on the
extent of MV-induced ion leakage in plants grown at rela-
tively high light intensities; in addition, APx seemed to
exert control on the extent of MV-induced decrease in
al1
in
such plants. In FeSOD (Fig. 5, A and C) and APx (Tables
I
and 11) the observed correlations agree with the observa-
tion that the activity of these enzymes increased after
growth at light intensities above
90
pmol
m-'s-'.
The activities
of
cytosolic Cu/ZnSOD and GR were not
significantly correlated with MV-induced damage. This ex-
tends the observation that the activity of these enzymes
Figure
8.
Correlations between the
abundance
of
antioxidants
and
MV-induced
oxygen
radical
damage
in
control
plants
(A-C)
and transgenic
plants
(D).
The
MV
concentration
was
2
p~.
The
variety
and
the
light
intensity
received
during
growth
(in
pmol
m-'s-')
are indicated
in
each
panel.
In
A
to
C,
the conductance increase
is
expressed
in
microsiemens
cm-'
(g
fresh
weight)-'.
In
D,
the
decrease
in
@,,
was
ex-
pressed
as
percentage
of
the
@,,
value
in
leaf
discs
without
MV.
was not or was only weakly dependent on the light inten-
sity received during growth (Fig. 6; Tables I and 11). Fur-
thermore, the activity of chloroplastic Cu/ZnSOD was al-
ways positively correlated with MV-induced damage. This
is in line with the fact that in var SR1 the activity of this
enzyme decreased with increasing light intensity received
during growth (Fig.
5,
B
and D).
The relatively strong type
1
and type 3 correlations for
glutathione and ascorbate in var SR1 and PBD6 (Table 111)
could not have been predicted from the average values in
Tables I and 11; the ascorbate and glutathione levels were
on the average the same, or nearly the same, in plants
grown at
40
and
90
pmol m-'s-'. Yet this is not a contra-
diction, since correlations between two quantities yield
more information than can be extracted from average val-
ues of a single quantity. However, it is not yet clear why
significant type
1
and type
3
correlations showed up only
in the substrates ascorbate and glutathione and not in the
enzymes.
The results obtained with transgenic plants are shown in
Table IV. In transgenic plants of var SR1 the activity
of
overexpressed MnSOD was significantly correlated with
MV-induced damage in a11 types of correlations. An exam-
ple is shown in Figure 8D. In addition, severa1 enzymes
exhibited significant correlations with MV-induced de-
crease in
@,,
in plants grown at relatively low light inten-
sities (correlation type
3)
in contrast to nontransgenic
plants. In the transgenic plants of var PBD6, none of the
listed components exhibited significant correlations with
MV-induced damage when the plants were grown at rela-
tively low light intensities (type
1
and
3
correlations). Un-
fortunately, it was not possible to define a sufficiently large
group of data from plants grown at relatively high light
intensities (type
2
and
4
correlations) that met the require-
ment that there be no significant correlation
of
the Chl
content or the fresh weight of the leaf discs with MV-
induced damage.
746
Slooten et
al. Plant Physiol.
Vol.
107,
1995
Table IV.
Correlation coefficients for linear correlations
of
enzyme activiiies
and
substrate concentrations
with
MV-induced conciuctance
increase and MV-induced decrease
in
a,,
in
transgenic plants
of
var
SR1
See legend to Table
111.
Cond.
Incr.,
Conductance increase.
Parameter
Cond. Incr. Cond. Incr.
A@,l
A%
Light
intensity
40
+
90
90
+
135
40
+
90 90
+
135
n
11
12
11
'I
2
Transgenic
MnSOD
-0.61 -0.64 -0.78a -0.59
FeSOD
-0.64 -0.58 -0.67
Chloroplastic Cu/ZnSOD
0.61 0.58 0.59
APx
-0.55 -0.62
-
3.66
DHAR
-0.57
MDHAR
-0.60
-0.51
Ascorbate
-0.60
Glutathione
-0.74
-0.70 -0.74
Chl
-0.33 -0.28 -0.33 -0.23
Fresh weight
-0.06
-0.36 0.06 -0.23
a
See Figure
8.
