Content uploaded by Yin-biao Wang
Author content
All content in this area was uploaded by Yin-biao Wang on Aug 24, 2022
Content may be subject to copyright.
Citation: Song, J.; Han, K.; Wang, Y.;
Qu, R.; Liu, Y.; Wang, S.; Wang, Y.;
An, Z.; Li, J.; Wu, H.; et al. Microglial
Activation and Oxidative Stress in
PM2.5-Induced Neurodegenerative
Disorders. Antioxidants 2022,11, 1482.
https://doi.org/10.3390/
antiox11081482
Academic Editor: Stanley Omaye
Received: 19 June 2022
Accepted: 27 July 2022
Published: 29 July 2022
Publisher’s Note: MDPI stays neutral
with regard to jurisdictional claims in
published maps and institutional affil-
iations.
Copyright: © 2022 by the authors.
Licensee MDPI, Basel, Switzerland.
This article is an open access article
distributed under the terms and
conditions of the Creative Commons
Attribution (CC BY) license (https://
creativecommons.org/licenses/by/
4.0/).
antioxidants
Review
Microglial Activation and Oxidative Stress in PM2.5-Induced
Neurodegenerative Disorders
Jie Song 1, Keyang Han 1, Ya Wang 2, Rongrong Qu 1, Yuan Liu 1, Shaolan Wang 1, Yinbiao Wang 1, Zhen An 1,
Juan Li 1, Hui Wu 1and Weidong Wu 1 ,*
1School of Public Health, Xinxiang Medical University, Xinxiang 453003, China; 171058@xxmu.edu.cn (J.S.);
50200209002@stu.xxmu.edu.cn (K.H.); 50200102025@stu.xxmu.edu.cn (R.Q.);
50200209006@stu.xxmu.edu.cn (Y.L.); 50200209008@stu.xxmu.edu.cn (S.W.); 151049@xxmu.edu.cn (Y.W.);
151037@xxmu.edu.cn (Z.A.); 171019@xxmu.edu.cn (J.L.); wuhui@xxmu.edu.cn (H.W.)
2Nursing School, Zhenjiang College, Zhenjiang 212028, China; wangya2021@zjc.edu.cn
*Correspondence: wuweidong@xxmu.edu.cn
Abstract:
Fine particulate matter (PM
2.5
) pollution remains a prominent environmental problem
worldwide, posing great threats to human health. The adverse effects of PM
2.5
on the respira-
tory and cardiovascular systems have been extensively studied, while its detrimental effects on
the central nervous system (CNS), specifically neurodegenerative disorders, are less investigated.
Neurodegenerative disorders are characterized by reduced neurogenesis, activated microglia, and
neuroinflammation. A variety of studies involving postmortem examinations, epidemiological inves-
tigations, animal experiments, and
in vitro
cell models have shown that PM
2.5
exposure results in
neuroinflammation, oxidative stress, mitochondrial dysfunction, neuronal apoptosis, and ultimately
neurodegenerative disorders, which are strongly associated with the activation of microglia. Mi-
croglia are the major innate immune cells of the brain, surveilling and maintaining the homeostasis of
CNS. Upon activation by environmental and endogenous insults, such as PM exposure, microglia can
enter an overactivated state that is featured by amoeboid morphology, the over-production of reactive
oxygen species, and pro-inflammatory mediators. This review summarizes the evidence of microglial
activation and oxidative stress and neurodegenerative disorders following PM
2.5
exposure. Moreover,
the possible mechanisms underlying PM
2.5
-induced microglial activation and neurodegenerative
disorders are discussed. This knowledge provides certain clues for the development of therapies that
may slow or halt the progression of neurodegenerative disorders induced by ambient PM.
Keywords: PM2.5; microglia; oxidative stress; neuroinflammation; neurodegeneration
1. Introduction
Air pollution is comprised of particulate matter (PM), gases, organic compounds, and
metals derived from both human activity and natural sources. PM is the most widespread
health threat and has been strongly implicated in diverse diseases [
1
]. An important con-
tributor to PM is traffic-related air pollution (TRAP), mostly ascribed to diesel exhaust
particles (DEP) [
2
]. Ambient PM can be split up in several size fractions based on the
aerodynamic diameter: coarse PM (2.5–10
µ
m), fine PM (<2.5
µ
m, PM
2.5
), and ultrafine
PM (<0.1
µ
m, UFPM) [
3
]. PM
2.5
exhibits tempo-spatial variations of complex components,
such as bacteria lipopolysaccharides (LPS), carbon-containing particles, sulfate, nitrate,
ammonium salt, and heavy metals [
4
,
5
]. PM
2.5
and UFPM are of particular concern, as
these particles can enter systemic circulation and be distributed in the brain and other
organs, posing significant potential danger to human health [
6
]. Globally, there are ap-
proximately 6.5 million excess deaths attributable to ambient PM
2.5
pollution annually [
7
].
Recent evidence indicates that PM
2.5
air pollution, in addition to causing respiratory and
cardiovascular diseases, also negatively affects the brain and contributes to central nervous
system (CNS) diseases [1].
Antioxidants 2022,11, 1482. https://doi.org/10.3390/antiox11081482 https://www.mdpi.com/journal/antioxidants
Antioxidants 2022,11, 1482 2 of 23
Since anatomopathological evidence from canine and human residents in Mexico City
was reported in the early 2000s [
8
–
10
], the neurotoxicity of PM
2.5
has received much atten-
tion. Many studies have reported association of PM
2.5
exposure with neurological disorders,
such as stroke, dementia, Alzheimer’s disease (AD), Parkinson’s disease (PD), and mild
cognitive impairment [
11
]. Thus far, the mechanisms underlying PM
2.5
-induced neurologi-
cal disorders have not been well elucidated, with oxidative stress and neuroinflammation
being two major recognized ones [
12
]. Due to its high metabolic demands, high energy use,
high lipid content, widespread axonal and dendritic networks, and low levels of endoge-
nous antioxidants, the brain is more susceptible to oxidative stress [
13
], the latter refers
to the imbalance between the production of free radicals, such as reactive oxygen species
(ROS) and reactive nitrogen species (RNS), and the antioxidant defense systems, which can
damage cellular biomolecules, including lipids, proteins, and DNA [
14
]. Oxidative stress
has been proposed as a hallmarker and major driving force for neurodegeneration [15].
Microglia are the principal players in the brain’s innate immune response [
16
]. Emerg-
ing evidence from recent studies has suggested that microglial activation, oxidative stress,
neuroinflammation, cerebrovascular damage, and abnormal protein aggregates may play
critical roles in the pathogenesis of neurodegenerative disorders triggered by ambient
PM
2.5
[
1
,
17
,
18
]. There are a few reviews elaborating on the association of PM exposure
with adverse neurological effects, especially neuroinflammation [
12
,
17
–
20
], but with none
centering on microglial involvement. This review systemically summarizes the evidence
of PM
2.5
-induced oxidative stress and neurodegenerative disorders from postmortem ex-
aminations, epidemiological investigations, animal experiments, and
in vitro
studies, with
an emphasis on microglial implications in these pathophysiological events. In addition,
the potential mechanisms underlying PM
2.5
-induced microglial activation and associated
oxidative stress and neurodegenerative disorders are also discussed.
2. Microglia: Physiological and Pathological Characteristics
Glial cells account for more than 90% of cells in the human brain and are divided into
two populations: the macroglia (i.e., astrocytes and oligodendrocytes) and microglia [
21
].
Microglia originate from immature yolk sac progenitor cells and are present in significant
numbers in normal brains, but their density varies by brain region in the adult human
and mice [
22
–
24
]. More microglia are found in the cortex than in the white matter, with the
highest concentrations found in the hippocampus, olfactory telencephalon, basal ganglia, and
substantia nigra [
25
,
26
]. Such distribution may explain the vulnerability of these brain areas.
Under resting conditions, microglia survey the microenvironment in real-time with
their ramified, motile, fine, and long cellular processes [
26
]. Meanwhile, diverse neu-
rotrophic factors are released from microglia and help to maintain neuronal cell survival
and circuit formation [
27
]. In contrast to neurons, microglial cells have the ability to
completely restore their population in the adult brain [
28
]. Microglia can be activated by
endogenous disease proteins, cytokines, neuronal death, and environmental toxicants in-
cluding components of air pollution [
1
]. Activated microglia in the CNS are heterogeneous
and can be categorized into two opposite phenotypes: classical (M1) or alternative (M2)
(Figure 1) [
29
]. The M1 phenotype characterized by amoeboid shape, high mobility, and
strong phagocytic capacity is mainly induced by LPS, interferon-
γ
(IFN-
γ
), amyloid
β
(A
β
),
and
α
-synuclein [
30
,
31
], and associated with the release of pro-inflammatory cytokines
and chemokines, such as tumor necrosis factor-
α
(TNF-
α
), interleukin (IL)-6, IL-1
β
, IL-12,
CC chemokine ligand-2 (CCL-2), monocyte chemoattractant protein-1 (MCP-1), and prosg-
landins [
31
–
33
], whose receptors are found on neurons, thus rendering neurotoxicity [
34
,
35
].
Moreover, activated microglia also over-express nicotinamide adenine dinucleotide phos-
phate (NADPH) oxidase (NOX) and inducible nitric oxide synthase (iNOS) that catalyze the
generation of ROS and nitric oxide (NO), respectively [
36
], and a major histocompatibility
complex-II that presents antigens, triggers and spreads further inflammatory response in
surrounding microglial cells [
28
], integrins, co-stimulatory molecules, Fc receptors, and
intracellular proteins (e.g., ionized calcium binding adapter molecule-1, Iba-1), contributing
Antioxidants 2022,11, 1482 3 of 23
to neurological damage [
37
]. In contrast, the M2 phenotype characterized by thin cell bod-
ies and branched processes can be induced by IL-4, IL-13, IL-10, or activated peroxisome
proliferator-activated receptors
γ
(PPAR
γ
) [
38
], resulting in the release of anti-inflammatory
cytokines, such as IL-10, transforming growth factor-
β
(TGF-
β
), growth factors, colony stim-
ulating factor
−
1 (CSF-1), neurotrophic growth factors, such as brain derived neurotrophic
factor (BDNF), a neuroprotective status [38–40].
Antioxidants 2022, 11, x FOR PEER REVIEW 3 of 25
that catalyze the generation of ROS and nitric oxide (NO), respectively [36], and a major
histocompatibility complex-II that presents antigens, triggers and spreads further inflam-
matory response in surrounding microglial cells [28], integrins, co-stimulatory molecules,
Fc receptors, and intracellular proteins (e.g., ionized calcium binding adapter molecule-1,
Iba-1), contributing to neurological damage [37]. In contrast, the M2 phenotype character-
ized by thin cell bodies and branched processes can be induced by IL-4, IL-13, IL-10, or
activated peroxisome proliferator-activated receptors γ (PPARγ) [38], resulting in the re-
lease of anti-inflammatory cytokines, such as IL-10, transforming growth factor-β (TGF-
β), growth factors, colony stimulating factor−1 (CSF-1), neurotrophic growth factors, such
as brain derived neurotrophic factor (BDNF), a neuroprotective status [38–40].
From the above, activated microglia show a broad spectrum of phenotypes ranging
from the pro-inflammatory, potentially cytotoxic M1 to the anti-inflammatory, scaveng-
ing, and regenerative M2.
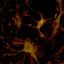
Figure 1. M1/M2 phenotypes and functions of microglia. The resting microglia can be activated by
toxic mediators, such as IFN-γ, LPS, and PM2.5, and display a M1 phenotype. In this condition, mi-
croglia induce neurotoxicity via release of neurotoxic mediators (TNF-α, PGE2, ROS, NO, etc.). The
factors secreted by the dead or damaged neurons in turn exacerbate the chronic activation of micro-
glia. Besides the M1 phenotype, in combination with IL-4, IL-10, and TGF-β, microglia could be
induced into the M2 phenotype, which plays a neuroprotective role through the generation and
release of anti-inflammatory cytokines (IL-13, IL-10, TGF-β, etc.).
