Content uploaded by Ligang Zhou
Author content
All content in this area was uploaded by Ligang Zhou on Nov 06, 2023
Content may be subject to copyright.
Content uploaded by Tijiang Shan
Author content
All content in this area was uploaded by Tijiang Shan on Aug 30, 2015
Content may be subject to copyright.
Endophytic fungi for producing bioactive compounds originally from
their host plants
J. Zhao
1
, L. Zhou
1,
*
, J. Wang
2
, T. Shan
1
, L. Zhong
1
, X. Liu
1
, and X. Gao
3
1
Department of Plant Pathology, College of Agronomy and Biotechnology, China Agricultural University, Beijing
100193, China
2
Department of Environmental Science and Engineering, College of Resource and Environmental Science, China
Agricultural University, Beijing 100193, China
3
Department of Entomology, College of Agronomy and Biotechnology, China Agricultural University, Beijing 100193,
China
Plant endophytic fungi are an important and novel resource of natural bioactive compounds with their potential
applications in agriculture, medicine and food industry. In the past two decades, many valuable bioactive compounds with
antimicrobial, insecticidal, cytotoxic and anticancer activities have been successfully discovered from the endophytic
fungi. During the long period of co-evolution, a friendly relationship was formed between each endophyte and its host
plant. Some endophytes have the ability to produce the same or similar bioactive compounds as those originated from their
host plants. This chapter mainly reviewed the research progress on the endophytic fungi for producing plant-derived
bioactive compounds such as paclitaxel, podophyllotoxin, camptothecine, vinblastine, hypericin and diosgenin etc. The
relations between the endophytic fungi and their host plants, some available strategies for efficiently promoting production
of these bioactive compounds, as well as their potential applications in the future are also discussed. It is beneficial for us
to better understand and take advantage of plant endophytic fungi.
Keywords endophytic fungi; bioactive compounds; host plants; co-evolution relations
1. Introduction
Plant endophytic fungi are defined as the fungi which spend the whole or part of their lifecycle colonizing inter-and/or
intra-cellularly inside the healthy tissues of the host plants, typically causing no apparent symptoms of disease. They are
important components of plant micro-ecosystems [1-3]. Plant endophytic fungi have been found in each plant species
examined, and it is estimated that there are over one million fungal endophytes existed in the nature [4]. Plant
endophytic fungi have been recognized as an important and novel resource of natural bioactive products with potential
application in agriculture, medicine and food industry [5-7]. Since the "gold" bioactive compound paclitaxel (taxol)
discovered from the endophytic fungus Taxomyces andreanae in 1993 [8], many scientists have been increasing their
interests in studying fungal endophytes as potential producers of novel and biologically active compounds. In the past
two decades, many valuable bioactive compounds with antimicrobial, insecticidal, cytotoxic and anticancer activities
have been successfully discovered from the endophytic fungi. These bioactive compounds could be classified as
alkaloids, terpenoids, steroids, quinones, lignans, phenols and lactones [2, 9]. During the long period of co-evolution, a
friendly relationship was gradually set up between each endophytic fungus and its host plant. The host plant can supply
plenteous nutriment and easeful habitation for the survival of its endophytes. On the other hand, the endophytes would
produce a number of bioactive compounds for helping the host plants to resist external biotic and abiotic stresses, and
benefiting for the host growth in return [3, 10]. Some endophytic fungi have developed the ability to produce the same
or similar bioactive substances as those originated from the host plants. This is beneficial for us to study the relations
between the endophytes and their host plants, and to develop a substitutable approach for efficiently producing these
scarce and valuable bioactive compounds [6, 11].
This chapter mainly describes the research progress on the endophytic fungi for producing bioactive compounds such
as paclitaxel, podophyllotoxin, camptothecine, vinblastine, hypericin and diosgenin (Fig. 1), which were also produced
by their host plants. The potential relationships of the endophytes with their host plants, some available strategies for
efficiently promoting production of these bioactive compounds, as well as their potential application in the future are
also discussed.
* Corresponding author: Ligang Zhou, e-mail: lgzhou@cau.edu.cn, Phone: +86 10 62731199, Fax: +86 10 62731062
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
©FORMATEX 2010
567
HO
O
O
HO
OHOH
CH
3
CH
3
OHOH
Hypericin
Paclitaxel Baccatin III: R=COCH
3
10-Deacetylbaccatin III: R=H
Podophyllotoxin
O
O
OH
NH
OOOH
O
O
O
OH
O
O
H
O
O
HO
OOH
O
O
O
OH
RO
H
O
O
O
O
O
OH
OCH
3
OCH
3
CH
3
O
O
Camptothecin: R
1
=R
2
=H
9-Methoxycamptothecin: R
1
=OCH
3
, R
2
=H
10-Hydroxycamptothecin: R
1
=H, R
2
=OH
N
N
O
O
O
OH
R
1
R
2
NH
N
OH
CH
2
CH
3
CH
3
OOC
H
N
N
R
CH
3
OHO COOCH
3
OCOCH
3
CH
2
CH
3
H
H
Vinblastine: R=CH
3
Vincristine: R=CHO
O
O
HO
Diosgenin
OH
O
O
O
O
O
O
OH
H
H
OH
O
O
O
NH
H
2
N
H
O
¦Á-Irone
Huperzine A
Toosendanin
¦Â-Irone
O
Fig. 1 Structures of the bioactive compounds from the endophytic fungi and their host plants.
