Content uploaded by Takahiro Shimada
Author content
All content in this area was uploaded by Takahiro Shimada on Mar 26, 2018
Content may be subject to copyright.
1 3
Mar Biol (2016) 163:8
DOI 10.1007/s00227-015-2771-0
ORIGINAL PAPER
Sea turtles return home after intentional displacement
from coastal foraging areas
Takahiro Shimada1,4 · Colin Limpus2 · Rhondda Jones1 · Julia Hazel1,4 ·
Rachel Groom3 · Mark Hamann1,4
Received: 30 January 2015 / Accepted: 17 December 2015
© Springer-Verlag Berlin Heidelberg 2016
their inferred home areas) for various reasons. All non-dis-
placed turtles (n = 54) remained at their home areas for the
duration of tracking. Among displaced turtles (n = 59), the
large majority travelled back to their respective home areas
(n = 52) or near home (n = 4). Homing turtles travelled
faster and adopted straighter routes in cooler water and
travelled faster by day than by night. Our results showed
that displacement up to 117.4 km and captivity up to
514 days did not disrupt homing ability nor diminish fidel-
ity to the home area. However, for homing turtles we infer
energetic costs and heightened risk in unfamiliar coastal
waters. Confirmed homing suggests that moving individu-
als away from danger might offer short-term benefit (e.g.
rescue from an oil spill), but moving turtles to a new forag-
ing area is unlikely to succeed as a long-term conservation
strategy. Priority must rather be placed on protecting their
original habitat.
Introduction
In diverse situations, wild animals may be removed from
their normal habitat and subsequently released at a new
location with expectation of a beneficial outcome. The
objective may be to establish threatened species in a new
area, reintroduce them in an area of local extinction, or
augment a locally diminished population (for examples
see Griffith et al. 1989; Fischer and Lindenmayer 2000).
In addition, a localised environmental catastrophe may
prompt the removal of vulnerable animals to a safer loca-
tion (e.g. Barham et al. 2006). Furthermore, animals that
have been temporarily held in captivity, e.g. for research
or rehabilitation, may be released at locations distant from
their area of origin for logistical feasibility or in expecta-
tion of more favourable conditions for the animals.
Abstract Vulnerable species may be removed from their
normal habitat and released at a new location for conserva-
tion reasons (e.g. re-establish or augment a local popula-
tion) or due to difficulty or danger in returning individu-
als to original sites (e.g. after captivity for research or
rehabilitation). Achieving the intended conservation ben-
efits will depend, in part, on whether or not the released
animals remain at the new human-selected location. The
present study tested the hypothesis that hard-shelled sea
turtles along the coast of north-eastern Australia (9–28°S,
142–153°E) would not remain at new locations and would
attempt to return to their original areas. We used satellite-
tracking data gathered previously for different purposes
over several years (1996–2014). Some turtles had been
released at their capture sites, inferred to be home areas,
while other turtles had been displaced (released away from
Responsible Editor: J.D.R. Houghton.
Reviewed by undisclosed experts.
Electronic supplementary material The online version of this
article (doi:10.1007/s00227-015-2771-0) contains supplementary
material, which is available to authorized users.
* Takahiro Shimada
takahiro.shimada@my.jcu.edu.au
1 College of Marine and Environmental Sciences, James Cook
University, Townsville, QLD 4811, Australia
2 Queensland Department of Environment and Heritage
Protection, Brisbane, QLD 4102, Australia
3 Marine Ecosystems Group, Flora and Fauna Division,
Northern Territory Department of Land Resource
Management, Darwin, NT 0828, Australia
4 Centre for Tropical Water and Aquatic Ecosystem Research,
James Cook University, Townsville, QLD 4811, Australia
Mar Biol (2016) 163:8
1 3
8 Page 2 of 14
Biological background knowledge is essential in assess-
ing the feasibility of moving vulnerable wild species
(Stamps and Swaisgood 2007; IUCN/SSC 2013). A fun-
damental question must be considered for a highly mobile
species (Stamps and Swaisgood 2007) namely: can the
displaced animals be expected to remain at the new loca-
tion? Clearly a positive answer is necessary to meet most
conservation goals, yet a negative answer must be assumed
if animals are expected to return home after displacement
for research or rehabilitation. For many species, no clear
answer is available.