3. Evidence from Postmortem Examinations
The first histopathological evidence for a link between air pollution and neuropathol-
ogy came from a necropsy study with canine residents naturally exposed to a highly pol-
luted environment in Mexico City [41]. In this study, the increased expression of neuroin-
flammatory and oxidative stress biomarkers, including nuclear factor-κB (NF-κB) and
iNOS in cortical endothelial cells occurred at ages 2 and 4 weeks of dogs with subsequent
neurodegenerative changes, such as the impairment of the blood–brain barrier (BBB) and
the extracellular deposition of Aβ peptide fibrils and intracellular neurofibrillary tangles
(NFTs) in the olfactory epithelium (OE) and the olfactory bulbs (OB), as well as in subcor-
tical and cortical structures [41]. In addition, dogs aged 8 months demonstrated pro-
nounced inflammatory effects, diffuse Aβ plaques, and a significant increase in DNA
damage in OB, frontal cortex, and hippocampus with ameboid microglia in the cortex and
white matter [8,41]. Moreover, feral dogs inhabiting in Mexico City presented brain tissue
damage and accumulated metals (nickel and vanadium) in a gradient fashion from OE >
OB > frontal cortex, indicating the nose as portal of entry [41]. Notably, those alterations
of the olfactory pathway were similar to the early olfactory pathology observed in AD.
The similar findings were also observed in autopsy examinations of human samples
[9]. Adult human residents living in Mexico City presented an increased expression of
inflammatory mediator cyclooxygenase-2 (COX-2) and the greater accumulation of neu-
ronal and astrocytic Aβ42, the 42-amino acid form of Aβ, in frontal cortex, hippocampus,
Figure 1.
M1/M2 phenotypes and functions of microglia. The resting microglia can be activated
by toxic mediators, such as IFN-
γ
, LPS, and PM
2.5
, and display a M1 phenotype. In this condition,
microglia induce neurotoxicity via release of neurotoxic mediators (TNF-
α
, PGE2, ROS, NO, etc.).
The factors secreted by the dead or damaged neurons in turn exacerbate the chronic activation of
microglia. Besides the M1 phenotype, in combination with IL-4, IL-10, and TGF-
β
, microglia could
be induced into the M2 phenotype, which plays a neuroprotective role through the generation and
release of anti-inflammatory cytokines (IL-13, IL-10, TGF-β, etc.).
From the above, activated microglia show a broad spectrum of phenotypes ranging
from the pro-inflammatory, potentially cytotoxic M1 to the anti-inflammatory, scavenging,
and regenerative M2.
3. Evidence from Postmortem Examinations
The first histopathological evidence for a link between air pollution and neuropathol-
ogy came from a necropsy study with canine residents naturally exposed to a highly
polluted environment in Mexico City [
41
]. In this study, the increased expression of neu-
roinflammatory and oxidative stress biomarkers, including nuclear factor-
κ
B (NF-
κ
B) and
iNOS in cortical endothelial cells occurred at ages 2 and 4 weeks of dogs with subsequent
neurodegenerative changes, such as the impairment of the blood–brain barrier (BBB) and
the extracellular deposition of A
β
peptide fibrils and intracellular neurofibrillary tangles
(NFTs) in the olfactory epithelium (OE) and the olfactory bulbs (OB), as well as in subcorti-
cal and cortical structures [
41
]. In addition, dogs aged 8 months demonstrated pronounced
inflammatory effects, diffuse A
β
plaques, and a significant increase in DNA damage in
OB, frontal cortex, and hippocampus with ameboid microglia in the cortex and white
matter [
8
,
41
]. Moreover, feral dogs inhabiting in Mexico City presented brain tissue dam-
age and accumulated metals (nickel and vanadium) in a gradient fashion from OE > OB
> frontal cortex, indicating the nose as portal of entry [
41
]. Notably, those alterations of the
olfactory pathway were similar to the early olfactory pathology observed in AD.
The similar findings were also observed in autopsy examinations of human sam-
ples [
9
]. Adult human residents living in Mexico City presented an increased expression of
inflammatory mediator cyclooxygenase-2 (COX-2) and the greater accumulation of neu-
ronal and astrocytic A
β42
, the 42-amino acid form of A
β
, in frontal cortex, hippocampus,
and OB [9]. In contrast, children and young adults exhibited a wide spectrum of neurode-
generative disorders, such as increases in microglial activation (CD68 and CD163), elevated
pro-inflammatory proteins (COX-2 and IL-1
β
) and the innate immunity receptor CD14,
Antioxidants 2022,11, 1482 4 of 23
accumulated AD or PD proteins (A
β42
and
α
-synuclein), oxidative stress in frontal and
infratentorial neurons and microglia (8-hydroxydeoxyguanosine (8-OHdG) and nitrotyro-
sine), and frontal BBB impairment, as well as the reduction of the neuroprotective cellular
prion protein (PrPC) in the frontal cortex [
42
]. Intriguingly, metals (manganese, nickel, and
chromium) were enriched in the frontal cortex with the high expression of COX-2, TGF-
β
,
CD14, and IL-1
β
[
42
]. Additionally, those children in Mexico City had brainstem abnor-
malities, such as inflammation,
α
-synuclein and/or A
β42
, deposition, activated microglia,
and reactive glial fibrillary acidic protein (GFAP) positive astrocytes throughout the brain-
stem [
43
]. Early olfactory abnormalities similar to the olfactory pathology in AD were also
observed in Mexico City children [
44
]. It is noteworthy that Apolipoprotein E4 (APOE4) is
the strongest genetic risk factor for AD [
45
], APOE4 carriers exposed to air pollution had
greater hyperphosphorylated tau, diffused A
β
plaques, and more pronounced olfactory
deficits than APOE3 carriers [42,46,47].
Taken together, the preliminary evidence of postmortem studies indicates that expo-
sure to ambient air pollutants is associated with microglia activation, oxidative stress, and
neurodegenerative alterations in brain tissues. However, due to the complex mixture of air
pollution, the causative association of air pollution exposure with the observed neurological
effects of CNS remains to be specified.
4. Evidence from Epidemiological Investigations
There is a growing body of epidemiological studies reporting ambient air pollution-
associated neurodegenerative disorders (see Table 1for details), such as cognitive decline,
AD, and PD. Regarding cognitive alteration, an early important aspect of AD, many
population-based studies with the elderly [
48
–
50
] have reported that PM air pollution,
particularly TRAP, is consistently associated with declined cognitive abilities [
51
]. However,
controlled animal studies in this aspect are still limited for further verification.
Several other epidemiological studies found that exposure to PM
2.5
was associated
with a significant risk for AD [
52
–
56
], which was consistent with the pathological findings
from the autopsy samples of individuals with AD-like pathologies in the highly polluted
Mexico City. These observations were supported by a follow-up study displaying a decrease
in A
β42
levels in the cerebrospinal fluids (CSF) of Mexico City children [
57
], a very early
change in AD [
58
]. In addition, Calderon-Garciduenas et al. recently reported elevated
levels of non-phosphorylated tau in the CSF, a marker of AD axonal pathology, or increases
in hyperphosphorylated tau and amyloid plaques in the OB of children and young adults
in Mexico City [
51
,
59
]. Given that aging is a risk factor for neurodegenerative diseases [
60
],
the aging brain is assumed to be particularly vulnerable to air pollution-induced neurotoxi-
city [
61
]. Therefore, it is not surprising to see an accelerated decline in episodic memory
among older females with late-life exposure to PM2.5 [62].
A few studies have examined the association of ambient PM
2.5
exposure with the risk for
PD. However, the epidemiological results appear inconsistent. For example, one study showed
opposite associations between ambient PM
2.5
exposure and the incidence of PD among two
populations from two locations with different severity of air pollution [
63
]. Another study
found an association between the concentrations of ambient PM
2.5
and PD risk in female
never-smokers [
64
]. However, in a large prospective study of women, Palacios et al. did not
see significant associations between PM10 or PM2.5 exposure and the incidence of PD [65].
Thus far, the epidemiological evidence specifically linking microglial activation to air
pollution-induced neurodegenerative disorders is mainly from the studies with children
and young adults in Mexico City [
66
–
68
]. Overall, these studies demonstrated that those
children presented early markers of neurodegeneration, neuroinflammation (e.g., elevated
macrophage inhibitory factor (MIF), IL-6, and IL-2 in CSF [
69
] and increased IL-6 and
Toll-like receptors (TLRs) expression in frontal cortex), olfactory dysfunction, and cognitive
deficits compared to control children from nearby non-polluted cities. Of these biomarkers
examined, MIF is a cytokine essential for microglial activation and the production of IL-6,
IL-1β, TNF-α, and iNOS [70].
Antioxidants 2022,11, 1482 5 of 23
Table 1. Major evidence from epidemiological investigations.
Study Design Location Subjects Exposure Outcome Results References
Cohort Taiwan, China 95,690 individuals’ age
≥65 PM2.5, PM10 and O3Newly diagnosed AD in
Taiwan from 2001–2010
A 138% risk of increase of AD per
increase of 4.34 g/m3in PM2.5 over the
follow-up period (95% CI: 2.21–2.56).
Jung et al. [52]
Cohort
the Ruhr area and
Southern
Muensterland, Germany
789 women Air pollution
(including PM2.5)
Cognitive performance
and function
PM2.5 was negatively associated with
cognitive function and cognitive
performance (β=−0.19 (95% CI: −0.36
to −0.02)).
Schikowski et al. [53]
Cohort Ontario, Canada
4.4 million adults for
a multiple
sclerosis cohort;
2.2 million adults for
dementia or Parkinson’s
disease cohort
Traffic-related air
pollution
(including PM2.5)
Residential proximity
to roads;
Incidence of multiple
sclerosis, dementia, and
Parkinson’s disease.
The incidence of dementia was
associated with the distance to roads:
(HR = 1.07, 95% CI: 1.06–1.08) for <50 m;
(HR = 1.04, 95% CI: 1.02–1.05) for
50–100 m;
(HR = 1.02, 95% CI: 1.01–1.03) for
101–200 m;
(HR = 1.00, 95% CI: 0.99–1.01) for
201–300 m.
Chen et al. [54]
Case-crossover Communities from
different sites in the USA
Medicare enrollees
(>65 years) PM2.5
The risk of hospitalization
for neurological disorders;
The association between
short-term exposure to
PM2.5 and
all-cause mortality.
Increased hospitalization risks for
Parkinson’s disease (3.23% increase,
95% CI: 1.08–5.43) for a 10 µg/m3
increase in the 2 days average.
Zanobetti et al. [55]
Cohort 50 northeastern
U.S. cities
9.8 million Medicare
enrollees (≥65 years) PM2.5
Time to first
hospitalization for
dementia, Alzheimer’s, or
Parkinson’s diseases.
Per 1-µg/m3increase in annual
PM2.5 concentrations:
HR of 1.08 (95% CI: 1.05–1.11)
for dementia;
HR of 1.15 (95% CI: 1.11–1.19) for AD;
HR of 1.08 (95% CI: 1.04–1.12) for
PD admissions
Kioumourtzoglou et al.
[56]
Antioxidants 2022,11, 1482 6 of 23
Table 1. Cont.
Study Design Location Subjects Exposure Outcome Results References
prospective
pilot study
Mexico City
metropolitan area
(MCMA) and small cities
with clean air for control
129 children and adults PM2.5
Neurodegenerative
biomarkers in CSF: Aβ42,
α-synuclein (t-α-syn and
d-α-synuclein).
Decreased levels of Aβ42 and BDNF in
MCMA children (p= 0.005 and 0.02,
respectively).