2. Endophytic fungi for producing paclitaxel and its analogues
Paclitaxel (taxol), as a well-known and highly functionalized tetracyclic diterpenoid bioactive compound found
originally from the bark of Taxus brevifolia in 1971 [12], has been proved with an efficient action against prostate,
ovarian, breast and lung cancers. Its primary mechanism of action is related to the ability to stabilize the microtubules
and to disrupt their dynamic equilibrium [13]. Up to now, the major supply of paclitaxel has been from the wild Taxus
plants. However, it is found in extremely low amounts in various parts such as the needles, barks and roots of Taxus
species. In order to satisfy the growing demand of market and make it more widely available, the alternative resource
and potential strategy should be developed. In the last 40 years, many efficient approaches such as field cultivation,
plant cell and tissue culture, chemical synthesize for paclitaxel production have been developed, and much progress has
been achieved [14]. However, it is not realistic for producing paclitaxel with these measures as the problems of time-
consuming, lower yield and non economic. Fortunately, a paclitaxel producing endophytic fungus Taxomyces
andreanae was successfully discovered from the Pacific yew (Taxus brevifolia) in 1993 [8]. This tremendous finding
firstly showed that the plant endophytic fungi also had the ability to produce paclitaxel, giving us a novel and promising
approach to produce this valuable compound. Since then, many scientists have been increasing their interests in
studying fungal endophytes as potential candidates for producing paclitaxel. Extensive research such as searching for
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
568
©FORMATEX 2010
paclitaxel-producing endophytic fungi from Taxus species as well as from other related plant species, microbial
fermentation processes and genetic engineering for improving paclitaxel production has been developed, and much
progress has been achieved during the past two decades. By now, at least 19 genera of endophytic fungi (i.e. Alternaria,
Aspergillus, Botryodiplodia, Botrytis, Cladosporium, Ectostroma, Fusarium, Metarhizium, Monochaetia, Mucor,
Ozonium, Papulaspora, Periconia, Pestalotia, Pestalotiopsis, Phyllosticta, Pithomyces, Taxomyces, Tubercularia) were
screened to have the ability to produce paclitaxel and its analogues (i.e. baccatin III, 10-deacetylbaccatin III) (Table 1).
The hosts of paclitaxel-producing fungi mainly include Taxus (i.e. T. baccata, T. cuspidata, T. media, and T.
yunnanensis) and non-Taxus species (i.e. Cardiospermum helicacabum, Citrus medica, Cupressus sp., Ginkgo biloba,
Hibiscus rosa-sinensis, Podocarpus sp., Taxodium distichum, Terminalia arjuna, Torreya grandifolia, and Wollemia
nobilis). Such a great number and wide range implies that both paclitaxel-producing fungi and their hosts have
biological diversity. These results also showed us a promising way that the endophytic fungi would be an alternative
paclitaxel-producing resource.
Table 1 Paclitaxel-producing endophytic fungi and their host plants.
Endophytic fungus Fungal strain Host plant Paclitaxel yield
(µg/L)
Reference
Alternaria sp. Ja-69 Taxus cuspidata 0.16 [15]
Alternaria sp. - Ginkgo biloba 0.12-0.26 [16]
Alternaria alternata TPF6 Taxus chinensis var. mairei 84.5 [17]
Aspergillus fumigatus EPTP-1 Podocarpus sp. 557.8 [18]
Aspergillus niger var. taxi HD86-9 Taxus cuspidata 273.6 [19]
Botryodiplodia theobromae BT115 Taxus baccata 280.5 [20]
Botrytis sp. XT2 Taxus chinensis var. mairei 161.24 [21]
Botrytis sp. HD181-23 Taxus cuspidata 206.34 [22]
Cladosporium cladosporioides MD2 Taxus media 800 [23]
Ectostroma sp. XT5 Taxus chinensis var. mairei 276.75 [21]
Fusarium arthrosporioides F-40 Taxus cuspidata 131 [24]
Fusarium lateritium Tbp-9 Taxus baccata 0.13 [15]
Fusarium mairei Y1117 Taxus chinensis var. mairei 2.7 [25]
Fusarium mairei UH23 Taxus chinensis var. mairei 286.4 [26]
Fusarium solani - Taxus celebica 1.6 [27]
Fusarium solani Tax-3 Taxus chinensis 163.35 [28]
Metarhizium anisopliae H-27 Taxus chinensis 846.1 [29]
Monochaetia sp. Tbp-2 Taxus baccata 0.10 [15]
Mucor rouxianus DA10 Taxus chinensis - [30]
Ozonium sp. BT2 Taxus chinensis var. mairei 4-18 [31]
Papulaspora sp. XT17 Taxus chinensis var. mairei 10.25 [21]
Periconia sp. No. 2026 Torreya grandifolia 0.03-0.83 [32]
Pestalotia bicilia Tbx-2 Taxus baccata 1.08 [15]
Pestalotiopsis guepinii W-1f-2 Wollemia nobilis 0.49 [33]
Pestalotiopsis microspora Ja-73 Taxus cuspidata 0.27 [15]
Pestalotiopsis microspora Ne-32 Taxus wallachiana 0.5 [15]
Pestalotiopsis microspora No. 1040 Taxus wallachiana 0.06-0.07 [34]
Pestalotiopsis microspora Cp-4 Taxodium distichum 0.05-1.49 [35]
Pestalotiopsis microspora Ne 32 Taxus wallachiana 0.34-1.83 [36]
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
©FORMATEX 2010
569
Table 1 Contd....