For hard-shelled sea turtles (Cheloniidae), there is evi-
dence of long-term fidelity to foraging sites, long-term
fidelity to breeding sites, and the capacity for migration
between these sites at irregular intervals (Miller 1997; Plot-
kin 2003). However, these common patterns can be subject
to variation. For example, seasonal and ontogenetic shifts
in foraging habitat have been reported for some species at
some locations (e.g. Musick and Limpus 1997; Morreale
and Standora 2005; Shimada et al. 2014). Consequently,
inference from natural behaviour offers uncertain guid-
ance about potential responses of sea turtles to unnatural
displacement.
Direct studies of displaced turtles have predominantly
investigated the ocean navigation ability of adult female
turtles after experimental displacement from breeding sites
(e.g. Luschi et al. 1996, 2001; Hays et al. 2003a; Lohm-
ann et al. 2008). Information about turtles displaced from
coastal foraging areas tends to be sparse and site-specific
(e.g. Limpus 1992; Avens et al. 2003) and largely reliant
on recapture of marked animals. Although some displaced
turtles in the Avens study (Avens et al. 2003) were radio-
tracked briefly, that technology was unsuitable for continu-
ous tracking over long duration and distance.
With satellite-linked devices, wild animals can be very
effectively tracked over extended time periods and almost
unlimited geographic range (Godley et al. 2008; Hazen
et al. 2012). Platform transmitter terminals (PTTs) allow
long-duration tracking with remote delivery of estimated
positions, but location accuracy is relatively low (Hays
et al. 2001; Hazel 2009). More accurate and more frequent
locations can be obtained from Fastloc GPS (FGPS) receiv-
ers (Hazel 2009) although these must be linked with the
Argos PTT system to allow remote data delivery. Despite
the technical capacity of satellite-linked systems, research
is typically limited by logistical and funding constraints.
For the present study, these two factors, as well as ethi-
cal considerations, precluded a large-scale displacement
experiment. Instead, we sought insight from tracking data
that had been gathered for diverse purposes at diverse times
during prior work with Cheloniidae in coastal foraging
areas of Queensland, Australia.
The primary objective for our study was to investigate
whether or not free-living sea turtles tend to remain at a
new location after displacement from their foraging areas.
Based on evidence of strong site fidelity at Australian
coastal foraging sites (Limpus 2008), we hypothesised that
the majority of our study turtles would not remain at their
new locations and would attempt to make their way back
to their original areas. However, we suspected that distance
of displacement or duration in captivity might reduce a
turtle’s motivation or ability to return to its original area.
We therefore wanted to investigate environmental variables
that could influence speed of travel and whether direct or
indirect routes were adopted. In combination, speed and
straightness of track would determine the overall duration
of successful return journeys.
We accepted that an opportunistic study sample would
not be comprehensive for all species or balanced for all var-
iables of interest. However, the present study encompassed
multiple species and a wide range of displacement situa-
tions that had occurred during prior work. By drawing on
existing tracking data, we aimed to gain new insights while
avoiding new deployment costs and additional intervention
in the lives of turtles.
Materials and methods
Study turtles
We assembled 113 tracks of turtles that had been captured
in shallow water (<10 m) in various tropical and subtropi-
cal foraging habitats of north-eastern Australia between
1996 and 2014 (Fig. 1; Table S1). Our complete data set
comprised 79 green turtles Chelonia mydas, 30 loggerhead
turtles Caretta caretta (one of them tracked twice), two
olive ridley turtles Lepidochelys olivacea and one hawks-
bill turtle Eretmochelys imbricata. Turtles were captured
for research by the rodeo method (Limpus 1978) (n = 105)
and captured incidentally on a baited drum-line set by the
Queensland Shark Control Program (n = 1). Other tur-
tles were taken into care after being found debilitated on
or near the shore (n = 6), hereafter termed rescued turtles.