Total synuclein showed an
PM2.5-dependent increase and then a
decrease after age 12 years, while
d-α-synuclein exhibited a tendency to
increase with cumulated PM2.5
(R2= 0.30).
Calderón-
Garcidueñas et al.
[57]
Prospective
pilot stud
MCMA and small cities
with clean air for control
507 healthy children
and adults
High vs. low
air pollution
Non-phosphorylated
tau(non-P-Tau) and Aβ42
in the cerebrospinal fluid.
A strong increase in Non-P-Tau with
age, which was faster among MCMA
children versus controls (p= 0.0055).
Aβ42 and BDNF concentrations were
lower in MMC children (p= 0.002 and
0.03, respectively).
Calderón-
Garcidueñaset al.
[59]
Prospective
cohort study
Communities from
different sites in the USA
1403
community-dwelling
older women
(71–89 years)
PM2.5
Volume of gray matter
(GM) and
normal-appearing white
matter (WM).
Older women with greater PM2.5
exposures had significantly
smaller WM.
A 4.47 cm3decrease (95% CI: 2.27–6.67)
in the volume of WM per increase of
3.49 µg/m3in PM2.5.
Chen et al. [61]
Prospective
cohort study 48 states of the USA 998 older females aged
(73–87 years) PM2.5
Tests of immediate free
recall/new learning (List
A Trials 1–3; List B) and
delayed free recall (short-
and long-delay).
PM2.5 was associated with greater
declines in immediate recall and new
learning: the annual decline rate was
significantly accelerated by 19.3% (95%
CI: 1.9–36.2%) for Trials 1–3 and 14.8%
(95% CI: 4.4–24.9%) for List B per
increase of 3.49 µg/m3in PM2.5.
Younan et al. [62]
Cohort
North Carolina and Iowa
of the USA 84,739 farmers PM2.5 and O3The incidence of
Parkinson’s disease.
A positive association of Parkinson’s
disease with PM2.5 (OR = 1.34; 95% CI:
0.93–1.93) in North Carolina but not
in Iowa.
Kirrane et al. [63]
Antioxidants 2022,11, 1482 7 of 23
Table 1. Cont.
Study Design Location Subjects Exposure Outcome Results References
Nested
case-control
Different states of
the USA
1556 Parkinson’s disease
cases and 3313 controls
PM2.5, PM10
and NO2
The incidence of
Parkinson’s disease.
A higher risk of PD was associated with
higher exposure to PM2.5
(ORQ5 vs. Q1 = 1.29; 95% CI: 0.94–1.76;
p= 0.04) among non-smokers.
Liu et al. [64]
Cohort 115,767 healthy women PM2.5 and PM10 The incidence of
Parkinson’s disease.
No statistically significant associations
between PM2.5 exposure and PD risk
(RR = 1.08, 95% CI: 0.81–1.45).
Palacios et al. [65]
Panel Jinan, China 76 people aged
60–69 years
High level of air
pollution (including
particulate matters)
Neurodegenerative
biomarkers: Aβ40, Aβ42 ,
α-synuclein, PRNT,
Tau(pThr181);
Activation of microglia:
S100B, TREM2).
Air pollution exposure induces the
alterations of neurodegenerative
biomarkers, such as Aβ40, Aβ42 ,
α-synuclein, PRNP, Tau (pThr181), and
the activation of microglia.
Tang et al. [71]
Panel Xinxiang, Chian
34 healthy retirees from
Xinxiang Medical
University
PM2.5
Biomarkers of neural
damage in serum: NfL,
NSE, PGP9.5, S100B.
PM2.5 and its key constituents were
significantly associated with neural
damage biomarkers: A 10 µg/m3
increase in PM2.5 concentration was
associated with 2.09% (95% CI:
39.3–76.5%), 100% (95% CI: 1.73–198%),
and 122% (95% CI: 20.7–222%)
increments in BDNF, NfL, and
PGP9.5, respectively.
Several constituents such as Cu, Zn, Ni,
Mn, Sn, V, Rb, Pb, Al, Be, Cs, Co, Th, U,
Cl−, and F−were significantly
associated with NfL.
Song et al. [72]
Antioxidants 2022,11, 1482 8 of 23
More recently, the association of PM
2.5
-associated microglial activation with neurode-
generative disorders have been investigated among the elderly. In a panel study, Tang et al.
examined the air pollutant (including PM) exposure in Chinese people aged 60–69 years,
showing that air pollution exposure could induce alterations of neurodegenerative biomark-
ers, such as A
β40
, A
β42
,
α
-synuclein, PrPC, Tau (pThr181), and the activation of microglia
represented by the over-expression of S100B and microglial triggering receptor expressed
on myeloid cells2 (TREM2) [
71
]. Our recent findings from a panel study on healthy retired
adults demonstrated that PM
2.5
concentration increments were associated with increases
in the biomarkers of neural damage, including a neurofilament light chain (NfL), neuron-
specific enolase, and the activation of microglia (S100B) in serum [
72
]. Meanwhile, several
constituents of ambient PM
2.5
, such as Cu, Zn, Ni, Mn, Sn, V, Rb, Pb, Al, Be, Cs, Co, Th, U,
Cl−, and F−were found to be significantly associated with serum levels of NfL [72].
In summary, the available evidence in humans, albeit limited and variable, is sugges-
tive of the association of PM2.5 exposure with neurodegenerative disorders.
5. Evidence from Animal Studies
In vivo
studies in general corroborate and expand the major pathophysiological find-
ings in human brain tissue and other accessible tissues [
73
,
74
], such as the markers of
oxidative stress, neuroinflammation, and neurodegeneration, and help decipher under-
lying mechanisms linking exposure to the development of neurodegenerative disorders.
Most of the animal studies have focused on the effects of PM
2.5
, especially DEP and UFPM,
on AD-like pathologies (see Table 2for details).
In AD condition [
75
], microglia-mediated neuroinflammation is a critical event charac-
terized by the release of IL-1
β
, IL-6, and TNF-
α
. This feature has been verified in animals
exposed to PM
2.5
under diverse exposure scenarios, mostly chronic exposure. For example,
children and dogs chronically exposed to severely polluted air pollution in Mexico City
displayed similar inflammatory neuropathological lesions [
10
,
76
]. The chronic inhalation of
airborne PM
2.5
caused time-dependent early AD-like changes in mice, such as an increase
in A
β40
, BACE (beta-site amyloid precursor protein (APP)-cleaving enzyme), and COX-2,
as well as a decrease in APP, with a minimal change of phosphorylated tau [
77
]. The
oropharyngeal aspiration of PM
2.5
for 4 weeks induced a dose-dependent increase in IL-1
β
and TNF-
α
in the blood and hippocampus of mice [
78
]. The whole-body exposure of rats to
PM
1.0
for 3 and 6 months resulted in microglia activation in the hippocampus [
79
]. In addi-
tion, short-term exposure showed similar neurological effects. For example, mice exposed
for 5 d to a traffic-polluted highway tunnel exhibited increased expression of microglia-
associated inflammatory genes (COX-2, iNOS, and nuclear factor) in the hippocampus, and
decreased BDNF expression in the OB [80].
DEP are a major constituent of ambient PM
2.5
and are commonly used as a surrogate
model of air pollution in health effects studies [
81
]. The short- or long-term inhalation
of DEP can induce the over-expression of pro-inflammatory factors in select brain re-
gions
[82,83]
. For example, the inhalation of DEP (0.5 and 2 mg/m
3
, for 1 month) increased
IL-1
β
, TNF-
α
, IL-6, MIP-1
α
(macrophage inflammatory protein-1
α
), fractalkine, and Iba-1
in most regions of Sprague Dawley (SD) rats, with the midbrain showing the greatest
DEP response [
84
]. Meanwhile, a single intratracheal administration of DEP increased
microglial Iba-1 levels in the substantia nigra and elevated serum and whole-brain TNF-
α
at 6 h post-treatment [
85
]. The susceptibility of the midbrain to DEP neuroinflammatory
effects was confirmed by another inhalation study over 6 months on male Fischer 344 rats
exposed to DEP (35–992 µg/m3), probably due to the most microglia in the midbrain [85].
In addition, TRAP-related PM has also been examined for neurological effects. For ex-
ample, SD rats exposed to PM
1
(250–300
µ
g/m
3
) for 3 and 6 months demonstrated that
PM
1
induced cytotoxicity, lipid peroxidation, microglial activation, and inflammation as
well as autophagy and caspase-3 up-regulation in microglia [
80
]. Wistar rats inhaling
DEP nanoPM (0.3–1.0 mg/L) for 3 months had higher levels of COX-2 and A
β42
in brain
regions [
86
]. An interesting finding came from transgenic mice with human APOE3 and E4
Antioxidants 2022,11, 1482 9 of 23
alleles, showing that chronic exposure to nanoPM over 15 weeks increased the cerebral A
β
production and deterioration of hippocampal CA1 neurons, with a more significant effect
in APOE4 carriers [87].
The role of microglia in DEP-induced neurodegenerative disorders has been explored
mostly in mouse models. The acute exposure of adult mice to DEP (250–300 mg/m
3
for
6 h) caused microglial activation, lipid peroxidation, and reduced neurogenesis in all brain
regions, particularly in the hippocampus and the OB [
88
]. The blockage of microglial activ-
ity with the PPAR-
γ
agonist pioglitazone inhibited the DEP-induced neuroinflammation
in cerebral cortex, oxidative stress, and neurogenesis reduction in the hippocampus [
89
].
The exposure of mice to TRAP-PM
0.2
(300
µ
g/m
3
) or of neuronal cells to the same nanoPM
(
1–10 µg/mL
) caused an increase in oxidative stress in lipid rafts associated with an in-
crease in A
β
, the latter was inhibited by the antioxidant N-acetyl cysteine, suggesting
that oxidative stress was involved in the pro-amyloidogenic effects of air pollution [
90
].
Interestingly, Cheng et al. examined the differential time course of oxidative stress and
inflammatory responses to UFPM between the OE and the brain. It was found that OE and
OB, but not the cerebral cortex and cerebellum, had rapid increases in microglial number,
and oxidative and nitrosative protein adducts in the nasal epithelium turbinate after 5 h
exposure, which precedes an increase in levels of TNF-
α
by 45 h [
91
]. These responses
corresponded to
in vitro
OE and mixed glial responses, with the rapid induction of nitrite
and iNOS preceding the induction of TNF-
α
[
91
] Furthermore, wild-type (WT) and Nrf2
knockout (Nrf2
−/−
) mice were subjected to the intranasal instillation of 1 mg/kg PM
2.5
for
28 days. Lower levels of antioxidant enzymes, oxidative stress, microglia activation, inflam-
mation, NF-
κ
B activation, and severe nerve injury were detected in the OB of Nrf2
−/−
mice
compared to the OB of WT mice [
92
]. In addition, PM
2.5
exposure-induced oxidative stress
and microglia activation was attributed to its metal contents and glutaminase-containing
extracellular vehicles (EVs) in the OB [93].
In PD condition,
α
-synuclein is a major component of Lewy bodies, a pathological
hallmark of PD [
94
]. A controlled study found that exposure of male Fischer 344 rats to
DEP (311
µ
g/m
3
or higher) for 6 months increased
α
-synuclein and A
β42
levels in the
midbrain [
85
]. In another study, an increase in
α
-synuclein levels was also found in the
cerebral cortex of C57BL6/J mice exposed to DEP (250 µg/m3) for 3 weeks [1].
In summary, animals exposed to PM ambiently or in controlled experiments reveals
the same pattern of neurotoxic effects as in humans although the crosstalk among these
events need further clarification.
Antioxidants 2022,11, 1482 10 of 23
Table 2. Evidence from main animal studies with PM2.5.
Animal Exposure Protocol Pathological Changes Conclusion References
Male C57BL/6 mice
(8 weeks)
PM2.5: 6 h/day, 5 days/week, for 3 and
9 months (65.7 ±34.2 µg/m3).