Endophytic fungus Fungal strain Host plant Paclitaxel yield
(µg/L)
Reference
Pestalotiopsis pauciseta CHP-11 Cardiospermum helicacabum 113.3 [37]
Pestalotiopsis sp. W-x-3 Wollemia nobilis 0.13 [33]
Pestalotiopsis sp. W-1f-1 Wollemia nobilis 0.17 [33]
Pestalotiopsis terminaliae TAP-15 Terminalia arjuna 211.1 [38]
Phyllosticta citricarpa No.598 Citrus medica 265 [39]
Phyllosticta dioscoreae No.605 Hibiscus rosa-sinensis 298 [40]
Phyllosticta spinarum No.625 Cupressus sp. 235 [41]
Pithomyces sp. P-96 Taxus sumatrana 0.095 [15]
Taxomyces andreanae - Taxus brevifolia 0.024-0.05 [8]
Taxomyces sp. - Taxus yunnanensis 2.3 [42]
Tubercularia sp. TF
5
Taxus chinensis var. mairei 185.4 [43]
Unidentified YF
1
Taxus yunnanensis - [44]
3. Endophytic fungi for producing podophyllotoxin
Podophyllotoxin (PDT), a well-known aryltetralin lignan with potent anticancer, antiviral, antioxidant, antibacterial,
immunostimulation and anti-rheumatic properties, mainly occurs in genera of Diphylleia, Dysosma, Sabina (also called
Juniperus), and Sinopodophyllum (also called Podophyllum) [45-52]. PDT has been used as a precursor for chemical
synthesis of the anticancer drugs like etoposide, teniposide and etopophose phosphate [48, 51]. At present, the major
supply of podophyllotoxin is from the natural Sinopodophyllum plants. As the over-exploitation, the Sinopodophyllum
plants have been declared to be endangered species. In order to satisfy the increasing demand and make it more
available, the alternative resource and strategy for efficiently producing this valuable compound should be developed.
Yang et al. first reported about six endophytic fungi obtained from Sinopodophyllum hexandrum, Diphylleia sinensis
and Dysosma veitchii that had the ability to produce podophyllotoxin [45]. Later, Lu et al. also declared that an
endophytic Alternaria sp. obtained from Sabina vulgaris could produce PDT [46]. Eyberger et al. successfully obtained
two endophytic Phialocephala fortinii strains PPE5 and PPE7 from the rhizomes of Sinopodophyllum peltatum that
could produce PDT with the yield of 0.5-189 µg/L in liquid suspension culture [51]. Puri et al. reported an endophytic
fungus Trametes hirsuta isolated from Sinopodophyllum hexandrum that could produce PDT and its glycoside in
Sabouraud broth culture [52]. Cao et al. examined an endophytic fungus Alternaria sp. isolated from Sinopodophyllum
hexandrum that could produce PDT [47]. Kour et al. also discovered a PDT-producing endophytic fungus Fusarium
oxysporum obtained from Sabina recurva in 2008 [48]. These results give us a promising way of exploring the
endophytic fungi as the alternative source to produce podophyllotoxin and its analogues.
Table 2 Podophyllotoxin-producing endophytic fungi and their host plants.
Endophytic fungus Fungal strain Host plant Podophyllotoxin
content or yield
Reference
Alternaria sp. - Sinopodophyllum hexandrum
(=Podophyllum hexandrum)
- [45]
Alternaria sp. SC13 Sabina vulgaris - [46]
Alternaria neesex Ty Sinopodophyllum hexandrum 2.4 µg/L [47]
Fusarium oxysporum JRE1 Sabina recurva
(=Juniperus recurva)
28 µg/g [48]
Monilia sp. - Dysosma veitchii - [45]
Penicillium sp. - Sinopodophyllum hexandrum - [45]
Penicillium sp. - Diphylleia sinensis - [45]
Penicillium sp. - Dysosma veitchii - [45]
Penicillium implicatum SJ21 Diphylleia sinensis - [49]
Penicillium implication 2BNO1 Dysosma veitchii - [50]
Phialocephala fortinii PPE5, PPE7 Sinopodophyllum peltatum 0.5-189 µg/L [51]
Trametes hirsuta - Sinopodophyllum hexandrum 30 µg/g [52]
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
570
©FORMATEX 2010
4. Endophytic fungi for producing camptothecine and its analogues
Camptothecin (CPT), a pentacyclic quinoline alkaloid, was firstly isolated from the wood of Camptotheca acuminata
(Nyssaceae) by Wall et al. in 1966 [53]. CPT and its analogue10-hydroxycamptothecin have been regarded as two of
the most effective antineoplastic agents. The primary action mechanism of CPT is by virtue of inhibiting the intra-
nuclear enzyme topoisomerase-1, which is required in DNA replication and transcription during molecular events [54].
Hycamtin (topotecan) and Camtostar (irinotecan), two of the famous CPT semi-synthetic drugs, have already been in
clinical use against ovarian, small lung and refractory ovarian cancers popularly all over the world [55]. At present, the
major supply of this bioactive compound CPT is still from the wild trees Camptotheca acuminata and Nothapodytes
nimmoniana (Icacinaceae). As the growing demand of this compound, it has resulted in extensive cropping of the trees
in China and India. It is necessary to further find high yielding candidates and alternative sources to produce this
bioactive compound and its analogues [56, 57].