The study turtles included adult and immature individu-
als of both sexes as identified by laparoscopic examina-
tion of the gonads, by curved carapace length (CCL), or
by combination of CCL and tail length (Limpus and Reed
1985; Limpus and Limpus 2003; Limpus 2008). Turtle
sizes ranged from 38.1 to 121.2 cm CCL, median 98.0 cm
(interquartile range 91.1–106.1 cm). Research turtles were
released within 5 days of original capture. Rescued turtles
were released after 69–514 days in rehabilitation centres
(Table S1).
Mar Biol (2016) 163:8
1 3
Page 3 of 14 8
Before release, each turtle was fitted with a tracking
device attached to the carapace with epoxy glue and fibre-
glass (e.g. Shimada et al. 2012). Some turtles received
a PTT (n = 27), while the majority (n = 86) received an
Argos-linked FGPS device that provided PTT locations
in addition to FGPS data. Turtles were released at <0.1 to
431.2 km from their capture locations. Tracking periods
ranged from 5 to 915 days (Table S1).
Data preparation
Preliminary screening was applied to all tracks (i.e. both
FGPS and PTT data), using the R package SDLfilter (avail-
able from https://github.com/TakahiroShimada/SDLfilter),
to remove temporal and spatial duplicates and retain only
a single fix (latitude/longitude pair) per time and location.
For concurrent FGPS fixes, the fix derived from the high-
est number of satellites was retained (Hazel 2009; Shimada
et al. 2012). For concurrent PTT fixes, the fix with highest
location class (LC) was retained (CLS 2011). When con-
current fixes had the same quality index, the fix with the
shortest summed distances to the previous and subsequent
fix was retained. We excluded any locations acquired dur-
ing breeding migrations. We also excluded any locations
on land (above high tide line) because, in eastern Australia,
foraging sea turtles rarely ascend beaches above the high
tide line, although some individuals may rest on intertidal
substrate where they become exposed at low tide (Limpus
et al. 2005). All analyses were conducted using R software
(R Core Team 2015).
Classification of displaced and non‑displaced turtles
To determine (a) whether a turtle had been displaced from
its original area and (b) whether displaced turtles returned
to their original areas, we used PTT locations because
these were available for all tracks (n = 113) and in some
instances the PTT data provided a longer tracking duration
than the corresponding FGPS data (in a device that used
both tracking systems, the PTT component could remain
functional after FGPS operation was halted by diminishing
battery power or by epibiont growth on the GPS receiver).
To improve the relatively low accuracy of raw PTT
locations, we fitted hierarchical Bayesian state space mod-
els (hSSM) following Jonsen et al. (2006). This technique
provides more accurate location estimates by accounting
for observation error and heterogeneity using tracking data
from multiple animals. Because the process involves highly
intensive computation, we balanced processing time against
the benefits gained from multiple tracks as follows: our PTT
data set was divided into 12 smaller portions with each sub-
set containing 9836–12,903 observations acquired from 9
to 13 turtles. The model was fit to each subset of PTT data
via two Markov chain Monte Carlo (MCMC) chains using
the R package bsam, provided by Jonsen et al. (2013). Each
MCMC chain was run for 300,000 iterations, excluding the
first 200,000 samples as a burn-in. Every 100th of the last
100,000 samples was retained to reduce autocorrelation.
Convergence and autocorrelation for hSSM were exam-
ined using diagnostic plots generated by the bsam package.
The hSSM locations were estimated at six hourly intervals
(mean interval of the raw Argos fixes). We dropped hSSM
locations that fell on land and locations for periods when
raw Argos fixes were absent for more than 5 days, the lat-
ter because error of hSSM locations appeared to inflate if
20 or more consecutive positions were missing (Bailey et al.
2008). Finally, the high-quality PTT locations (LC 3, 2, 1)
were merged with the hSSM data. These locations, with an
expected mean error of 2.2 km (Hoenner et al. 2012), are
hereafter referred to as post-processed hSSM data.