Filtered air for controls.
9 months: increased COX-1, COX-2, APP, BACE,
Aβ1–40, PSD-95 and cytokines levels.
3 months: no difference of all these biomarkers.
Long-term exposure to high dose PM
2.5
could
alter brain inflammatory phenotype, induce
synapse damage and promote
AD-like pathology.
Bhatt et al. [77]
Male C57BL/6 mice
(8 weeks)
Oropharyngeal aspiration of PM2.5
(1 and 5 mg/kg bw) every other day for
4 weeks.
Saline for controls.
A dose-dependent increase in IL-1βand TNF-α
in the blood and hippocampus.
Increased BACE1 (biomarker of synaptic
function) expression.
Chronic exposure to PM2.5 causes
neuroinflammation, deteriorated synaptic
function integrity.
Ku et al. [78]
Male SD rats
(6 months)
Traffic-related PM1(aerodynamic
diameter < 1 µm): 6 h/day, for 3 and
6 months (16.3 ±8.2 µg/m3)
Filtered air for controls.
Elevated levels of TBARSs, PGE2, TNF-α
and Iba-1.
Traffic-related PM exposure causes microglia
activation, neuroinflammation and oxidative
stress in the brain.
Bai et al. [79]
C57BL/6 mice
(6 weeks)
Traffic-polluted highway tunnel for
5 days (mean PM 2.5 55.1 µg/m3, mean
elemental carbon 13.9 µg/m3).
Filtered air for controls.
Increases in COX-2, NOS2, and NOS3 genes
(encoding the COX-2, iNOS, and eNOS,
respectively) in the hippocampus.
Decreased level of BDNF in the olfactory bulb.
Short-term exposure to traffic-related air
pollution induces the differential expression
of inflammatory and oxidative genes in
different brain regions.
The olfactory bulb may display a lower
neurotrophic support in response to
air pollution.
Bos et al. [80]
SD rats
(12 weeks)
DEP: 4 h/day, 5 days/week, for 1
month (0.5 or 2 mg/m3) and 0 mg/m3
for controls.
Elevated levels of whole-brain IL-6, nitrated
proteins and Iba-1 (biomarker of
microglia activation).
The midbrain displayed a higher sensibility
to DEP.
Inhalation of DEP causes various degrees of
microglia activation and neuroinflammation
in different brain regions.
Levesque et al. [84]
Male Fischer 344 rats
(10–12 weeks)
DEP: 6 h/day, 7 days/week, for 6
months (35, 100, 311 and 992 µg/m3)
Filtered air for controls.
Elevated level of TNF-αat high concentrations
(most at 992 µg/m3) in all regions, with the
exception of the cerebellum.
Increased level of TNF-
α
at 100
µ
g/m
3
midbrain.
The midbrain may be more sensitive to the
neuroinflammatory effects of DEP exposure. Levesque et al. [85]
Female EFAD
transgenic mice
(E3FAD, E4FAD at
3 months)
nPM: 5 h/day, 3 days/week, for
15 weeks (10 µg/mL).
Filtered air for controls.
In both genotypes: increased levels of Aβ
generation and deposition in the cerebral and
CA1 neurites atrophy, decreased glutamate
GluR1 subunit level.
E4FAD mice displayed more significant
neurotoxicity of nPM.
Long-term nPM exposure could promote the
generation and accumulation of Aβand the
neuronal damage, which further leads
to neurodegeneration.
Cacciottolo et al. [87]
Antioxidants 2022,11, 1482 11 of 23
Table 2. Cont.
Animal Exposure Protocol Pathological Changes Conclusion References
Adult mice
(both sexes at 8 weeks)
DEP: 250–300 µg/m3for 6 h.
Filtered air for controls.
Increased levels of IL-1
β
, TNF-
α
and MDA in all
brain regions, especially the OB and
hippocampus.
Decreased level of BrdU in the hippocampus.
Male mice showed higher increase in IL-1β,
TNF-α, MDA levels.
Acute exposure to DEP may cause
neurotoxicity (neuroinflammation, oxidative
stress, and neurodegeneration).
Males may be more sensitive to the
neurotoxicity of DEP.
Costa et al. [88]
C57BL/6J mice
(both sexes at 8 weeks)
DEP: 250–300 µg/m3for 6 h.
Filtered air for controls.
Decreased numbers of new neurons in the SGZ,
SVZ, and OB, while only in the OB in females.
Elevated numbers of activated microglia and the
levels of TNF-αand MDA in the cortex and
hippocampus, which was decreased by
pioglitazone treatment.
Acute DEP exposure leads to
neuroinflammation, oxidative stress and
disordered neurons genesis, which was more
severe in males and seems to be associated
with the activation of microglia.
Coburn et al. [89]
Male C57BL/6J mice
(3 months)
nPM: for 5, 20, and 45 h over 3 weeks.
Filtered air for controls.
Rapid increases of 4-HNE and 3-NT protein in
OB and OE at 5 h.
Increased numbers of microglia in OB and nasal
epithelium turbinate.
Elevated level of TNF-αin all brain regions at
45 h, with an earlier increased level of TNF-α
mRNA in the OB and OE.
Acute nPM exposure could induce the
activation of microglia, neuroinflammation,
and oxidative stress in different brain regions,
especially the OE and OB.
Cheng et al. [91]
Male C57BL/6 and
Nrf2−/−mice
Intranasal instillation of PM2.5 for
28 days (1 mg/kg bw).
Deionized water for controls.
Decreased levels of antioxidant enzymes (GSH,
SOD) and increased levels of MAD,
inflammatory cytokines, and activation of
microglia and NFκB in the OB.
Increased neuron apoptosis in the olfactory bulb.
Nrf2 may play a neuroprotective role in
response to PM2.5 exposure. Chen et al. [92]
Male C57BL/6 mice
(6 weeks)
Daily intranasal instillation of PM2.5
(0.1 or 1 mg/kg bw), Chelex-treated
PM2.5 (1 mg/kg bw), PM2.5 (1 mg/kg
bw) plus CB-839 (glutaminase inhibitor)
for 28 days.
Deionized water for controls.
Elevated levels of ROS generation, microglia
activation, EVs release, and GAC expression in
the OB.
Treated with CB-839 significantly decreased the
number of EVs and the expression of GAC.
PM2.5 exposure could activate microglia and
may mediate its neurotoxicity by promoting
the production of
glutaminase-containing EVs.
Chen et al. [93]
Abbreviations: COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; APP, amyloid precursor protein; BACE, beta-site APP cleaving enzyme; PSD-95, pre- and post- synaptic marker;
nPM, nanosized particulate matter; DEP, diesel exhaust particle; Iba-1, ionized calcium-binding adaptor molecule 1; TBARSs, thiobarbituric acid-reactive substances; PGE2, prostaglandin
E2; NTS, nucleus of solitary tract; MDA, malondialdehyde; ROS, reactive oxygen species; OB, olfactory bulb; BrdU, brmodeoxyuridine; SGZ, hippocampal subgranular zone; SVZ, the
subventricular zone; 4-HNE, 4-hydroxy-2-nonenal; 3-NT, 3-nitrotyrosine; OE, olfactory neuroepithelium; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase;
EVs, extracellular vesicles; GAC, glutaminase C.
Antioxidants 2022,11, 1482 12 of 23
6. Evidence from In Vitro Studies
In vitro
studies provide insights into in-depth cellular and molecular mechanisms by
which PM exposure promotes cellular damage and abnormality, linking to neurodegener-
ative disorders, such as the alterations of cell viability and apoptosis, the dysfunction of
mitochondria, the production of ROS, or the release of pro-inflammatory mediators [
1
,
17
].
Thus far, neuronal and microglial cell lines, primary cultures or co-culture of those cells
have been introduced for exposure to concentrated ambient air particles, DEP, and LPS,
among others [95] (Table 3).
A mouse microglial cell line (BV2) exposed to concentrated ambient PM
2.5
displayed
the upregulated mRNA of pro-inflammatory cytokines, such as IL-1
β
and TNF-
α
[
96
].
Moreover, the inhibition of Nrf2 activity significantly blocked the PM
2.5
-induced decrease
in cell viability, the increase in the intracellular ROS generation, and the NF
κ
B phosphory-
lation in BV2 cells [
92
]. The acute exposure of microglial cells to high-dose PM
2.5
decreased
cell survival as a result of neuroinflammation and the production of ROS [
97
,
98
]. In ad-
dition, an
in vitro
neuron-microglia culture model exposed to PM
2.5
presented elevated
apoptosis, IL-1
β
, and caspase-1 activity, which could be alleviated by the addition of
IL-1 receptor antagonists and ROS inhibitors [
98
]. Together, these findings suggest that
PM
2.5
has a role in AD pathogenesis, the underlying mechanisms possibly being PM
2.5
-
induced microglial activation, neuroinflammation, increased ROS activity, and even A
β
production [
95
]. Notably, there is evidence that metals associated with PM
2.5
may activate
microglia, since microglia can be activated in vitro by manganese [99].
Microglia were first shown to recognize and respond to PM in an
in vitro
study using
DEP [
100
]. Generally,
in vitro
studies support
in vivo
observations by showing that DEP
can activate microglia, resulting in oxidative stress and neuroinflammation
[86,100,101]
.
With BV2 cells, DEP were shown to reduce cell viability and increase microglial activa-
tion, lipid peroxidation, the production of pro-inflammatory mediators, including IL-6,
TNF-
α
and prosglandin E2 (PGE2), and cytotoxicity [
80
]. Intriguingly, exposure to DEP
(
25–100 µg/cm2
) did not affect the viability of mouse primary cerebellar granule neurons
in vitro
. However, the death of these neurons was increased two- or three-fold with simul-
taneous exposure to DEP and microglia [
101
]), suggesting that microglia play an essential
role in DEP-induced neurotoxicity.
In the PD condition, primary neuron–glia co-cultures and the HAPI (highly aggres-
sively proliferating immortalized) microglial cell line were pre-treated with DEP (5
µ
g/mL)
followed by LPS (2.5 ng/mL) and synergistically amplified NO production, TNF-
α
re-
lease, and DA neurotoxicity [
84
]. Pre-treatment with fractalkine (50 pg/mL), a chemokine
from neurons as a soluble anti-inflammatory signal for microglia [
102
], ameliorated DEP
(50
µ
g/mL)-induced H
2
O
2
production from microglia and protected against DEP-induced
DA neurotoxicity in midbrain neuron–glia cultures [
84
]. Another study with mesencephalic
neuron–glia cultures treated with DEP (5–50
µ
g/mL) resulted in a dose-dependent mi-
croglial activation determined by changes in morphology and increase in superoxide
production and a decrease in DA neurons, with no TNF-
α
, NO, or prostaglandin PGE2
detected [
100
]. Noticeably, the selective DA neurotoxicity only occurred in the presence
of microglia, indicating that microglia mediated the neuron damage. In addition, a study
revealed that microglia cultures derived from mice missing functional NADPH oxidase,
the enzyme responsible for microglial extracellular superoxide production, were insen-
sitive to DEP-induced neurotoxicity, indicating that microglia-derived ROS are key for
DEP-induced DA neurotoxicity [
100
]. Thus, it is assumed that the neurotoxic effects of DEP
on DA neurons could be either direct or indirect via the release of inflammatory mediators
and ROS from activated microglial cells [84,100,102].
Overall,
in vitro
studies suggest PM-induced oxidative stress and microglial cell medi-
ated inflammatory and/or oxidative responses as potential mechanisms leading to neu-
rotoxicity, and the increased risk of neurodegenerative disease as seen in epidemiological
and animal studies.
Antioxidants 2022,11, 1482 13 of 23
Table 3. Evidence from main in vitro studies with PM2.5.