Puri et al. first reported an endophytic fungus Entrophospora infrequens obtained from Nothapodytes foetida that had
the ability to produce camptothecin in 2005 [58]. Later, Amna et al. performed the kinetic studies of the growth and
CPT accumulation of the endophyte E. infrequens in suspension culture with the either shake flasks or bioreactor, and
demonstrated that this endophyte would be a potential alternate microorganism source to produce CPT [56]. Rehman et
al. successfully discovered a CPT-producing endophytic fungus Neurospora sp. from the seeds of Nothapodytes foetida
in 2008 [59]. More recently, Kusari et al. reported an endophytic fungus Fusarium solani obtained from Camptotheca
acuminata could produce CPT, 9-methoxycamptothecin and 10-hydroxycamptothecin in Sabouraud dextrose broth [60].
Min and Wang reported an unidentified endophytic fungal strain XK001 could produce 10-hydroxycamptothecin with
the yield of 677 µg/L [61]. Shweta et al. successfully found two endophytic Fusarium solani strains MTCC9667 and
MTCC9668 had the ability to produce CPT and 9-methoxycamptothecin (0.45 µg/g), and the endophyte MTCC9668
could also produce 10-hydroxycamptothecin as much as 0.08 µg/g [57]. These findings showed that the endophytic
fungi could be an alterative resource to produce CPT and its analogues.
Table 3 Camptothecin-producing endophytic fungi and their host plants.
5. Endophytic fungi for producing vinblastine and its analogues
Vinblastine and vincristine, the terpenoid indole alkaloids derived from the coupling of vindoline and catharanthine
monomers, are two of the well-known anticancer agents [62, 63]. The primary action mechanism of vincristine is via
interference with microtubule formation and mitotic spindle dynamics, disruption of intracellular transport and
decreased tumour blood flow, with the latter probably as a consequence of anti-angiogenesis [62, 64]. Guo et al. first
reported an endophytic fungus Alternaria sp. isolated from the phloem of Catharanthus roseus that had the ability to
produce vinblastine in 1998 [65]. Later, Zhang et al. successfully discovered an endophytic Fusarium oxysporum from
the pholem of C. roseus that could produce vincristine [66]. Yang et al. also found an unidentified vincristine-producing
endophytic fungus from the leaves of C. roseus in 2004 [67]. These results indicate that some endophytic fungi could be
a potential source to produce either vinblastine or vincristine.
6. Endophytic fungi for producing other bioactive compounds originally from their
host plants
Other pronounced bioactive compounds originated from the host plants could also be biosynthesized by their
endophytic fungi mainly include huperzine A, α-irone, β-irone, diosgenin, hypericin and toosendanin (shown in Table
4). Li et al first reported an endophytic fungus Acremonium (2F09P03B) obtained from Huperzia serrata that could
produce huperzine A that was a lycopodium alkaloid. They further optimized its fermentation conditions [68]. Zhou et
al. reported an endophytic fungus Penicillium chrysogenum obtained from Lycopodium serratum could also produce
huperzine A as much as 4.761 mg/L in liquid culture [73]. Ju et al. successfully discovered two endophytic fungi
Blastomyces sp. (HA15) and Botrytis sp. (HA23) from Phlegmariurus cryptomerianus that had the ability to produce
huperzine A [69].
Endophytic fungus Fungal strain Host plant Camptothecin
content or yield
Reference
Entrophospora infrequens RJMEF 001 Nothapodytes foetida - [58]
Entrophospora infrequens 5124 Nothapodytes foetida 49.6 µg/g [56]
Fusarium solani INFU/Ca/KF/3 Camptotheca acuminata - [60]
Fusarium solani MTCC 9667 Apodytes dimidiata 0.37 µg/g [57]
Fusarium solani MTCC 9668 Apodytes dimidiata 0.53 µg/g [57]
Neurospora sp. ZP5SE Nothapodytes foetida - [59]
Unidentified XK001 Camptotheca acuminata - [61]
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
©FORMATEX 2010
571
Zhou and his co-workers screened a few diosgenin-producing endophytic fungi from Paris polyphylla var.
yunnanensis [70, 71]. Zhang et al. reported an endophytic fungus Rhizopus oryzae (94Y-01) from the rhizomes of Iris
germanica that could produce α- and β-irones for which the culture conditions were then optimized [74]. Wang et al.
discovered three endophytic fungal isolates from Melia azedarach that had the ability to produce toosendanin [75].
Kusari et al. reported an endophytic fungus isolated from the stems of Hypericum perforatum (St. John's Wort) had the
ability to produce hapericin and emodin in rich mycological medium with shake flasks [72]. All the results mentioned
above clearly showed that a promising way that the endophytic fungi would be an alternative resource for efficiently
producing valuable bioactive compounds in the future.
Table 4 Other bioactive compounds-producing endophytic fungi and their host plants.