DISCUSSION
Severa1 groups have generated transgenic plants that
overexpress SODs in order to enhance tolerance to oxida-
tive stress. These attempts have been successful to various
degrees. Overexpression of chloroplastic Cu/ZnSOD from
Petunia hybrida
in tobacco
(N.
tabacum)
did not increase the
tolerance of the plants to MV (Tepperman and Dunsmuir,
1990) or ozone (Pitcher et al., 1991). Tomato plants
(Lyco-
persicon
esculentum)
transformed with the same construct
did not exhibit an increased resistance to chilling-induced
photoinhibition (Tepperman and Dunsmuir, 1990). How-
ever, overexpression of a mitochondrial MnSOD from
N.
plumbaginifolia
in the chloroplasts of
N.
tabacum
resulted in
an increased tolerance of the plants to MV (Bowler et al.,
1991) as well as to ozone (Van Camp et al., 1994). Alfalfa
plants
(M.
sativa)
transformed with the same construct
were more tolerant to freezing stress (McKersie et al., 1993).
Potato plants
(S.
tuberosum)
overexpressing Cu/ZnSOD
from tomato had an increased tolerance to
MV
(Perl et al.,
1993); and overexpression of chloroplastic Cu/ZnSOD
from pea in the chloroplasts of
N.
tabacum
rendered the
plants more tolerant to MV as well as to photoinhibition
(Sen Gupta et al., 1993a). The present results confirm that
overexpression of mitochondrial MnSOD in the chloro-
plasts of tobacco enhanced the resistance of the plants to
MV-dependent, light-induced oxidative stress. However,
the degree to which the MV tolerance is enhanced is
strongly dependent on the growth conditions of the plants.
This is a factor not taken into account in previous studies,
and it may be partly responsible for the different results
obtained by various workers, at least with regard to en-
hancement of MV tolerance.
It has become clear that there are two major mechanisms
of photoinhibition: acceptor-side and donor-side photoin-
hibition. The latter type occurs when electron donation to
the
PSII
reaction center is inefficient; it has been demon-
strated only in subcellular systems, but it might occur in
vivo
as a consequence of acceptor-side photoinhibition (see
Prasil et al. [1992] for review). It is currently thought that
acceptor-side photoinhibition involves the generation of
singlet oxygen following inactivation of the boimd electron
acceptor
QA
(Barber and Andersson, 1992; Vass et al.,
1992). On the other hand, superoxide radical:; have been
implicated in the generation of chilling-induced photoin-
hibition in subcellular systems (Richter et al.. 1990), and
unspecified oxygen free radicals different froni singlet ox-
ygen were iinplicated in the light-dependent Aegradation
of the 32-kD protein of the PSII reaction center (Sopory et
al., 1990). Furthermore, a mutant of
Conyza
bomriensis
with
elevated levels of SOD, APx, and
GR
was reported to
exhibit an enhanced tolerance to photoinhibition (Jansen et
al., 1989). In our hands the overexpressed MnSOD did not
provide any significant protection against chilling-induced
photoinhibition, as determined from measureinents of
(not shown). Thus, if superoxide anion radicds are at a11
involved in the generation of chilling-induced photoinhi-
bition, they are not accessible to the overexpressed Mn-
SOD. This is in contrast with data from Sen Gupta et al.
(1993a), who found that transgenic tobacco plants overex-
pressing chloroplastic Cu/ZnSOD from pea were more
resistant to chilling-induced photoinhibition,
(3s
well as to
MV, than control plants.
Abundance
of
SODs
and the Effect
of
Transgeiiic MnSOD
on Activities
of
Other Antioxidant Enzymes
The activities of cytosolic and chloroplastic Cu/ZnSOD
and of mitochondrial MnSOD were, in general, lower in
transgenic (MnSOD-overexpressing) plants
t
han in non-
transgenic plants, especially in var SR1 (Figs.
E;
and
6).
This
suggests that overexpression of MnSOD some how leads to
suppression of the activity of cytosolic and chloroplastic
Cu/ZnSOD and of mitochondrial MnSOD. Sen Gupta et al.
(1993a), who designed experiments in which chloroplastic
Cu/ZnSOD from pea was overexpressed ir. the chloro-
plasts of tobacco, noted an even stronger decline in the
activity of the endogenous chloroplastic Cu/i:nSOD in the
transgenic plants.
Mn
Superoxide Dismutase-Overproducing Tobacco
747
Chloroplastic Cu/ZnSOD is especially prominent in
young, expanding leaves. It shares this characteristic with
endogenous, mitochondrial MnSOD and (to a lesser extent)
with cytosolic Cu/ZnSOD (Fig.