Cell Type Species Exposure Protocol Pathological Changes Conclusion References
Microglia cell
line (BV2) Mouse
DEP: 50 and 100 µg/mL for
24 h.
0µg/mL for control.
Increased levels of ROS, LDH, TBARSs, IL-6, PGE2,
and TNF-αand decreased cell viability.
Microglia activation.
Acute exposure to DEP could induce
cytotoxicity, lipid peroxidation, microglial
activation and inflammation.
Bai et al. [79]
Microglia cell line
(HAPI) and
primary neurons
Rat
DEP: 5–50 µg/mL for 3 and
24 h.
0µg/mL for control.
Increased levels of NO, TNF-αand DA injury
(5 µg/mL group) and H2O2generation in microglia
co-treatment with LPS (2.5 ng/mL).
DEP exposure causes neuroinflammation,
oxidative stress and neuron death, which
may be associated with the activation
of microglia.
Levesque et al. [84]
Microglia cell
line (BV2) Murine PM2.5: 50 µg/mL for 24 h.
0µg/mL for control.
Decreased cell viability and increased intracellular
ROS generation and NF-κB phosphorylation when
the Nrf2 activity was inhibited.
Nrf2 may play anti-oxidation and
anti-inflammation roles in response to PM
2.5
exposure in the neurons.
Chen et al. [93]
Microglia cell
line (BV2) Mouse
CAPs (≤2.5 µm): 75 µg/mL
for 4 h and 25–100
µ
g/mL for
1.5 h or 6 h.
0µg/mL for control.
Decreased levels of intracellular ATP (≥250 mg/mL)
and depolarized mitochondrial membranes
(≥6 mg/mL).
Release of pro-inflammatory cytokines (TNF-α
and IL-6).
Up-regulated expression of inflammatory genes.
CAPs exposure could induce an
inflammatory response and regulate the
gene expression in BV2, and the
mitochondrial injury may be key to
CAPs-induced neurotoxicity.
Sama et al. [96]
Microglia cell
line (BV2) Rat
PM2.5: 5, 10, 25, 50,
100 µg/mL for 1 h and 24 h.
0µg/mL for control.
Increased levels of NO and ROS generation and the
genes expression of IL-1β, IL-6, COX-2, and iNOS,
especially in high dose groups.
Microglia activation of M1 phenotype.
Decreased cell viability.
Acute PM
2.5
exposure probably mediates its
neurotoxicity through inflammation and
oxidative stress in the microglia.
Kim et al. [97]
Primary microglial
cells and neurons mouse PM2.5: 50 µg/mL for 4 h.
0µg/mL for control.
Elevated levels of IL-1β, caspase-1 activation and
ROS generation.
Inhibition of IL-1 receptor and ROS generation
decreased the levels of inflammatory cytokines and
cell apoptosis.
Acute PM2.5 exposure would cause
neuroinflammation and oxidative stress,
which may induce neurons apoptosis.
Wang et al. [98]
Microglia cell line
(HAPI) and
primary microglial
Rat
MnCl2: 0.33, 1, 3.33, 10, 33
µM for 0.25, 1, 3, 6 and 24 h.
0µM for control.
An increased time- and concentration-dependent
release of hydrogen peroxide (H2O2) in microglia.
MnCl2is capable of activating microglia to
release ROS. Zhang et al. [99]
Antioxidants 2022,11, 1482 14 of 23
Table 3. Cont.
Cell Type Species Exposure Protocol Pathological Changes Conclusion References
Primary microglial
cells and neurons Rat DEP (0.22 µm): 5–50 µg/mL.
0µg/mL for control.
Dose-dependent microglia activation.
Selective dopaminergic neuron (DA) death induced
by DEP treatment was reinstated with the addition
of microglia.
Microglia from mice missing functional NADPH
oxidase displayed insensitive response to
DEP treatment.
Microglia may play a key role in
DEP-induced neurotoxicity. Block et al. [100]
Primary microglia
cells and cerebellar
granule
neurons (CGNs)
Mouse
DEP: 25, 50, 100
µ
g/2 cm
2
for
24 h.
0µg/2 cm2for control.
DEP treatment did not affect the viability of CGNs.
Neuronal cell death increased by 2–3-fold after
co-treatment with microglia.
Elevated level of ROS genes expression of IL-1βand
IL-6 in microglia.
Microglia may mediate DEP-induced
neuronal toxicity through oxidative stress
and neuroinflammatory mechanisms.
Roquéet al. [101]
Abbreviations: CAPs, concentrated ambient particles; ROS, reactive oxygen species; NF-
κ
B, nuclear factor kappa B; iNOS, inducible nitric oxide synthase; DEP, diesel exhaust particle;
HAPI, highly aggressively proliferating immortalized; TBARSs, thiobarbituric acid-reactive substances; PGE2, prostaglandin E2.
Antioxidants 2022,11, 1482 15 of 23
7. Potential Mechanisms for PM2.5 -Induced Microglial Oxidative Stress and
Neuronal Toxicity
The aforementioned evidence suggests that exposure to PM
2.5
has a crucial role in
neurodegenerative disorders possible through the activation of microglial cells [
95
]. How-
ever, the responsible mechanisms remain large unknown. The four critical issues are of
significant relevance and need to be addressed: (1) The routes through which PM
2.5
access
CNS; (2) the receptors microglia use to relay PM
2.5
neurotoxic signals; (3) PM
2.5
-induced
microglial oxidative stress; and (4) the interactions between microglia and neurons.
Route of CNS Effects
: Multiple routes for PM
2.5
impact on the CNS have been pro-
posed, two of them are predominant (Figure 2). First, PM
2.5
, UFPM, and their components
can enter the olfactory receptor neurons that extend their dendrites into the mucous layer
covering the OE through pinocytosis, simple diffusion, or receptor-mediated endocytosis,
and is further transported along the axons to the OB and olfactory cortex [
9
,
103
]. In addi-
tion, UFPM exposure has been shown to rapidly increase the products of lipid peroxidation,
such as 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosine (3-NT) protein adducts in the
OE and OB [
91
], further leading to oxidative inflammation from nose to brain. Second,
PM
2.5
inhaled into the deep lung successively penetrates the alveolar–blood barrier and
the BBB and finally reaches brain regions. Meanwhile, lung-derived circulating cytokines
induced by PM
2.5
exposure could also enter the brain [
18
]. In both cases, PM
2.5
directly or
indirectly activates microglia and induces the release of pro-inflammatory cytokines and
ROS, leading to neurodegeneration [
66
,
104
]. Alternatively, PM
2.5
may potentially affect the
CNS via the gut microbiota–brain axis [1,105] or lung microbiota [106].
Antioxidants 2022, 11, x FOR PEER REVIEW 17 of 25
7. Potential Mechanisms for PM2.5-Induced Microglial Oxidative Stress and Neuronal
Toxicity
The aforementioned evidence suggests that exposure to PM2.5 has a crucial role in
neurodegenerative disorders possible through the activation of microglial cells [95]. How-
ever, the responsible mechanisms remain large unknown. The four critical issues are of
significant relevance and need to be addressed: (1) The routes through which PM2.5 access
CNS; (2) the receptors microglia use to relay PM2.5 neurotoxic signals; (3) PM2.5-induced
microglial oxidative stress; and 4) the interactions between microglia and neurons.
Route of CNS Effects: Multiple routes for PM2.5 impact on the CNS have been pro-
posed, two of them are predominant (Figure 2). First, PM2.5, UFPM, and their components
can enter the olfactory receptor neurons that extend their dendrites into the mucous layer
covering the OE through pinocytosis, simple diffusion, or receptor-mediated endocytosis,
and is further transported along the axons to the OB and olfactory cortex [9,103]. In addi-
tion, UFPM exposure has been shown to rapidly increase the products of lipid peroxida-
tion, such as 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosine (3-NT) protein adducts in
the OE and OB [91], further leading to oxidative inflammation from nose to brain. Second,
PM2.5 inhaled into the deep lung successively penetrates the alveolar–blood barrier and
the BBB and finally reaches brain regions. Meanwhile, lung-derived circulating cytokines
induced by PM2.5 exposure could also enter the brain [18]. In both cases, PM2.5 directly or
indirectly activates microglia and induces the release of pro-inflammatory cytokines and
ROS, leading to neurodegeneration [66,104]. Alternatively, PM2.5 may potentially affect
the CNS via the gut microbiota–brain axis [1,105] or lung microbiota [106].
Figure 2. The routes that PM2.5 enters the brain. The lung–brain axis and olfactory pathway are two
recognized predominant routes that PM2.5 takes into the brain. Once inhaled, PM2.5 can quickly dif-
fuse throughout the alveoli and lead to lung inflammation. These circulating cytokines (IL-6, TNF-
α, IL-1β, etc.), in combination with soluble components of PM2.5, cross the BBB directly or via a
disruption to the permeability of the BBB, and then induce microglia activation and neurotoxicity.
Meanwhile, with a consequence of lipid peroxidation (4-HNE and 3-NT protein adduction), PM2.5
could also gain access to the olfactory bulb through the olfactory epithelium and then move into the
deep regions of the brain. Moreover, the gut–brain axis is potentially another route through which
PM2.5 exerts its neurotoxicity, which is probably associated with the dysbiosis of gut microbiota.
Figure 2.
The routes that PM
2.5
enters the brain. The lung–brain axis and olfactory pathway are two
recognized predominant routes that PM
2.5
takes into the brain. Once inhaled, PM
2.5
can quickly
diffuse throughout the alveoli and lead to lung inflammation. These circulating cytokines (IL-6,
TNF-
α
, IL-1
β
, etc.), in combination with soluble components of PM
2.5
, cross the BBB directly or via
a disruption to the permeability of the BBB, and then induce microglia activation and neurotoxicity.
Meanwhile, with a consequence of lipid peroxidation (4-HNE and 3-NT protein adduction), PM
2.5
could also gain access to the olfactory bulb through the olfactory epithelium and then move into the
deep regions of the brain. Moreover, the gut–brain axis is potentially another route through which
PM2.5 exerts its neurotoxicity, which is probably associated with the dysbiosis of gut microbiota.
Antioxidants 2022,11, 1482 16 of 23
Sensing receptors on microglia
: Microglia monitor the brain environment by inter-
preting and processing stimuli through pattern recognition receptors (PRRs) (Figure 3),
which mainly include TLRs [
107
,
108
], scavenger receptors (e.g., SR-A1 and SR-B1) [
109
],
macrophage antigen complex 1 (MAC1), and receptor complexes (CD36,
α
6
β
1 integrin,
and CD47) [
16
], for diverse neurotoxic and pro-inflammatory ligands, respectively. Both
nanoPM and LPS have been shown to strongly activate TLR4 and NF-
κ
B in mixed glial
cultures. TLR4 siRNA attenuated TNF-
α
and other inflammatory responses to nanoPM via
the MyD88-dependent pathway [
110
]. Thus, PPRs expressed on the microglial surface seem
to be one of the primary common pathways by which ambient PM signals are transduced
into ROS production [16].
Antioxidants 2022, 11, x FOR PEER REVIEW 19 of 25
Figure 3. Proposed mechanisms for PPR-mediated microglia activation and neuronal toxicity in-
duced by PM2.5. Microglia monitor the brain environment by interpreting and processing stimuli
through pattern recognition receptors (PRRs), which mainly include TLRs, scavenger receptors,
MAC1, and receptor complex for diverse neurotoxic and pro-inflammatory ligands, respectively.
Exogenous and endogenous insults bind to diverse PPRs and result in microglial activation and
release of soluble factors, such as cytokines, PGE2, and neurotrophins (BDNF), which bind to neu-
ronal receptors. Meanwhile, neuronal metabolites and damaged neuron components could also ac-
tivate microglia. Together, microglia-neuron interactions further promote the pathogenesis of neu-
rodegenerative disorders.