Endophytic fungus Fungal strain Host plant Bioactive
compounds
Reference
Acremonium sp. 2F09P03B Huperzia serrata Huperzine A [68]
Blastomyces sp. HA15 Phlegmariurus cryptomerianus Huperzine A [69]
Botrytis sp. HA23 Phlegmariurus cryptomerianus Huperzine A [69]
Cephalosporium sp. 84 Paris polyphylla var.
yunnanensis
Diosgenin [70, 71]
Chaetomium globosum INFU/Hp/KF/34B Hypericum perforatum Hypericin,
Emodin
[72]
Paecilomyces sp. 80 Paris polyphylla var.
yunnanensis
Diosgenin [70, 71]
Penicillium chrysogenum SHB Lycopodium serratum Huperzine A [73]
Rhizopus oryzae 94Y-01 Iris germanica α-Irone, β-Irone [74]
Unidentified O-L-5, O-SC II-4,
O-RC-3
Melia azedarach Toosendanin [75]
7. Conclusions and future perspectives
Plant endophytic fungi, as a novel and abundant microorganism resource, owning the special ability to produce the
same or similar compounds originated from their host plants, as well as other bioactive compounds, have increased
many investigators׳ interesting in both basic research and applied fields. In the past two decades, scientists mainly
focused on the investigation of endophytic fungal diversity, relationships between endophytic fungi and their host
plants, seeking for natural bioactive compounds originated from the endophytic fungi, and improving the productivity
of some potential candidates by taking advantage of genetic engineering, microbial fermentation projects and other
measures [5]. Up to now, hundreds of plants have been investigated for their endophytic fungi, and most of them have
been proved to be rich with endophytic fungi. Many novel and valuable bioactive compounds with antimicrobial,
insecticidal, cytotoxic and anticancer activities have been successfully obtained from the endophytic fungi [76]. The
evidence of plant-associated microbes discovered in the fossilized tissues of stems and leaves indicated that the
endophytic associations may have evolved from the time that higher plants first appeared on the earth, hundreds of
millions of years ago [77]. Carroll suggested that some phytopathogens in the environment were related to endophytes
and had an endophytic origin [78]. A few microorganisms appear actively to penetrate plant tissues through invading
openings or wounds, as well as proactively using hydrolytic enzymes such as cellulase and pectinase [2]. During the
long period of co-evolution, the endophytic fungi have adapted themselves to their special microenvironments gradually
by genetic variation, including uptake of some plant DNA segments into their own genomes, as well as insertion their
own DNA segments into the host genomes. This could have led to certain endophytes own the ability to biosynthesize
some ״phytochemicals״ originated from their host plants [2, 8]. One typical example was the production of gibberellins
from both fungi and plants [79]. The outline of the bioactive compounds from both endophytic fungi and their host
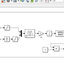
plants along with their potential applications is shown in Fig. 2.
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
572
©FORMATEX 2010
Host plants Endophytic
fungi
Bioactive
compounds
Plant
biotechnology
Genetic
engineering
Microbial
fermentation
Phytopathogenic
fungi
Co-evolution
relations
Chemical
process
Applications in agriculture,
medicine and food industry
Fig. 2 Outline of the bioactive compounds from both endophytic fungi and their host plants along with their potential applications.
It is believed that the plant endophytic fungi as a novel mine of natural bioactive compounds have their great
potential applications in agriculture, medicine and food industry [5, 7, 80]. Taking advantage of modern biotechnology
such as genetic engineering, metabolic technology and microbial fermentation process, we can better understand and
manipulate this important microorganism resource, and make it more benefit for the mankind. First, the most important
step is to search for potential endophytic fungi resources from the nature. And then, through mutations, protoplast
fusion, gene manipulation and other DNA recombination techniques, the high productivity candidates suitable for
industrial fermentation could be established. Furthermore, colonizing and expression of relevant functional genes in the
biosynthetic pathways are also beneficial for improving the productivity of the candidates. It is well known that
microorganism fermentation is a sophisticated project, and it has been widely used in many occasions for a long period
of time. Penicillin, avermectin, validamycin and other well-known antibiotics have been successfully developed through
fermentation process. Compared with plant cell culture, the culture medium for the fungal cells is simple, inexpensive
with the abundant supply, and the production cost is relatively low. Moreover, the period of fermentation is short, and
the microbial fermentation process can provide the best growth and breeding conditions, and the various culture
parameters can be strictly controlled according to our requests. In addition, the microbial fermentation conditions can be
easily optimized, and many feasible strategies could be adopted for efficiently enhancing the bioactive compound
production during the fermentation process, such as feeding precursors, adding biotic and abiotic elicitors, appending
inhibitors, using special enzymes and other substances through metabolic investigation.
In summary, plant endophytic fungi, as a novel and important microbial resource for producing bioactive compounds
originally from their hosts, have attracted many researchers' attentions on their theoretical study as well as potential
applications. After more than two decades of research, much progress has been achieved though there are still many
issues (i.e. increasing compound yield in fermentation culture, elucidating biosynthetic pathway of the compounds in
the endophytic fungi, etc.) needed to be further clarified and resolved.
Acknowledgements This work was partially supported by the grants from the Hi-Tech R&D Program of China (2006AA10Z423 and
2006AA10A209), the National Key Technology R&D Program of China (2008BADA5B03 and 2006BAD08A03), the National
Natural Science Foundation of China (30871662 and 31071710) and Natural Science Foundation of Beijing (6092015).
References
[1] Tan RX, Zhou WX. Endophytes: a rich source of functional metabolites. Natural Product Reports. 2001; 18: 448-459.
[2] Zhang HW, Song YC, Tan RX. Biology and chemistry of endophytes. Natural Product Reports. 2006; 23: 753-771.
[3] Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytologist. 2009;
182: 314-330.