7).
The reason for this may
be that in tobacco these three SODs are associated mainly
with dark metabolism. Young, expanding leaves exhibit a
high metabolic activity, which presumably entails an in-
creased risk of oxygen radical formation. It remains to be
determined whether chlororespiration (a cyanide-sensitive
respiratory pathway in chloroplasts, branching off from the
photosynthetic pathway at the level
of
the plastoquinone
pool) is involved in the putative increased rate of oxygen
radical formation in young leaves. Garab et al. (1989) found
evidence for the occurrence of this pathway in tobacco,
especially in young leaves. This might explain the high
activity of chloroplastic Cu/ZnSOD in young tobacco
leaves. The fact that the initially high activity of the Cu/
ZnSODs and
of
endogenous MnSOD decreases to much
lower or even undetectable levels when the leaves mature
may indicate that these enzymes have a relatively high
turnover rate.
It may be hypothesized that the expression of the endo-
genous Mn- and Cu/ZnSODs is induced by superoxide
radicals, or by a more mobile "signal molecule" produced
by reaction with superoxide. A decline in the production of
superoxide, due to a decreased metabolic activity in mature
leaves, would limit the expression
of
these SODs. Overex-
pression of MnSOD in the chloroplasts would likewise
reduce the concentration
of
the signal molecule and would
thus bring about a similar decrease in the expression
of
the
Cu/ZnSODs and of endogenous MnSOD. This would im-
ply that the hypothetical signal molecule cannot be hydro-
gen peroxide, because the concentration of that component
would be higher in transgenic than in nontransgenic
plants. It must be noted, however, that Tsang et al. (1991),
who studied induction of SODs in
N.
plumbaginifolia
at the mRNA level, observed that stress conditions can
be imposed leading only to induction of cytosolic Cu/
ZnSOD (e.g. during illumination at high temperatures).
Combined with our data this suggests that different
mechanisms for induction of SODs may exist, depending
on the developmental stage of the leaf and on the type
of
imposed stress.
The abundance of FeSOD is not strongly dependent on
leaf age or position (Fig.
7)
but increases with increasing
light intensity received during growth, at least above a
threshold value of 90 Fmol m-'
s-'
(Fig. 5). Tsang et al.
(1991) interpreted their results as indicating that light-
dependent oxidative stress causes induction
of
FeSOD.
This is not inconsistent with our data, although we found
no clear evidence for the involvement of superoxide in the
induction of FeSOD. If this were the case, one would expect
that overexpression of MnSOD would also suppress the
induction of FeSOD. This was not consistently observed
(Fig. 5).
The activities of APx, DHAR, GR, and MDHAR were not
affected by the overexpression of MnSOD. As for APx, this
observation is at variance with data from Sen Gupta et al.
(1993b), who noted a 3-fold increase in activity of APx in
transgenic tobacco plants overexpressing SOD in the chlo-
roplasts. However, these authors used chloroplastic
Cu/
ZnSOD instead of MnSOD, which was used in this study.
We found that transgenic plants overexpressing FeSOD in
the chloroplasts of var SR1 did not show a change in APx
activity either (our unpublished data). The question of how
two functionally similar, although structurally distinct, en-
zymes can have such dissimilar effects on the activity of
other enzymes is interesting but as yet unanswered.
Enhancement
of
MV
Tolerance
by
Overexpressed MnSOD
The overexpressed MnSOD did not significantly enhance
the tolerance of the plants to MV when the plants were
grown at low-light intensities
(40
or
90
pmol
m-*
s-').
A
simple explanation would be that in plants grown at low-
light intensities, the
oxygen-radical-scavenging
capacity is
limited by the low concentrations
of
other components
involved in oxygen-radical scavenging. Under those con-
ditions overexpression of MnSOD would not have much
effect. The increase in the concentration
of
these other
components, in response to higher-light intensities re-
ceived during growth, would enable MnSOD to exert a
beneficia1 effect on the MV tolerance. The data shown in
Figure
2
and Tables I and
I1
are in agreement with this
notion. In var PBD6 most enzymes and substrates known
to be involved in oxygen-radical scavenging were present
at relatively high levels only in plants grown at 135 pmol
m-2
s-l
(Table I). The effect of overexpressed MnSOD on
the tolerance to MV was significant only in these plants
(Fig.