8. Conclusions and Future Directions
In summary, air pollution, together with the increasing age of the global population,
pose great threats to public health. Thus far, the mechanisms responsible for PM2.5-in-
duced neurodegenerative diseases remain largely unknown. The CNS effects are chronic,
beginning in childhood, and may take time to accumulate pathology. Specifically, air pol-
lution has been shown to cause neuroinflammation, oxidative stress, cerebral vascular
damage, and neurodegenerative pathology, which all involve microglial activation. Evi-
dence from epidemiological and experimental studies suggests that exposure to ambient
PM, especially PM2.5, is associated with neurodegenerative disorders. The interpretation
of the intracellular and extracellular pathways participating in the generation of oxidative
stress in microglia may be important not only for comprehending the pathophysiological
basis for neuron damage in neurodegenerative diseases but also for designing effective
strategies to mitigate or even prevent PM2.5-induced neural neurodegenerative damage.
While epidemiology has linked an increased risk of stroke, AD, and PD with exposure to
Figure 3.
Proposed mechanisms for PRR-mediated microglia activation and neuronal toxicity induced
by PM
2.5
. Microglia monitor the brain environment by interpreting and processing stimuli through
pattern recognition receptors (PRRs), which mainly include TLRs, scavenger receptors, MAC1, and
receptor complex for diverse neurotoxic and pro-inflammatory ligands, respectively. Exogenous and
endogenous insults bind to diverse PPRs and result in microglial activation and release of soluble
factors, such as cytokines, PGE2, and neurotrophins (BDNF), which bind to neuronal receptors. Mean-
while, neuronal metabolites and damaged neuron components could also activate microglia. Together,
microglia-neuron interactions further promote the pathogenesis of neurodegenerative disorders.
Microglia-associated oxidative stress:
ROS are critical components of the pro-
inflammatory signaling pathway in microglia [
110
]. Activated microglia by exogenous and
endogenous insults can become a chronic source of pro-inflammatory factors and oxidative
stress in the brain, driving neurodegenerative diseases [
16
]. In microglia, ROS primarily
Antioxidants 2022,11, 1482 17 of 23
from both NOX and the mitochondria, may act as second messengers to propagate im-
mune activation, excessive inflammation, and oxidative stress [
111
]. Ambient particles
transported into the brain could be phagocytized by microglia, leading to NOX and mi-
croglial activation, and ROS production [
84
]. Mitochondrial dysfunction in microglia has
been proposed to play a role in the progression of neurodegenerative diseases [
112
]. The
elevated generation of ROS and the loss of mitochondrial membrane potential through
various mechanisms have been observed in AD. A
β
interacts with microglial receptors,
such as TREM2, activating downstream pathways, causing mitochondrial damage, and
aggravating inflammation and cytotoxicity. Fibrillar A
β
activates NOX in microglia leading
to the elevated induction of mitochondrial ROS, which further causes neurotoxicity [112].
Microglia-neuron interactions:
The bi-directional communication between microglia
and neurons has been recognized to be critical for maintaining a healthy environment
in the CNS and also for the chronic development of neuroinflammation [
113
]. The air
pollution-induced loss of neurons has been detected in postmortem and experimental
studies, as described earlier, and neuronal cell death may be direct or indirect via microglia
activation [
12
]. Thus far, the mechanisms for microglia–neuron interaction remain elusive.
Activated microglia can release soluble factors, such as cytokines (IL-1
β
; TNF-
α
), PGE2, and
neurotrophins (BDNF), which bind to neuronal receptors [114]. With a primary cerebellar
granule neuron (CGN) model, DEP showed minimal effect on neuron viability after 24 h of
treatment. In the presence of primary cortical microglia neuronal cell death increased by
2–3 fold after co-treatment with DEP, suggesting that microglia are important contributors
to DEP-induced CGN neurotoxicity, possibly due to soluble intermediates since microglia-
conditioned medium by DEP treatment was also toxic to CGNs [
101
]. In addition, Block
et al. showed that DEP could damage DA neurons through microglia-derived oxidant
species [
104
]. However, another study reported that DEP caused a significant increase in
ROS in microglia, antioxidants failed to protect neurons from DEP/microglia-induced toxi-
city [
105
]. From the above, the mechanisms underlying PM
2.5
-induced microglial activation
and its interaction with neurons are still unclear and warrant further investigation.
8. Conclusions and Future Directions
In summary, air pollution, together with the increasing age of the global population,
pose great threats to public health. Thus far, the mechanisms responsible for PM
2.5
-induced
neurodegenerative diseases remain largely unknown. The CNS effects are chronic, begin-
ning in childhood, and may take time to accumulate pathology. Specifically, air pollution
has been shown to cause neuroinflammation, oxidative stress, cerebral vascular damage,
and neurodegenerative pathology, which all involve microglial activation. Evidence from
epidemiological and experimental studies suggests that exposure to ambient PM, espe-
cially PM
2.5
, is associated with neurodegenerative disorders. The interpretation of the
intracellular and extracellular pathways participating in the generation of oxidative stress
in microglia may be important not only for comprehending the pathophysiological basis
for neuron damage in neurodegenerative diseases but also for designing effective strate-
gies to mitigate or even prevent PM
2.5
-induced neural neurodegenerative damage. While
epidemiology has linked an increased risk of stroke, AD, and PD with exposure to PM
2.5
,
further epidemiological and mechanistic studies regarding the association between the
components of air pollution and the development of CNS diseases are of pressing concern
for human health.
Author Contributions:
Conceptualization, W.W. and J.S.; methodology, J.S., K.H., Y.W. (Ya Wang) and
R.Q.; software, H.W., Y.L. and S.W.; validation, Z.A., Y.W. (Yinbiao Wang) and J.L.; formal analysis,
J.S., K.H. and Y.W. (Ya Wang); investigation, J.S., K.H., Y.W. (Ya Wang) and R.Q.; resources, J.S., K.H.
and Y.W. (Ya Wang); data curation, K.H. and Y.W. (Ya Wang); writing—original draft preparation, J.S.
and K.H.; writing—review and editing, W.W.; visualization, J.S., H.W. and K.H.; supervision, W.W.;
project administration, W.W.; funding acquisition, W.W. and J.S. All authors have read and agreed to
the published version of the manuscript.
Antioxidants 2022,11, 1482 18 of 23
Funding:
This research was funded by the National Natural Science Foundation of China (grant
number: 81961128031; 81573112) and the Key Scientific and Technological Research Projects of Henan
Province (222102310600; 212102310335).
Conflicts of Interest:
The authors declare no conflict of interest. The funders had no role in the design
of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or
in the decision to publish the results.
Abbreviations
PM
2.5
, PM with a diameter less than 2.5
µ
m; UFPM, ultrafine PM; CNS, central nervous sys-
tem; TRAP, traffic-related air pollution; DEP, diesel exhaust particles; LPS, lipopolysaccharides; AD,
Alzheimer’s disease; PD, Parkinson’s disease; ROS, reactive oxygen species; RNS, nitric oxide species;
IFN-
γ
, interferon-
γ
; A
β
, amyloid
β
; TNF-
α
, tumor necrosis factor-
α
, IL-6, interleukin 6; IL-1
β
, in-
terleukin 1
β
; IL-12, interleukin 12; CCL-2, chemokine ligand-2; MCP-1, monocyte chemoattractant
protein-1; PGE, prosglandin E; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, nicoti-
namide adenine dinucleotide phosphate oxidase; iNOS, inducible nitric oxide synthase; NO, nitric
oxide; Iba-1, ionized calcium binding adapter molecule-1; PPAR
γ
, peroxisome proliferator-activated
receptors
γ
; TGF-
β
, transforming growth factor-
β
; CSF-1, gcolony stimulating factor-1; BDNF, brain
derived neurotrophic factor; NF-
κ
B, nuclear factor-
κ
B; BBB, blood-brain barrier; NFTs, neurofibrillary
tangles; OE, olfactory epithelium; OB, olfactory bulbs; PrPC, cellular prion protein; GFAP, glial fibril-
lary acidic protein; APOE4, Apolipoprotein E4; APOE3, Apolipoprotein E3; COX-2, cyclooxygenase-2;
8-OHdG, 8-hydroxydeoxyguanosine; CSF, cerebrospinal fluids; MIF, macrophage inhibitory factor;
TLRs, Toll-like receptors; TREM2, triggering receptor expressed on myeloid cells 2; NfL, neurofila-
ment light chain; BACE, beta-site amyloid precursor protein (APP)-cleaving enzyme; APP, amyloid
precursor protein; MIP-1
α
, macrophage inflammatory protein-1
α
; SD, Sprague–Dawley; PM
1
, PM
with a diameter less than 1
µ
m; nanoPM, nanosized PM; WT, wild-type; EVs, extracellular vehicles;
Nrf2, nuclear factor erythroid 2-related factor 2; PGE2, prosglandin E2; 4-HNE, 4-hydroxy-2-nonenal;
3-NT, 3-nitrotyrosine; PRRs, pattern recognition receptors; MAC1, macrophage antigen complex 1;
CGNs, cerebellar granule neuron; DA, dopaminergic.
References
1.
Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its
possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther.
2020
,210, 107523. [CrossRef] [PubMed]
2.
Ghio, A.J.; Smith, C.B.; Madden, M.C. Diesel exhaust particles and airway inflammation. Curr. Opin. Pulm. Med.
2012
,18, 144–150.
[CrossRef] [PubMed]
3.
Wu, W.; Jin, Y.; Carlsten, C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol.
2018
,
141, 833–844. [CrossRef] [PubMed]
4.
Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and temporal variation in PM
2.5
chemical composition in the
United States for health effects studies. Environ. Health Perspect. 2007,115, 989–995. [CrossRef]
5.
Gao, J.; Wang, K.; Wang, Y.; Liu, S.; Zhu, C.; Hao, J.; Liu, H.; Hua, S.; Tian, H. Temporal-spatial characteristics and source
apportionment of PM
2.5
as well as its associated chemical species in the Beijing-Tianjin-Hebei region of China. Environ. Pollut.
2018,233, 714–724. [CrossRef]
6.
Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM
2.5
exposures
and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019,174, 344–352. [CrossRef]
7.
Figueres, C.; Landrigan, P.J.; Fuller, R. Tackling air pollution, climate change, and NCDs: Time to pull together. Lancet
2018
,392,
1502–1503. [CrossRef]
8.
Calderón-Garcidueñas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henríquez-Roldán, C.; Schoonhoven, R.; Acuña-Ayala, H.;
Villarreal-Calderón, A.
; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA damage in nasal and brain tissues of canines
exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol. Pathol.
2003
,
31, 524–538. [CrossRef]
9.
Calderón-Garcidueñas, L.; Reed, W.; Maronpot, R.R.; Henríquez-Roldán, C.; Delgado-Chavez, R.; Calderón-Garcidueñas, A.;
Dragustinovis, I.; Franco-Lira, M.; Aragón-Flores, M.; Solt, A.C.; et al. Brain inflammation and Alzheimer’s-like pathology in
individuals exposed to severe air pollution. Toxicol. Pathol. 2004,32, 650–658. [CrossRef]
10.
Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.;
Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with
children and dogs. Brain Cogn. 2008,68, 117–127. [CrossRef]
Antioxidants 2022,11, 1482 19 of 23
11.
Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM
2.5
exposure and neurological disorders: A systematic
review and meta-analysis. Sci. Total Environ. 2019,655, 1240–1248. [CrossRef]
12.
Wang, J.; Ma, T.; Ma, D.; Li, H.; Hua, L.; He, Q.; Deng, X. The impact of air pollution on neurodegenerative diseases. Ther. Drug
Monit. 2021,43, 69–78. [CrossRef]
13. Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020,21, 7152. [CrossRef]
14.
Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining
their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci.
2021
,22, 4642.
[CrossRef]
15.