[4] Petrini O. Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS, eds. Microbial Ecology of Leaves. New York: Spring
Verlag; 1991: 179-197.
[5] Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. Journal of Natural Products.
2004; 67: 257-268.
[6] Gunatilaka AAL. Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and
implications of their occurrence. Journal of Natural Products. 2006; 69: 505-526.
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
©FORMATEX 2010
573
[7] Verma VC, Kharmar RN, Strobel GA. Chemical and functional diversity of natural products from plant associated endophytic
fungi. Natural Product Communications. 2009; 4: 1511-1532.
[8] Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew.
Science. 1993; 260: 214-216.
[9] Xu L, Zhou L, Zhao J, Jiang W. Recent studies on the antimicrobial compounds produced by plant endophytic fungi. Natural
Product Research and Development. 2008; 20: 731-740.
[10] Silvia F, Sturdikova M, Muckova M. Bioactive secondary metabolites produced by microorganisms associated with plants.
Biologia. 2007; 62: 251-257.
[11] Zhou L, Zhao J, Xu L, Huang Y, Ma Z, Wang J, Jiang W. Antimicrobial compounds produced by plant endophytic fungi. In:
De Costa P, Bezerra P, eds. Fungicides: Chemistry, Environmental Impact and Health Effects. New York: Nova Science
Publishers; 2009: 91-119.
[12] Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents VI: the isolation and structure of taxol, a novel
antilekemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society. 1971; 93: 2325-2327.
[13] Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a
review. Cancer Chemotherapy and Pharmacology. 1999; 44: 355-361.
[14] Zhou L, Wu J. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in
China. Natural Product Reports. 2006; 23: 789-810.
[15] Strobel GA, Hess WM, Ford E, Sidhu RS, Yang X. Taxol from fungal endophytes and issue of biodiversity. Journal of
Industrial Microbiology. 1996; 17: 417-423.
[16] Kim SU, Strobel GA, Ford E. Screening of taxol-producing endophytic fungi from Ginkgo biloba and Taxus cuspidata in
Korea. Agricultural Chemistry and Biotechnology. 1999; 42: 97-99.
[17] Tian R, Yang Q, Zhou G, Tan J, Zhang L, Fang C. Taxonomic study on a taxol producing fungus isolated from bark of Taxus
chinensis var. mairei. Journal of Wuhan Botanical Research. 2006; 24: 541-545.
[18] Sun D, Ran X, Wang J. Isolation and identification of a taxol-producing endophytic fungus from Podocrapus. Acta
Microbiologica Sinica. 2008; 48: 589-595.
[19] Zhao K, Ping W, Li Q, Hao S, Zhao L, Gao T, Zhou D. Aspergillus niger var. taxi, a new species variant of taxol-producing
fungus isolated from Taxus cuspidata in China. Journal of Applied Microbiology. 2009; 107: 1202-1207.
[20] Venkatachalam R, Subban K, Paul MJ. Taxol from Botryodiplodia theobromae (BT 115)-an endophytic fungus of Taxus
baccata. Journal of Biotechnology. 2008; 136: S189-S190.
[21] Hu K, Tan F, Tang K, Zhu S, Wang W. Isolation and screening of endophytic fungi synthesizing taxol from Taxus chinensis
var. mairei. Journal of Southwest China Normal University (Natural Science Edition). 2006; 31: 134-137.
[22] Zhao K, Zhao L, Jin Y, Wei H, Ping W, Zhou D. Isolation of a taxol-producing endophytic fungus and inhibiting effect of the
fungus metabolites on HeLa cell. Mycosystema. 2008; 27: 735-744.
[23] Zhang P, Zhou P, Yu L. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2.
Current Microbiology. 2009; 59: 227-232.
[24] Li C-T, Li Y, Wang Q-J, Sung C-K. Taxol production by Fusarium arthrosporioides isolated from yew, Taxus cuspidata.
Journal of Medical Biochemistry. 2008; 27: 454-458.
[25] Cheng L, Ma Q, Tao G, Tao W. Systemic identification of a paclitaxel-producing endophytic fungus. Industrial Microbiology.
2007; 37: 23-30.
[26] Dai W, Tao W. Preliminarly study on fermentation conditions of taxol-producing endophytic fungus. Chemical Industry and
Engineering Progress. 2008; 27: 883-886.
[27] Chakravarthi BVSK, Das P, Surendranath K, Karande AA, Jayabaskaran C. Production of paclitaxel by Fusarium solani
isolated from Taxus celebica. Journal of Biosciences. 2008; 33: 259-267.
[28] Deng BW, Liu KH, Chen WQ, Ding XW, Xie XC. Fusarium solani, Tax-3, a new endophytic taxol-producing fungus from
Taxus chinensis. World Journal of Microbiology and Biotechnology. 2009; 25: 139-143.
[29] Liu K, Ding X, Deng B, Chen W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis.
Journal of Industry Microbiology and Biotechnology. 2009; 36: 1171-1177.
[30] Miao Z, Wang Y, Yu X, Guo B, Tang K. A new endophytic taxane production fungus from Taxus chinensis. Applied
Biochemistry and Microbiology. 2009; 45: 81-86.
[31] Guo BH, Wang YC, Zhou XW, Hu K, Tan F, Miao ZQ, Tang KX. An endophytic taxol-producing fungus BT2 isolated from
Taxus chinensis var. mairei. African Journal of Biotechnology. 2006; 5: 875-877.