2).
In var SR1, plants grown at
90
pmol mP2
s-'
had
somewhat elevated levels of ascorbate, DHAR, and
MDHAR, compared with plants grown at
40
pmol m-'
s-'
(Table II), but this was not accompanied by an increase in
the protection provided by overexpressed MnSOD against
MV. Apparently, raising the levels of these components
was not sufficient to increase the protection provided by
MnSOD against MV. An additional increase
of
these, as
well
as
other, components occurred after growth at 135
pmol
m-'
s-'
(Table
111,
and in these plants the transgenic
MnSOD did have
a
positive, albeit weak, effect on
MV
tolerance (Fig.
2).
Plants grown at
800
pmol
m-'s-'
ex-
hibited an even greater increase in the levels
of
most anti-
oxidant enzymes and substrates compared with plants
grown at 135 pmol m-'s-' (Table 11), with the exception of
GR and chloroplastic and cytosolic Cu/ZnSOD, none of
which were correlated with MV tolerance (see below).
In
agreement with this, MnSOD overexpression caused a
more substantial enhancement of MV tolerance in plants
grown at 800 pmol
m-'
s-l
than in plants grown at 135
pmol
mP2
s-'.
In conclusion, our results suggest that en-
hancement of the oxidative-stress tolerance by overexpres-
sion
of
MnSOD requires that other antioxidant enzymes
and substrates be present at relatively high levels. In the
present experiments this requirement was met by growing
the plants at high-light intensities.
As mentioned in the introduction, transgenic plants
of
var PBD6, overexpressing MnSOD in the chloroplasts, also
exhibited an enhanced ozone tolerance. Interestingly, the
decrease in ozone injury observed in these plants was
748
Slooten
et
al.
Plant Physiol.
Vol.
107,
1995
likewise light dependent, ranging between 45 and 75%. The
largest decrease was observed after acclimatization (4 d)
and ozone treatment
(7
d) at high-light intensities (Van
Camp et al., 1994). The decrease in MV-induced ion leakage
that we observed in var PBD6 after growth at 135 pmol
(around 60%, Fig. 2A) was within the range ob-
m-z
s-l
served by Van Camp et al. (1994).
Differential Attenuation
of
Two Types
of
MV-lnduced
Oxygen-Radical Damage by Overexpressed MnSOD
Taking average values, we found that in transgenic
plants of var SR1, the enhancement of MV tolerance was
expressed only in the conductance measurements (Fig. 2C)
and not in the fluorescence measurements (Fig. 2D). One
might argue that the MV-induced decrease in
aI1
arises
because reduced MV, after having received an electron
from
PSI,
occasionally diffuses toward the reaction center
of
PSII
and inactivates it without involvement of superox-
ide. SOD would be unable to attenuate this type of damage.
However, this argument can be dismissed on several
grounds. First, MnSOD overexpressed in the chloroplasts
of var PBD6 caused a pronounced and significant attenu-
ation of the MV-induced decrease in
QII
in at least two
independent transgenic lines (Bowler et al., 1991; Slooten et
al., 1992). To a lesser extent this was also the case in a third
transgenic line that was used in the present work. Second,
overexpression
of
chloroplastic FeSOD from
Arabidopsis
thaliana
in the chloroplasts of var SRl attenuated the de-
crease in
QII
as well as the ion leakage induced by MV (data
not shown). This indicates that these two types of damage
are both due to superoxide but can be differentially atten-
uated, depending on the identity of the overexpressed
enzyme as well as on the target variety. Interestingly,
experiments currently in progress suggest that in trans-
genic plants of var SR1, the overexpressed FeSOD has a
higher membrane affinity than the overexpressed MnSOD
(our unpublished data).
The major site of electron donation to MV has been
shown to be the Fe-S center
B
in
PSI
(Fujii et al., 1990). From
there the oxygen radicals must propagate, on the one hand
out of the chloroplasts and to the cell membrane, resulting
in ion leakage, and on the other hand to the reaction center
of
PSII,
resulting in
loss
of
QI1.