Simpson, D.S.A.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative
disease. Antioxidants 2020,9, 743. [CrossRef]
16.
Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci.
2007,8, 57–69. [CrossRef]
17.
Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci.
2009
,
32, 506–516. [CrossRef]
18.
Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol.
2012
,
2012, 782462. [CrossRef]
19.
Zhu, X.; Ji, X.; Shou, Y.; Huang, Y.; Hu, Y.; Wang, H. Recent advances in understanding the mechanisms of PM
2.5
-mediated
neurodegenerative diseases. Toxicol. Lett. 2020,329, 31–37. [CrossRef]
20.
Calderón-Garcidueñas, L.; Leray, E.; Heydarpour, P.; Torres-Jardón, R.; Reis, J. Air pollution, a rising environmental risk factor for
cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev. Neurol.
2016
,172, 69–80.
[CrossRef]
21. Greter, M.; Merad, M. Regulation of microglia development and homeostasis. Glia 2013,61, 121–127. [CrossRef]
22. Wieghofer, P.; Knobeloch, K.P.; Prinz, M. Genetic targeting of microglia. Glia 2015,63, 1–22. [CrossRef]
23. Priller, J.; Prinz, M. Targeting microglia in brain disorders. Science 2019,365, 32–33. [CrossRef]
24.
Mittelbronn, M.; Dietz, K.; Schluesener, H.J.; Meyermann, R. Local distribution of microglia in the normal adult human central
nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001,101, 249–255. [CrossRef]
25.
Ong, W.Y.; Leong, S.K.; Garey, L.J.; Tan, K.K.; Zhang, H.F. A light and electron microscopic study of HLA-DR positive cells in the
human cerebral cortex and subcortical white matter. J. Hirnforsch. 1995,36, 553–563.
26.
Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult
mouse brain. Neuroscience 1990,39, 151–170. [CrossRef]
27.
Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial
support for survival during postnatal development. Nat. Neurosci. 2013,16, 543–551. [CrossRef]
28.
Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of general and discriminating markers of differential microglia phenotypes.
Front. Cell Neurosci. 2020,14, 198. [CrossRef] [PubMed]
29.
Zhang, L.; Zhang, J.; You, Z. Switching of the Microglial Activation Phenotype Is a Possible Treatment for Depression Disorder.
Front. Cell Neurosci. 2018,12, 306. [CrossRef] [PubMed]
30.
Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed.
J. Neuroinflammation 2014,11, 98. [CrossRef] [PubMed]
31.
Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev.
Immunol. 2017,35, 441–468. [CrossRef]
32.
Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases.
Semin. Cell Dev. Biol. 2019,94, 112–120. [CrossRef]
33.
Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.L. Role of microglia in neurological disorders and their potentials as
a therapeutic target. Mol. Neurobiol. 2017,54, 7567–7584. [CrossRef]
34.
Lee, K.H.; Cha, M.; Lee, B.H. Crosstalk between neuron and glial cells in oxidative injury and neuroprotection. Int. J. Mol. Sci.
2021,22, 13315. [CrossRef]
35.
Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat.
Neurosci. 2007,10, 1387–1394. [CrossRef]
36.
van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys.
Acta 2011,1812, 141–150. [CrossRef]
37.
Garza-Lombó, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity linked to dysfunctional metal ion
homeostasis and xenobiotic metal exposure: Redox signaling and oxidative stress. Antioxid. Redox Signal.
2018
,28, 1669–1703.
[CrossRef]
38.
Saijo, K.; Crotti, A.; Glass, C.K. Regulation of microglia activation and deactivation by nuclear receptors. Glia
2013
,61, 104–111.
[CrossRef]
39.
He, Y.; Gao, Y.; Zhang, Q.; Zhou, G.; Cao, F.; Yao, S. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates
neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience 2020,437, 161–171. [CrossRef]
40.
Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl.
Neurodegener. 2020,9, 42. [CrossRef]
Antioxidants 2022,11, 1482 20 of 23
41.
Calderón-Garcidueñas, L.; Azzarelli, B.; Acuna, H.; Garcia, R.; Gambling, T.M.; Osnaya, N.; Monroy, S.; Delt, M.R.; Carson, J.L.;
Villarreal-Calderon, A.; et al. Air pollution and brain damage. Toxicol. Pathol. 2002,30, 373–389. [CrossRef]
42.
Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.;
Osnaya, N.; Stone, I.; García, R.; et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate
immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42
and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008,36, 289–310.e5.
43.
Calderón-Garcidueñas, L.; D’Angiulli, A.; Kulesza, R.J.; Torres-Jardón, R.; Osnaya, N.; Romero, L.; Keefe, S.; Herritt, L.;
Brooks, D.M.
; Avila-Ramirez, J.; et al. Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem
auditory evoked potentials. Int. J. Dev. Neurosci. 2011,29, 365–375. [CrossRef]
44.
Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.;
Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: Influences on olfactory function and pathology
in exposed children and young adults. Exp. Toxicol. Pathol. 2010,62, 91–102. [CrossRef] [PubMed]
45.
Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid. Biochem. Mol. Biol.
2016,160, 134–147. [CrossRef]
46.
Calderón-Garcidueñas, L.; Kavanaugh, M.; Block, M.; D’Angiulli, A.; Delgado-Chávez, R.; Torres-Jardón, R.; González-Maciel, A.;
Reynoso-Robles, R.; Osnaya, N.; Villarreal-Calderon, R.; et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid
plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J. Alzheimers Dis.
2012,28, 93–107. [CrossRef] [PubMed]
47.
Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Kulesza, R.J.; Mukherjee, P.S.; Torres-Jardón, R.;
Rönkkö, T.
;
Doty, R.L. Alzheimer ’s disease and alpha-synuclein pathology in the olfactory bulbs of infants, children, teens and adults
≤
40 years in Metropolitan Mexico City. APOE4 carriers at higher risk of suicide accelerate their olfactory bulb pathology. Environ.
Res. 2018,166, 348–362. [CrossRef] [PubMed]
48.
Cipriani, G.; Danti, S.; Carlesi, C.; Borin, G. Danger in the air: Air pollution and cognitive dysfunction. Am. J. Alzheimers Dis Other
Demen 2018,33, 333–341. [CrossRef] [PubMed]
49.
Paul, K.C.; Haan, M.; Mayeda, E.R.; Ritz, B.R. Ambient air pollution, noise, and late-life cognitive decline and dementia risk.
Annu. Rev. Public Health 2019,40, 203–220. [CrossRef] [PubMed]
50.
Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function,
cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology
2016
,56, 235–253.
[CrossRef] [PubMed]
51.
Costa, L.G. Traffic-related air pollution and neurodegenerative diseases: Epidemiological and experimental evidence and potential
underlying mechanisms-ScienceDirect. Adv. Neurotoxicol. 2017,1, 1–46.
52.
Jung, C.R.; Lin, Y.T.; Hwang, B.F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based
cohort study in Taiwan. J. Alzheimers Dis. 2015,44, 573–584. [CrossRef]
53.
Schikowski, T.; Vossoughi, M.; Vierkötter, A.; Schulte, T.; Teichert, T.; Sugiri, D.; Fehsel, K.; Tzivian, L.; Bae, I.S.; Ranft, U.; et al.
Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ. Res.
2015,142, 10–16. [CrossRef]
54.
Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.;
Jessiman, B.; et al.
Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort
study. Lancet 2017,389, 718–726. [CrossRef]
55.
Zanobetti, A.; Dominici, F.; Wang, Y.; Schwartz, J.D. A national case-crossover analysis of the short-term effect of PM
2.5
on
hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health 2014,13, 38. [CrossRef]
56.
Kioumourtzoglou, M.A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-term PM
2.5
exposure and neurological hospital admissions in the Northeastern United States. Environ. Health Perspect.
2016
,124, 23–29.
[CrossRef]
57.
Calderón-Garcidueñas, L.; Avila-Ramírez, J.; Calderón-Garcidueñas, A.; González-Heredia, T.; Acuña-Ayala, H.; Chao, C.K.;
Thompson, C.; Ruiz-Ramos, R.; Cortés-González, V.; Martínez-Martínez, L.; et al. Cerebrospinal fluid biomarkers in highly
exposed PM
2.5
urbanites: The risk of Alzheimer ’s and Parkinson’s diseases in young Mexico City residents. J. Alzheimers Dis.
2016,54, 597–613. [CrossRef]
58.
Blennow, K.; Dubois, B.; Fagan, A.M.; Lewczuk, P.; de Leon, M.J.; Hampel, H. Clinical utility of cerebrospinal fluid biomarkers in
the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015,11, 58–69. [CrossRef]
59.
Calderón-Garcidueñas, L.; Mukherjee, P.S.; Waniek, K.; Holzer, M.; Chao, C.K.; Thompson, C.; Ruiz-Ramos, R.;
Calderón-Garcidueñas, A.
;
Franco-Lira, M.; Reynoso-Robles, R.; et al. Non-phosphorylated Tau in cerebrospinal fluid is a marker of Alzheimer’s disease
continuum in young urbanites exposed to air pollution. J. Alzheimers Dis. 2018,66, 1437–1451. [CrossRef]
60.
Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative
disease. Nat. Rev. Neurol. 2019,15, 565–581. [CrossRef]
61.
Chen, J.C.; Wang, X.; Wellenius, G.A.; Serre, M.L.; Driscoll, I.; Casanova, R.; McArdle, J.J.; Manson, J.E.; Chui, H.C.;
Espeland, M.A.
Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann. Neurol.
2015,78, 466–476. [CrossRef]
Antioxidants 2022,11, 1482 21 of 23
62.
Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.;
Rapp, S.R.; et al.
Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s
disease. Brain 2020,143, 289–302. [CrossRef]
63.
Kirrane, E.F.; Bowman, C.; Davis, J.A.; Hoppin, J.A.; Blair, A.; Chen, H.; Patel, M.M.; Sandler, D.P.; Tanner, C.M.;
Vinikoor-Imler, L.; et al.
Associations of Ozone and PM
2.5
concentrations with Parkinson’s disease among participants in
the Agricultural Health Study. J. Occup. Environ. Med. 2015,57, 509–517. [CrossRef]
64.
Liu, R.; Young, M.T.; Chen, J.C.; Kaufman, J.D.; Chen, H. Ambient air pollution exposures and risk of Parkinson disease. Environ.
Health Perspect. 2016,124, 1759–1765. [CrossRef]
65.
Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Particulate matter and risk
of Parkinson disease in a large prospective study of women. Environ. Health 2014,13, 80. [CrossRef]
66.
Calderón-Garcidueñas, L.; Calderón-Garcidueñas, A.; Torres-Jardón, R.; Avila-Ramírez, J.; Kulesza, R.J.; Angiulli, A.D. Air
pollution and your brain: What do you need to know right now. Prim. Health Care Res. Dev. 2015,16, 329–345. [CrossRef]
67.
Xu, X.; Ha, S.U.; Basnet, R. A review of epidemiological research on adverse neurological effects of exposure to ambient air
pollution. Front. Public Health 2016,4, 157. [CrossRef] [PubMed]
68.
Delgado-Saborit, J.M.; Guercio, V.; Gowers, A.M.; Shaddick, G.; Fox, N.C.; Love, S. A critical review of the epidemiological
evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Total Environ.
2021,757, 143734. [CrossRef] [PubMed]
69.
Calderón-Garcidueñas, L.; Cross, J.V.; Franco-Lira, M.; Aragón-Flores, M.; Kavanaugh, M.; Torres-Jardón, R.; Chao, C.K.;
Thompson, C.; Chang, J.; Zhu, H.; et al. Brain immune interactions and air pollution: Macrophage inhibitory factor (MIF), prion
cellular protein (PrP(C)), Interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1Ra), and interleukin-2 (IL-2) in cerebrospinal
fluid and MIF in serum differentiate urban children exposed to severe vs. low air pollution. Front. Neurosci. 2013,7, 183.