[32] Li JY, Sidhu RS, Ford EJ, Long DM, Hess WM, Strobel GA. The induction of taxol production in the endophytic fungus-
Periconia sp. from Torreya grandifolia. Journal of Industrial Microbiology and Biotechnology. 1998; 20: 259-264.
[33] Strobel GA, Hess WM, Li JY, Ford E, Sears J, Sidhu RS, Summerell B. Pestalotiopsis guepinii, a taxol-producing endophyte of
the Wollemi pine, Wollemia nobilis. Australian Journal of Botany. 1997; 45: 1073-1082.
[34] Strobel G, Yang XS, Sears J, Kramer R, Sidhu RS, Hess WM. Taxol from Pestalotiopsis microspora, an endophytic fungus of
Taxus wallachiana. Microbiology. 1996; 142: 435-440.
[35] Li JY, Strobel GA, Sidhu R, Hess WM, Ford EJ. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum.
Microbiology. 1996; 142: 2223-2226.
[36] Li JY, Sidhu RS, Bollon A, Strobel GA. Stimulation of taxol production in liquid cultures of Pestalotiopsis microspora .
Mycological Research. 1998; 102: 461-464.
[37] Gangadevi V, Murugan M, Muthumary J. Taxol derermination from Pestalotiopsis pauciseta, a fungal endophyte of a
medicinal plant. Chinese Journal of Biotechnology. 2008; 24: 1433-1438.
[38] Gangadevi V, Muthumary J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun
tree). Biotechnology and Applied Biochemistry. 2009; 52: 9-15.
[39] Kumaran RS, Muthumary J, Hur BK. Taxol from Phyllosticta citricarpa, a leaf spot fungus of the Angiosperm Citrus medica.
Journal of Bioscience and Bioengineering. 2008; 106: 103-106.
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
574
©FORMATEX 2010
[40] Kumaran RS, Muthumary J, Kim EK, Hur BK. Production of taxol from Phyllosticta dioscoreae, a leaf spot fungus isolated
from Hibiscus rosa-sinensis. Biotechnology and Bioprocess Engineering. 2009; 14: 76-83.
[41] Kumaran RS, Muthumary J, Hur BK. Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp..
Engineering in Life Sciences. 2008; 8: 438-446.
[42] Wang B, Li A, Wang X. An endophytic fungus for producing taxol. Science in China (Series C). 2001; 31: 271-274.
[43] Wang J, Lu H, Huang Y, Zheng Z, Su W. A taxol-producing endophytic fungus isolated from Taxus mairei and its antitumor
activity. Journal of Xiamen University (Natural Science Edition). 1999; 38: 485-487.
[44] Qiu D, Huang M, Fang X, Zhe C. Isolation of an endophytic fungus associated with Taxus yunnanensis et L.K.Fu. Acta
Mycologica Sinica. 1994; 13: 314-316.
[45] Yang X, Guo S, Zhang L, Shao H. Selection of producing podophyllotoxin endophytic fungi from podophyllin plant. Natural
Product Research and Development. 2003; 15: 419-422.
[46] Lu L, He J, Yu X, Li G, Zhang X. Studies on isolation and identification of endophytic fungi strain SC13 from harmaceutical
plant Sabina vulgaris Ant. and metabolites. Acta Agriculturae Boreali-occidentalis Sinica. 2006; 15: 85-89.
[47] Cao L, Huang J, Li J. Fermentation conditions of Sinopodophyllum hexandrum endophytic fungus on production of
podophyllotoxin. Food and Fermentation Industries. 2007; 33: 28-32.
[48] Kour A, Shawl AS, Rehman S, Sultan P, Qazi PH, Suden P, Khajuria RK, Verma V. Isolation and identification of an
endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World Journal of Microbiology
and Biotechnology. 2008; 24: 1115-1121.
[49] Zeng S, Shao H, Zhang L. An endophytic fungus producing a substance analogous to podophyllotoxin isolated from Diphylleia
sinensis. Journal of Microbiology. 2004; 24: 1-2.
[50] Guo S, Jiang B, Su Y, Liu S, Zhang L. Podophyllotoxin and its analogues from the endophytic fungi drrivativd from Dysosma
veitchii. Biotechnology. 2004; 14: 55-57.
[51] Eyberger AL, Dondapati R, Porter JR. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin.
Journal of Natural Products. 2006; 69: 1121-1124.
[52] Puri SC, Nazir A, Chawla R, Arora R, Riyaz-ul-Hasan S, Amna T, Ahmed B, Verma V, Singh S, Sagar R, Sharma A, Kumar R,
Sharma RK, Qazi GN. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related
aryl tetralin ligans. Journal of Biotechnology. 2006; 122: 494-510.
[53] Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I.the isolation and structure of
camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. Journal of the American
Chemical Society. 1966; 88: 3888-3890.
[54] Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA
topoisomerase-I. Journal of Biological Chemistry. 1985; 260: 14873-14878.
[55] Sirikantaramas S, Asano T, Sudo H, Yamazaki M, Saito K. Camptothecin: therapeutic potential and biotechnology. Current
Pharmaceutical Biotechnology. 2007; 8: 196-202.
[56] Amna T, Puri SC, Verma V, Sharma JP, Khajuria RK, Musarrat J, Spiteller M, Qazi GN. Bioreactor studies on the endophytic
fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Canadian Journal of
Microbiology. 2006; 52: 189-196.