From Figure
2
it appears
that in var SRl the overexpressed MnSOD interferes more
extensively with the former pathway of damage propaga-
tion than with the latter. According to recent data, the
chloroplasts possess two separate oxygen-radical-scaveng-
ing systems: a stromal system comprising APx, DHAR, GR,
and MDHAR, and a membrane-bound system that is func-
tionally linked to
PSI
and comprises at least .APx (Miyake
and Asada, 1992, 1994). It seems reasonable to speculate
that the stromal system intercepts the oxygen radicals
spreading outward, thus reducing ion leakage, but has
little influence on the spreading of oxygen radicals along or
within the membrane, leading to
PSII
inactivation. The
membrane-bound system, which is linked to
PSI,
would
intercept the toxic oxygen species very close to the site of
formation, and hence would prevent both kinds of damage.
To our knowledge there are no data available concerning
the spatial distribution of either endogenous
01'
transgenic
SODs
within the chloroplast. However, to enhance the
scavenging capacity of the membrane-bound system, the
transgenic SOD must probably meet two conditions: it
must be membrane bound and it must be bocnd in close
association with
PSI.
We speculate that in var SRl, at least
the second condition is not sufficiently met, siich that the
overexpressed MnSOD enhances primarily t he oxygen-
radical-scavenging capacity of the stromal sysi em.
Correlation
of
Abundance
of
Antioxidants with
MV Tolerance
The abundances of FeSOD, DHAR, and MIIHAR were
correlated predominantly with attenuation of IvlV-induced
ion leakage. The location of FeSOD in the chioroplasts is
not known, but DHAR and MDHAR are stronial enzymes
(Asada and Takahashi, 1987). APx, on the other hand,
occurs in the chloroplasts in a membrane-bourtd as well as
in a stromal form, the membrane-bound form being func-
tionally linked to
PSI
(Miyake and Asada, 1992,1994). This
enzyme was, at least in var SRl, correlated Mith attenua-
tion of MV-induced ion leakage as well as MV-induced loss
of
QII.
AI1 this supports our interpretation, given in the
preceding paragraph, that in var SRl the o1,erexpressed
MnSOD enhances primarily the oxygen-radica -scavenging
capacity
of
the stromal system.
In transgenic plants of var SRl the activity of overex-
pressed MnSOD was significantly correlated with attenu-
ation of MV-induced damage under a11 conditi ons studied.
This was surprising because, at least in plants Srown at the
two lowest light intensities, transgenic plants were on the
average no more tolerant of MV, and sometimes less tol-
erant of MV, than control plants (Fig. 2). This suggests in
fact that low levels of overexpressed MnSOD
è
ecreased the
tolerance
of
the leaf discs to MV, and that Iiigher levels
increased this tolerance.
As
outlined previous Ly (Bowler et
al., 1991), the reason may be that during illumination of
MV-treated leaf discs, low levels of MnSOD ,ire sufficient
to allow generation of H,O, at fairly high rates but are not
sufficient to deplete the superoxide that arise
5
from reoxi-
dation of photoreduced MV. Under those conditions,
H,O,
and
0,-
may react together in a metal-catalyzed Haber-
Weiss reaction. This would result
in
the forriation of the
hydroxyl radical, OH (see introduction), and in enhance-
ment rather than attenuation of MV-induced tlamage. This
deleterious effect could be prevented either b
y
an increase
in the leve1 of overexpressed MnSOD or FeSOD (resulting
in more complete depletion of
OJ
or by an increase in the
rate of
H,O,
scavenging. This may explain why in trans-
genic plants of var SRl several enzymes exhibited signifi-
cant correlations with attenuation of MV-induced decrease
in
aI1
in plants grown at relatively low-light intensities
(correlation type 3), in contrast to nontransgmic plants.
Chloroplastic CuZnSOD was always negttively corre-
lated with MV tolerance, in some cases significantly
so
(Tables
111
and IV). Apparently the leaves wii h higher MV
tolerance were physiologically somewhat older and had
lost more of their chloroplastic Cu/ZnSOD tl- an the leaves
with lower MV tolerance. The activities
of
cytosolic Cu/
Mn
Superoxide Dismutase-Overproducing Tobacco 749
ZnSOD and GR were not significantly correlated with MV-
induced damage, in spite
of
the fact that
a
severalfold
variation occurred in the activity of these enzymes in indi-
vidual experiments (similar to the variations shown in Fig.
8).