We used the post-processed hSSM data to calculate the
utilisation distribution (UD) for each turtle. To avoid prob-
lems of autocorrelation, we applied the movement-based
kernel density method of Benhamou (2011) as implemented
in the R package adehabitatHR (Calenge 2006, 2015a). To
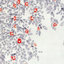
Fig. 1 Release sites of study turtles were dispersed widely along the
coast of Queensland, Australia
Mar Biol (2016) 163:8
1 3
8 Page 4 of 14
define the resettlement area of each turtle, we used the 95 %
contour of the UD, with a buffer of width 2.2 km (expected
mean error of our post-processed hSSM data). A turtle was
deemed to have settled in the buffered 95 % UD provided
the turtle did not move outside the 95 % UD for longer than
1 day. In cases where the 95 % UD comprised two or more
disjunct polygons, an earlier polygon was excluded if the
turtle had moved out of it and did not return to it.
A turtle was classified as displaced if its release loca-
tion was outside its resettlement area, and classified as non-
displaced if its release location was within its resettlement
area (Fig. 2). Provided the capture location was contained
within the resettlement area, the resettlement area was
deemed to represent the original area of that turtle. Thus,
a displaced turtle that subsequently returned to its original
area was regarded as returning home (Fig. 2b). If a turtle
did not return to its original area, the distance between
capture and resettlement was measured to the periphery of
the resettlement area. In the special case where transmis-
sion ceased while a turtle was still travelling (n = 2), the
resettlement area could not be estimated. In this situation,
we classified the turtle as non-displaced if the distance
between its capture and release locations was shorter than
95th percentile diameters of circularised resettlement areas
of all other turtles (16.8 km, n = 111). If the distance was
greater than this, the turtle was classified as displaced.
Detailed analyses for homing turtles
Displaced turtles that returned to their original areas were
classified as homing turtles. For these turtles, we merged
FGPS locations with high-quality PTT locations (LC 3, 2,
1) and then used the R package SDLfilter to apply addi-
tional filtering as follows. In order to remove locations
above the high tide line, the water depth at track locations
was estimated using bathymetry models and tidal data.
Horizontal resolution of the bathymetry models was 110 m
for one release site (site 11, see Fig. 1) (Daniell 2008) and
100 m for the other release sites (Beaman 2010). Tidal data
were obtained from the Australian Bureau of Meteorology
and Queensland Department of Transport and Main Roads.
Filtering according to water depth was applied to the high-
quality PTT locations and to the FGPS fixes derived from
four satellites. Filtering by water depth was deemed unnec-
essary for FGPS fixes derived from >4 satellites because
these fixes had estimated accuracy <64 m at site 1 and
<33.1 m at site 8 (Hazel 2009; Shimada et al. 2012), thus
higher accuracy than the bathymetry models. After filter-
ing by water depth, we applied a data-driven filter following
the method of Shimada et al. (2012). Location fixes were
removed if the speed both from a preceding location and
to a subsequent location exceeded the maximum realistic
swimming speed (Vmax) or if all of the following applied: (a)
fixes were derived from only four GPS satellites or from the
PTT system, the inner angle (180° minus the animal’s turn-
ing angle) was <90°, and the speed either from a preceding
location or to a subsequent location exceeded a maximum
“loop trip” speed (Vlp) estimated for each species (Table 1).
Estimated error (mean ± SD) for high-quality data filtered
by this method was 47.1 ± 61.0 m (Shimada et al. 2012).
To investigate homing behaviour in detail, our analyses
focused on the homing segment of the track, that is, from
Fig. 2 At site 1, a loggerhead turtle T53800 was tracked twice. a On
the first occasion in 1998, the turtle was not displaced. After release
it remained in its original foraging area. b On the second occasion in
2010, the turtle was displaced by 18.3 km from its capture location.
It travelled back to its original area and thus was regarded as a hom-
ing turtle. Square capture location, triangle release location, cross-
hatched polygon resettlement area. Grey line is the travelling path
after displacement. Empty circle location of relatively low residency,
filled circle location of relatively high residency
Mar Biol (2016) 163:8
1 3
Page 5 of 14 8
point of release to the first location of relatively high resi-
dency within the resettlement area (Fig. 2b). We used the
residence time method (Barraquand and Benhamou 2008),
implemented in R package adehabitatLT (Calenge 2006,
2015b) to distinguish locations of relatively high and low
residency. We excluded from our detailed analysis any
homing tracks that included locations <100 m from land,
other than during the first 3 h after release. This was nec-
essary because very close proximity to land would restrict
direction of travel and introduce a confounding effect on
straightness of the track. We calculated straightness index
(Batschelet 1981) equal to straight-line distance from first
to last location (beeline distance) divided by summed
track length. Summed track length was simply the sum
of distances between successive locations along the track.