70.
Cox, G.M.; Kithcart, A.P.; Pitt, D.; Guan, Z.; Alexander, J.; Williams, J.L.; Shawler, T.; Dagia, N.M.; Popovich, P.G.;
Satoskar, A.R.; et al.
Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J. Immunol.
2013,191, 1043–1054. [CrossRef]
71.
Tang, S.; Li, T.; Fang, J.; Chen, R.; Cha, Y.; Wang, Y.; Zhu, M.; Zhang, Y.; Chen, Y.; Du, Y.; et al. The exposome in practice:
An exploratory panel study of biomarkers of air pollutant exposure in Chinese people aged 60–69 years (China BAPE Study).
Environ. Int. 2021,157, 106866. [CrossRef] [PubMed]
72.
Song, J.; Qu, R.; Sun, B.; Chen, R.; Kan, H.; An, Z.; Jiang, J.; Li, J.; Zhang, Y.; Wu, W. Associations of short-term exposure to
fine particulate matter with neural damage biomarkers: A panel study of healthy retired adults. Environ. Sci. Technol.
2022
,56,
7203–7213. [CrossRef] [PubMed]
73.
Kilian, J.; Kitazawa, M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease-Evidence
from epidemiological and animal studies. Biomed. J. 2018,41, 141–162. [CrossRef] [PubMed]
74. Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roque, P. Neurotoxicants are in the air: Convergence of human, animal,
and in vitro studies on the effects of air pollution on the brain. Biomed. Res. Int. 2014,2014, 736385. [CrossRef]
75.
Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.;
Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000,21, 383–421. [CrossRef]
76.
Calderón-Garcidueñas, L.; Reynoso-Robles, R.; Vargas-Martínez, J.; Gómez-Maqueo-Chew, A.; Pérez-Guillé, B.; Mukherjee, P.S.;
Torres-Jardón, R.; Perry, G.; Gónzalez-Maciel, A. Prefrontal white matter pathology in air pollution exposed Mexico City young
urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ.
Res. 2016,146, 404–417. [CrossRef]
77.
Bhatt, D.P.; Puig, K.L.; Gorr, M.W.; Wold, L.E.; Combs, C.K. A pilot study to assess effects of long-term inhalation of airborne
particulate matter on early Alzheimer-like changes in the mouse brain. PLoS ONE 2015,10, e0127102. [CrossRef]
78.
Ku, T.; Li, B.; Gao, R.; Zhang, Y.; Yan, W.; Ji, X.; Li, G.; Sang, N. NF-
κ
B-regulated microRNA-574-5p underlies synaptic and
cognitive impairment in response to atmospheric PM2.5 aspiration. Part. Fibre Toxicol. 2017,14, 34. [CrossRef]
79.
Bai, K.J.; Chuang, K.J.; Chen, C.L.; Jhan, M.K.; Hsiao, T.C.; Cheng, T.J.; Chang, L.T.; Chang, T.Y.; Chuang, H.C. Microglial
activation and inflammation caused by traffic-related particulate matter. Chem. Biol. Interact. 2019,311, 108762. [CrossRef]
80.
Bos, I.; De Boever, P.; Emmerechts, J.; Buekers, J.; Vanoirbeek, J.; Meeusen, R.; Van Poppel, M.; Nemery, B.; Nawrot, T.; Panis, L.I.
Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal. Toxicol. 2012,24, 676–686. [CrossRef]
81.
Hesterberg, T.W.; Long, C.M.; Lapin, C.A.; Hamade, A.K.; Valberg, P.A. Diesel exhaust particulate (DEP) and nanoparticle
exposures: What do DEP human clinical studies tell us about potential human health hazards of nanoparticles? Inhal. Toxicol.
2010,22, 679–694. [CrossRef]
82.
Gerlofs-Nijland, M.E.; van Berlo, D.; Cassee, F.R.; Schins, R.P.; Wang, K.; Campbell, A. Effect of prolonged exposure to diesel
engine exhaust on proinflammatory markers in different regions of the rat brain. Part. Fibre Toxicol. 2010,7, 12. [CrossRef]
83.
van Berlo, D.; Albrecht, C.; Knaapen, A.M.; Cassee, F.R.; Gerlofs-Nijland, M.E.; Kooter, I.M.; Palomero-Gallagher, N.;
Bidmon, H.J.
;
van Schooten, F.J.; Krutmann, J.; et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine
exhaust on rat lung and brain. Arch. Toxicol. 2010,84, 553–562. [CrossRef]
84.
Levesque, S.; Taetzsch, T.; Lull, M.E.; Kodavanti, U.; Stadler, K.; Wagner, A.; Johnson, J.A.; Duke, L.; Kodavanti, P.;
Surace, M.J.; et al.
Diesel exhaust activates and primes microglia: Air pollution, neuroinflammation, and regulation of
dopaminergic neurotoxicity. Environ. Health Perspect. 2011,119, 1149–1155. [CrossRef]
Antioxidants 2022,11, 1482 22 of 23
85.
Levesque, S.; Surace, M.J.; McDonald, J.; Block, M.L. Air pollution and the brain: Subchronic diesel exhaust exposure causes
neuroinflammation and elevates early markers of neurodegenerative disease. J. Neuroinflamm. 2011,8, 105. [CrossRef]
86.
Durga, M.; Devasena, T.; Rajasekar, A. Determination of LC50 and sub-chronic neurotoxicity of diesel exhaust nanoparticles.
Environ. Toxicol. Pharmacol. 2015,40, 615–625. [CrossRef]
87.
Cacciottolo, M.; Wang, X.; Driscoll, I.; Woodward, N.; Saffari, A.; Reyes, J.; Serre, M.L.; Vizuete, W.; Sioutas, C.; Morgan, T.E.; et al.
Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis
in experimental models. Transl. Psychiatry 2017,7, e1022. [CrossRef]
88.
Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roqué, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology
2017,59, 133–139. [CrossRef]
89.
Coburn, J.L.; Cole, T.B.; Dao, K.T.; Costa, L.G. Acute exposure to diesel exhaust impairs adult neurogenesis in mice: Prominence
in males and protective effect of pioglitazone. Arch. Toxicol. 2018,92, 1815–1829. [CrossRef]
90.
Cacciottolo, M.; Morgan, T.E.; Saffari, A.A.; Shirmohammadi, F.; Forman, H.J.; Sioutas, C.; Finch, C.E. Traffic-related air pollutants
(TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic. Biol. Med.
2020
,147, 242–251.
[CrossRef]
91.
Cheng, H.; Saffari, A.; Sioutas, C.; Forman, H.J.; Morgan, T.E.; Finch, C.E. Nanoscale particulate matter from urban traffic rapidly
induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ. Health Perspect.
2016,124, 1537–1546. [CrossRef] [PubMed]
92.
Chen, X.; Liu, S.; Zhang, W.; Wu, C.; Liu, H.; Zhang, F.; Lu, Z.; Ding, W. Nrf2 deficiency exacerbates PM
2.5
-induced olfactory bulb
injury. Biochem. Biophys. Res. Commun. 2018,505, 1154–1160. [CrossRef] [PubMed]
93.
Chen, X.; Guo, J.; Huang, Y.; Liu, S.; Huang, Y.; Zhang, Z.; Zhang, F.; Lu, Z.; Li, F.; Zheng, J.C.; et al. Urban airborne PM
2.5
-activated
microglia mediate neurotoxicity through glutaminase-containing extracellular vesicles in olfactory bulb. Environ. Pollut.
2020
,
264, 114716. [CrossRef] [PubMed]
94.
Chen, R.; Gu, X.; Wang, X.
α
-Synuclein in Parkinson’s disease and advances in detection. Clin. Chim. Acta
2022
,529, 76–86.
[CrossRef] [PubMed]
95.
Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM
2.5
exposure in association with AD-related neuropathology and cognitive
outcomes. Environ. Pollut. 2022,292 Pt A, 118320. [CrossRef] [PubMed]
96.
Sama, P.; Long, T.C.; Hester, S.; Tajuba, J.; Parker, J.; Chen, L.C.; Veronesi, B. The cellular and genomic response of an immortalized
microglia cell line (BV2) to concentrated ambient particulate matter. Inhal. Toxicol. 2007,19, 1079–1087. [CrossRef]
97.
Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin suppresses PM
2.5
-induced neuroinflammation by regulating Akt
phosphorylation in BV-2 microglial cells. Int. J. Mol. Sci. 2020,1, 7227. [CrossRef]
98.
Wang, B.R.; Shi, J.Q.; Ge, N.N.; Ou, Z.; Tian, Y.Y.; Jiang, T.; Zhou, J.S.; Xu, J.; Zhang, Y.D. PM
2.5
exposure aggravates oligomeric
amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an
in vitro
model of Alzheimer’s
disease. J. Neuroinflamm. 2018,15, 132. [CrossRef]
99.
Zhang, P.; Hatter, A.; Liu, B. Manganese chloride stimulates rat microglia to release hydrogen peroxide. Toxicol. Lett.
2007
,173,
88–100. [CrossRef]
100.
Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer size diesel exhaust
particles are selectively toxic to dopaminergic neurons: The role of microglia, phagocytosis, and NADPH oxidase. FASEB J.
2004
,
18, 1618–1620. [CrossRef]
101.
Roqué, P.J.; Dao, K.; Costa, L.G. Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroin-
flammatory mechanisms. Neurotoxicology 2016,56, 204–214. [CrossRef]
102.
Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.;
Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006,9, 917–924. [CrossRef]
103.
Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of Ambient Air Pollution Exposure on Olfaction: A Review. Environ. Health Perspect.
2016,124, 1683–1693. [CrossRef]
104.
Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature
2006
,443, 787–795.
[CrossRef]
105.
Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.;
Jakate, S.; et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol.
2011,8, 19. [CrossRef]
106.
Hosang, L.; Canals, R.C.; van der Flier, F.J.; Hollensteiner, J.; Daniel, R.; Flügel, A.; Odoardi, F. The lung microbiome regulates
brain autoimmunity. Nature 2022,603, 138–144. [CrossRef]
107.
Jack, C.S.; Arbour, N.; Manusow, J.; Montgrain, V.; Blain, M.; McCrea, E.; Shapiro, A.; Antel, J.P. TLR signaling tailors innate
immune responses in human microglia and astrocytes. J. Immunol. 2005,175, 4320–4330. [CrossRef]
108.
Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs.
J. Immunol. 2004,173, 3916–3924. [CrossRef]
109.
Husemann, J.; Loike, J.D.; Kodama, T.; Silverstein, S.C. Scavenger receptor class B type I (SR-BI) mediates adhesion of neonatal
murine microglia to fibrillar beta-amyloid. J. Neuroimmunol. 2001,114, 142–150. [CrossRef]
110.
Qin, L.; Liu, Y.; Wang, T.; Wei, S.J.; Block, M.L.; Wilson, B.; Liu, B.; Hong, J.S. NADPH oxidase mediates lipopolysaccharide-
induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem.
2004
,279, 1415–1421. [CrossRef]
Antioxidants 2022,11, 1482 23 of 23
111. Pinti, M.; Ferraro, D.; Nasi, M. Microglia activation: A role for mitochondrial DNA? Neural Regen. Res. 2021,16, 2393–2394.
112.
Agrawal, I.; Jha, S. Mitochondrial Dysfunction and Alzheimer’s Disease: Role of Microglia. Front. Aging Neurosci.
2020
,12, 252.
[CrossRef]
113.
Pósfai, B.; Cserép, C.; Orsolits, B.; Dénes, Á. New insights into microglia-neuron interactions: A neuron’s perspective. Neuroscience
2019,405, 103–117. [CrossRef] [PubMed]
114.
Kim, Y.S.; Choi, J.; Yoon, B.E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells
2020
,9, 2176. [CrossRef]
[PubMed]