This could mean either that these enzymes are not
involved in oxygen-radical scavenging or, more likely, that
they are involved but do not limit the oxygen-radical-
scavenging capacity under the growth and assay condi-
tions used in this work. As for GR, this is at variance with
the results of Aono et al. (1993), who reported that tobacco
plants overexpressing GR in the chloroplasts exhibited an
increased tolerance to MV,
as
judged by visual inspection
of MV-induced pigment bleaching. It may be noted, how-
ever, that GR overexpression in poplar chloroplasts re-
sulted in an increased size of the glutathione pool in the
leaves (Foyer et al., 1994). It is not clear to what extent this
effect may have been responsible for the results obtained
by Aono et al. (1993).
CONCLUDINC
REMARKS
It cannot be excluded that other factors besides the com-
ponents studied here affect the tolerance of the leaves to
MV. Consequently, the correlations discussed above can-
not be used as evidence for causal relationships. On the
other hand, such relationships may exist. It has been shown
in maize inbreeds that lines that have elevated levels of
both SOD and GR exhibit an increased tolerance to drought
as well
as
to
MV
(Malan
et
al., 1990). A mutant of
Conyza
bonariensis
with elevated levels of
SOD,
APx, and GR had
an increased tolerance to MV (Shaaltiel and Gressel, 1986)
as well as to photoinhibition (Jansen et al., 1989). The
present results suggest,
as
indicated above, that overex-
pression of
SOD
resulted in an increased tolerance to MV
only if other components were also present at high levels.
The correlation studies suggest that APx, DHAR, and
MDHAR are among those components. Therefore, it will be
of interest to attempt to further enhance the oxidative-
stress tolerance of the plants by overexpressing, in addition
to SOD, one or more
of
the latter three enzymes. The
strongest case in this respect is that for APx: for this en-
zyme, correlations with MV tolerance were observed in
both varieties, SR1 and PBD6. The correlations of the ascor-
bate and glutathione pools with MV tolerance, observed in
both tobacco varieties, suggest that it will also be of interest
to enlarge the pools of these components. In the case of
poplar it has been shown that enlarged
pools
of glutathione
(Foyer et al., 1994) occur after overexpression of GR in the
chloroplasts.
ACKNOWLEDCMENT
The skillful technical assistance of
S.
Vandenbranden is grate-
fully acknowledged.
Received August 22, 1994; accepted November 14, 1994.
Copyright Clearance Center:
0032-0889/95/107/0737/14,
LITERATURE
CITED
Aono M, Kubo A, Saji H, Tanaka K, Kondo
N
(1993) Enhanced
tolerance to photooxidative stress of transgenic
Nicotiana
taba-
cum
with high chloroplastic glutathione reductase activity. Plant
Cell Physiol
34
129-135
Arnon
DI
(1949) Copper enzymes in isolated chloroplasts. Poly-
phenol oxidase in
Beta
vulgaris.
Plant Physiol
24
1-15
Asada
K
(1984) Chloroplasts: formation of active oxygen and its
scavenging. Methods Enzymol
105:
422429
Asada
K,
Takahashi M
(1987) Production and scavenging of active
oxygen in photosynthesis.
In
DJ
Kyle, CB Osmond, CJ Arntzen,
eds, Photoinhibition. Elsevier Science Publishers, Amsterdam,
Barber
J,
Andersson
B
(1992)
Too
much of a good thing: light can
be bad for photosynthesis. Trends Biochem Sci
1:
61-66
Beauchamp C, Fridovich
I
(1973) Isozymes of superoxide dis-
mutase from wheat germ. Biochim Biophys Acta
317:
50-64
Bowler C, Slooten
L,
Vandenbranden
S,
De Rycke
R,
Botterman
J,
Sybesma C, Van Montagu M, Inzé
D
(1991) Manganese
superoxide dismutase can reduce cellular damage mediated by
oxygen radicals in transgenic plants. EMBO
J
10
1723-1732
Bowler
C,
Van Montagu M, Inzé D
(1992) Superoxide dismutase
and stress tolerance. Annu Rev Plant Physiol Plant Mo1 Biol
43:
Bowyer
JR,
Camilleri
P
(1985) Spin-trap study of the reactions of
ferredoxin with reduced oxygen species in pea chloroplasts.