Thus, a turtle swimming in a straight line all the way
would have straightness index = 1 and a turtle swimming
along a more circuitous path would have a straightness
index <1.
We used generalised linear models (GLMs) to model
travelling speed and straightness index during the over-
all homing trip as functions of displacement distance,
sea surface temperature (SST) at release, season, lati-
tude, and species. We also checked correlations between
travelling speed and straightness. We obtained SST as
daily estimates derived from satellite data at a resolution
of 0.1° (NASA Earth Observations 2014). The Austral-
ian seasons were defined as: spring September–Novem-
ber, summer December–February, autumn March–May,
and winter June–August (Bureau of Meteorology 2015).
Potential effects of bathymetry were not considered
because estimated water depths were consistently shallow
(mean ± SD = 7.3 ± 4.0 m, n = 1046) and the bathymetry
models (resolution 100–110 m) would not identify small
features in the complex substrate at our study sites.
We also evaluated travelling speed and straightness
index during diurnal and nocturnal periods, using track seg-
ments between the first and last fixes of each day and night.
To differentiate day and night periods, we estimated time
of sunrise and sunset at each location using the R pack-
age StreamMetabolism (Sefick 2015). We again examined
factors affecting travelling speed and straightness index
in generalised linear mixed effects models (GLMMs) and
included day/night as a fixed effect, together with other
significant effects identified in the preceding analyses.
Individual turtles were treated as random effects because
some turtles required multiple day/night periods for their
journey.
We chose distributions for response variables in the
GLMs and GLMMs as follows: travelling speed (con-
tinuous, positive, and skewed to right) was fitted with the
gamma distribution, and straightness index (proportion)
was fitted with the beta distribution. We used the R pack-
age stats to fit gamma GLMs (R Core Team 2015), pack-
age betareg to fit beta GLMs (Cribari-Neto and Zeileis
2010), and package glmmADMB to fit both gamma and
beta GLMMs (Fournier et al. 2011; Skaug et al. 2015). For
each model, we computed the variance inflation factors
(VIF) among the covariates using the R package car (Fox
and Weisberg 2011). We considered collinearity was not an
issue if the values were <3 (Zuur et al. 2010). Homogeneity
of variance was assessed by plotting residuals versus fitted
values. Transformations were applied to data when neces-
sary to meet assumptions of the models. Response varia-
bles were centred to have a mean of zero for analyses with
GLMs and GLMMs (Becker et al. 1988). We used the R
package MuMIn (Barton 2015) to rank all possible models
by second-order Akaike information criterion (AICc). We
selected a set of models within two AICc units of the best-
ranked model to identify models with similar explanatory
power (Burnham and Anderson 2002). AICc model weights
(ωi) were computed as the weights of evidence in favour of
each model i within the “best subset”. We compared each
model in the “best subset” to a null model by likelihood
ratio test using the R package lmtest (Zeileis and Hothorn
2002).
We originally wanted to examine the relationship with
speed and straightness of travel for all variables of interest
(species, displacement distance, season, SST, latitude, and
day/night period). However, it emerged that the relevant
portion of our data set (i.e. displaced turtles that returned
home and had tracks not restricted by very close proximity
to land) suffered from collinearity and was highly unbal-
anced with respect with season, species, and latitude; for
example, none of these turtles were released during the
summer months; season was highly correlated with SST
(VIF > 3); green turtle tracks began at six different sites
spread over a wide latitudinal range, but all loggerhead
tracks began at one site. We were therefore obliged to ana-
lyse different combinations of variables for separate subsets
Table 1 Data preparation for
detailed analyses of homing
turtle behaviour: threshold
speed (km h−1) used in the data-
driven filter
Vmax, maximum swimming speed; Max. Vlp, maximum “loop trip” speed, as defined in Shimada et al.