[57] Shweta S, Züehlke S, Ramesha BT, Priti V, Mohana Kunar P, Ravikanth G, Spiteller M, Vasudeva R, Shaanker RU.
Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin,
10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry. 2010; 71: 117-122.
[58] Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. An endophytic fungus from Nothapodytes foetida that produces
camptothecin. Journal of Natural Products. 2005; 68: 1717-1719.
[59] Rehman S, Shawl AS, Kour A, Andrabi R, Sudan P, Sultan P, Verma V, Qazi GN. An endophytic Neurospora sp. from
Nothapodytes foetida producing camptothecin. Applied Biochemistry and Microbiology. 2008; 44: 203-209.
[60] Kusari S, Zühlke S, Spiteller M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and
analogues. Journal of Natural Products. 2009; 72: 2-7.
[61] Min C, Wang X. Isolation and identification of the 10-hydroxycamptothecin-producing endophytic fungi from Camptotheca
acuminata Decne. Acta Botanica Boreali- Occidentalia Sinica. 2009; 29: 0614-0617.
[62] Perez J, Pardo J, Gomez C. Vincristine: an effective treatment of corticoid-resistant life-threatening infantile hemangiomas.
Acta Oncologica. 2002; 41: 197-199.
[63] Wang Q, Yuan F, Pan Q, Li M, Wang G, Zhao J, Tang K. Isolation and functional analysis of the Catharanthus roseus
deacetylvindoline-4-O-acetyltransferase gene promoter. Plant Cell Report. 2010; 29: 185-192.
[64] Moore A, Pinkerton R. Vincristine: can its therapeutic index be enhanced? Pediatric & Blood Cancer. 2009; 53: 1180-1187.
[65] Guo B, Li H, Zhang L. Isolation of the fungus producing vinblastine. Journal of Yunnan University (Natural Science Edition).
1998; 20: 214-215.
[66] Zhang L, Guo B, Li H, Zeng S, Shao H, Gu S, Wei R. Preliminary study on the isolation of endophytic fungus of Catharanthus
roseus and its fermentation to produce products of therapeutic value. Chinese Traditional and Herbal Drugs. 2000; 31: 805-
807.
[67] Yang X, Zhang L, Guo B, Guo S. Preliminary study of a vincristine-producing endophytic fungus isolated from leaves of
Catharanthus roseus. Chinese Traditional and Herbal Drugs. 2004; 35: 79-81.
[68] Li W, Zhou J, Lin Z, Hu Z. Study on fermentation condition for production of huperzine A from endophytic fungus 2F09P03B
of Huperzia serrata. Chinese Medicinal Biotechnology. 2007; 2: 254-259.
[69] Ju Z, Wang J, Pan S. Isolation and preliminary identification of the endophytic fungi which produce hupzine A from four
species in Hupziaceae and determination of huperzine A by HPLC. Fudan University Journal (Medicinal Science Edition).
2009; 36: 445-449.
[70] Zhou L, Cao X, Yang C, Wu X, Zhang L. Endophytic fungi of Paris polyphylla var. yunnanensis and steroid analysis in the
fungi. Natural Product Research and Development. 2004; 16: 198-200.
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
©FORMATEX 2010
575
[71] Cao X, Li J, Zhou L, Xu L, Li J, Zhao J. Determination of diosgenin content of the endophytic fungi from Paris polyphylla var.
yunnanensis by using an optimum ELISA. Natural Product Research and Development. 2007; 19: 1020-1023.
[72] Kusari S, Lamshöft M, Zühlke S, Spiteller M. An endophytic fungus from Hypericum perforatum that produces hypericin.
Journal of Natural Products. 2008; 71: 159-162.
[73] Zhou S, Yang F, Lan S, Xu N, Hong Y. Huperzine A producing conditions from endophytic fungus in SHB Huperzia serrata.
Journal of Microbiology. 2009; 29: 32-36.
[74] Zhang L, Gu S, Shao H, Wei R. Isolation, determination and aroma product characterization of fungus producing irone.
Mycosystema. 1999; 18: 49-54.
[75] Wang Q, Fu Y, Gao J, Wang Y, Li X, Zhang A. Preliminary isolation and screening of the endophytic fungi from Melia
azedarach L. Acta Agriculturae Boreali-occidentalis Sinica. 2007; 16: 224-227.
[76] Schneider P, Misiek M, Hoffmeister D. In vivo and in vitro production options for fungal secondary metabolites. Molecular
Pharmaceutics. 2008; 5: 234-242.
[77] Taylor TN, Taylor EL. The rhynie chert ecosystem: a model for understanding fungal interactions, in: Bacon CW, White JF,
eds. Microbial Endophytes. New York: Marcel Decker; 2000: 31-48.
[78] Carroll G. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology. 1988; 69: 2-9.
[79] Choi W-Y, Rim S-O, Lee J-H, Lee J-M, Lee I-J, Cho K-J, Rhee I-K, Kwon J-B, Kim J-G. Isolation of gibberellins-producing
fungi from the root of several Sesamum indicum plants. Journal of Microbiology and Biotechnology. 2005; 15: 22-28.
[80] Zhou L, Zhao J, Shan T, Cai X, Peng Y. Spirobisnaphthalenes from fungi and their biological activities. Mini-Reviews in
Medicinal Chemistry. 2010; 10: 977-989.
_______________________________________________________________________________________
Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology
A. Méndez-Vilas (Ed.)
576
©FORMATEX 2010