Biochim Biophys Acta
808:
235-242
Chen GX, Asada
K
(1989) Ascorbate peroxidase in tea leaves:
occurrence of two isozymes and the differences in their enzy-
matic and molecular properties. Plant Cell Physiol
30
987-998
Elstner EF, Frommeyer
D
(1978) Production of hydrogen peroxide
by photosystem
I1
of spinach chloroplast lamellae. FEBS Lett
86:
Elstner
EF,
Konze JR
(1974) Light-dependent ethylene production
by isolated chloroplasts. FEBS Lett
45
18-21
Elstner
EF,
Saran M, Bors
W,
Lengfelder
E
(1978) Oxygen activa-
tion in isolated chloroplasts. Mechanism
of
ferredoxin-depen-
dent ethylene formation from methionine. Eur
J
Biochem
89
Foyer CH, Halliwell
B
(1976) The presence of glutathione and
glutathione reductase in chloroplasts: a proposed role in ascor-
bic acid metabolism. Planta
133:
21-25
Foyer CH, Lelandais M, Jouanin
L,
Kunert
KJ
(1994) Overexpres-
sion of enzymes of glutathione metabolism in poplar
(Populus
tremula
X
P.
alba).
Bulletin de la Societie Luxembourgeoise de
Biologie Clinique, Special Issue, pp 229-240
Fujii T, Yokoyama
E,
Inoue
K,
Sakurai H
(1990) The site of
electron donation of photosystem
I
to methyl viologen. Biochim
Biophys Acta
1015:
41-48
Furbank RT, Badger MA
(1982) Oxygen exchange associated with
electron transport and photophosphorylation
in
spinach chloro-
plasts. Biochim Biophys Acta
723:
400409
Garab G, Lajko
F,
Mustardy
L,
Marton
L
(1989) Respiratory
control over photosynthetic electron transport in chloroplasts of
higher-plant cells: evidence for chlororespiration. Planta
179:
Geller BI, Winge
DR
(1984) Subcellular distribution of superoxide
dismutases in rat liver. Methods Enzymol
105
105-114
Griffith
OW
(1980) Determination of glutathione and glutathione
disulfide using glutathione reductase and 2-vinylpyridine. Ana1
Biochem
106
207-212
Halliwell
B
(1987) Oxidative damage, lipid peroxidation and an-
tioxidant protection in chloroplasts. Chem Phys Lipids
44:
Hosein
B,
Palmer G
(1983) The kinetics and mechanism of oxida-
tion of reduced spinach ferredoxin by molecular oxygen and its
reduced products. Biochim Biophys Acta
723:
383-390
Jansen MAK, Shaaltiel
Y,
Kazzes
D,
Canaani
O,
Malkin
S,
Gres-
se1
J
(1989) Increased tolerance to photoinhibitory light
in
para-
quat-resistant
Conyza
bonariensis
measured by photoacoustic
spectroscopy and I4CO, fixation. Plant Physiol
91:
1174-1178
Lichtenthaler HK
(1987) Chlorophylls and carotenoids: pigments
of
photosynthetic membranes. Methods Enzymol
148:
350-382
Malan C, Greyling MM, Gressel
J
(1990) Correlation between
Cu/Zn superoxide dismutase and glutathione reductase, and
pp 227-287
83-116
143-146
61-66
349-358
327-340
750
Slooten
et
al
Plant
Physiol.
Vol.
107,
1995
environmental and xenobiotic stress tolerance in maize in-
breeds. Plant Sci
69:
157-166
Marsho TV, Behrens PW, Radmer RJ (1979) Photosynthetic oxy-
gen reduction in isolated intact chloroplasts and cells from
spinach. Plant Physiol
64
656-659
McKersie BD, Chen
Y,
de Beus M, Bowley
SR,
Bowler C, Inzé D,
D’Halluin K, Botterman J (1993) Superoxide dismutase en-
hances tolerance of freezing stress in transgenic alfalfa
(Medicago
sativa
L.). Plant Physiol
103:
1155-1163
Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase
in spinach chloroplasts and photoreduction
of
its primary oxi-
dation product monodehydroascorbate radicals in thylakoids.
Plant Cell Physiol
33:
541-553
Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction
of
the monodehydroascorbate radical in spinach thylakoids.
Plant Cell Physiol
35
539-549
Nakano
Y,
Asada K (1981) Hydrogen peroxide is scavenged by
ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell
Physiol
22:
867-880
Okamura M (1980) An improved method for determination of
L-ascorbic acid and L-dehydroascorbic acid in blood plasma.
Clin Chim Acta
103:
259-268
Perl A, Perl-Treves R, Galili
S,
Aviv D, Shalgi
E,
Malkin
S,
Galun
E
(1993) Enhanced oxidative-stress defense in transgenic
potato expressing tomato Cu,Zn superoxide dismutases. Theor
Appl Genet
85
568-576
Pitcher LH, Brennan E, Hurley A, Dunsmuir P, Tepperman JM,
Zilinskas BA (1991) Overproduction of petunia copper/zinc
superoxide dismutase does not confer ozone tolerance in trans-
genic tobacco. Plant Physiol
97:
452455
Polle A, Chakrabarti K, Schiirmann
W,
Rennenberg H (1990)
Composition and properties of hydrogen peroxide decomposing
systems in extracellular and total extracts from needles of Nor-
way spruce
(Picea
abies
L.,
Karst.). Plant Physiol
94
312-319
Prasil
O,
Adir N, Ohad.
I
(1992) Dynamics of photosystem
11:
mechanism of photoinhibition and recovery processes.
In
J
Bar-
ber, ed, The Photosystems: Structure, Function and Molecular
Biology. Elsevier Science Publishers, Amsterdam, pp 295-348
Richter M, Riihle W, Wild
A
(1990)
Studies
on
the mechanism
of
photosystem
I1
photoinhibition.
11.
The involvement of toxic
oxygen species. Photosynth Res
24
237-243
Schaedle
M,
Bassham JA (1977) Chloroplast glutathione reduc-
tase. Plant Physiol
59
1011-1012
Sen Gupta A, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993a)
Increased resistance to oxidative stress in transgenic: plants that
overexpress chloroplastic Cu/Zn superoxide dismutase. Proc
Natl Acad Sci USA
90:
1629-1633
Sen Gupta
A,
Webb RP, Holaday AS, Allen AR (1993b) Overex-
pression of superoxide dismutase protects plants frcm oxidative
stress. l’lant Physiol
103:
1067-1073
Shaaltiel
Y,
Gressel J (1986) Multienzyme oxygen radical detoxi-
fication system correlated with paraquat resistance in
Conyza
bonariensis.
Pestic Biochem Physiol
26:
22-28
Slooten L, Capiau K, Vandenbranden
S,
Sybesma
C,
Van Mon-
tagu M,
Inzé
D (1992) Improvement
of
the resistance
of
higher
plants against oxidative stress. Mededelingen van de Faculteit
Landbouwwetenschappen van de Universiteit Ge:it
57:
1477-
1485
Sopory SK, Greenberg BM, Mehta RA, Edelman
M,
Mattoo AK
(1990) Free radical scavengers inhibit light-dependimt degrada-
tion of the 32 kDa photosystem
I1
reaction center protein.
Z
Naturforsch 45c: 412-417
Takahashi M, Asada K (1988) Superoxide production in the
aprotic interior
of
thylakoid membranes. Arch Biochem Biophys
Tanaka K, Suda
Y,
Kondo
N,
Sugahara K (1985)
O,
t.Aerance and
the ascorbate-dependent
H,O,
decomposing system in chloro-
plasts. Plant Cell Physiol
26
1425-1431
Tepperman JM, Dunsmuir P (1990) Transformed plmts with el-
evated levels of chloroplastic SOD are not more resistant to
superoxide toxicity. Plant Mo1 Biol
14
501-511
Tsang EWT, Bowler
C,
Hérouart D, Van Camp W, Villaroel R,
Genetello C, Van Montagu
M,
Inzé D (1991) Diffc.rentia1 regu-
lation of superoxide dismutases in plants exposecl to environ-
mental stress. Plant Cell
3
783-792
Van Camp
W,
Willekens
H,
Bowler C, Van Montagu M,
Inzé
D,
Reupold-Popp
R,
Sandman H Jr, Langebartels
(2
(1994) Ele-
vated levels of superoxide dismutase protect traniigenic plants
against ozone damage. Biotechnology
12
165-168
Vass
I,
Styring
S,
Hundal T, Koivuniemi A, Aro
E-M,
Andersson
B (1992) Reversible and irreversible intermediates during pho-
toinhibition of photosystem
11:
stable reduced
QA
species pro-
mote chlorophyll triplet formation. Proc Natl Acacl Sci USA
89:
1408-1412
267:
714-722