(2012)
Species Vmax Max. Vlp Data source
Chelonia mydas (n = 12) 9.9 (10,189 fixes) 2.0 (716 loop trips) This study
Caretta caretta (n = 8) 8.9 (3921 fixes) 1.8 (57 loop trips) Shimada et al. (2012)
Mar Biol (2016) 163:8
1 3
8 Page 6 of 14
of the homing turtles. See “Homing behaviour of displaced
turtles” section for details of turtle subsets and the variables
addressed for each subset.
Results
Outcomes for displaced turtles
Fifty-nine turtles were classified as displaced. They com-
prised 44 green turtles (including two rescued), 13 logger-
head turtles (one rescued), and two olive ridley turtles (both
rescued). These displaced turtles had been retained for
<1 to 514 days (median = 1 day, interquartile range 0.9–
1.5 days), and they had been displaced from their capture
locations by 6.6–432.1 km (median = 17.5 km, interquar-
tile range 13.3–21.3 km) (Table S1).
Most displaced turtles (n = 52 or 88 %), including two
rescued turtles, returned home and resettled in their original
areas (e.g. Figs. 2b, 3a, b). Another four displaced turtles
moved towards their respective capture areas and settled
within 1.8–14.1 km of their capture location, but their
resettlement areas (95 % UD) did not include the capture
location: these comprised two green turtles (one research,
one rescued), an olive ridley turtle (rescued), and a logger-
head turtle (rescued) (e.g. Fig. 3c).
Two displaced turtles (research) travelled towards their
capture locations, but satellite transmission ceased before
these turtles reached their area of capture. For one individ-
ual (K89296 green turtle, displaced by 19.2 km, Fig. S1j
red), the transmissions abruptly ceased on the 31st day, at
which time the turtle had reached a point 10.7 km from the
capture location. The other individual (QA12903 logger-
head turtle, displaced by 432.1 km, Fig. S1h) had moved
53.6 km towards its capture location when transmission
ceased on the 54th day. Detail of this turtle’s track showed
that during the first 20 days after release it moved 44.5 km
towards its capture location. For the next 5 days, its move-
ments were localised along the coast. For the last 29 days,
most locations indicated a nearby beach. There was a nota-
ble change in the PTT data quality: during the first 20 days
of travel, only 9 % of the data were high-quality fixes (LC
Fig. 3 Representative tracks
of turtles after displacement.
Square capture location,
triangle release location, grey
line travelling path, grey filled
polygon resettlement area. a,
b At site 1, these green turtles
were displaced and returned to
their areas of capture. c At site
2, this olive ridley turtle was
found debilitated and displaced
after rehabilitation. The turtle
moved towards its capture area
but its resettlement area did not
include the capture location. d
At site 5, this green turtle was
displaced and resettled away
from its capture location
Mar Biol (2016) 163:8
1 3
Page 7 of 14 8
3, 2, 1) whereas during the last 34 days, 86 % of the data
were high-quality fixes.
Only one displaced turtle (research) did not move towards
its capture location during its tracking period of 120 days.
Instead this turtle (QA45689 green turtle, displaced by 7.8 km)
settled in an area 35.1 km from its capture location (Fig. 3d).
Outcomes for non‑displaced turtles
Fifty-four turtles were classified as non-displaced. They
comprised 35 green turtles, 18 loggerhead turtles and
one hawksbill turtle (one rescued green turtle, all others
research). The non-displaced turtles had been held for <1 to
170 days (median = 1 day, interquartile range 0.9–1.9 days)
and released at locations <0.1 to 8.9 km (median = 4.2 km,
interquartile range 1.9–6.1 km) away from their capture
locations. After release, all non-displaced turtles remained
in their original areas (95 % UD) (e.g. Fig. 2a).
Homing behaviour of displaced turtles
Of the displaced turtles that returned home, 29 qualified for
detailed analyses because they were tracked with Argos-
linked FGPS devices and their homing tracks were unre-
stricted by very close proximity to land (e.g. Figs. 2b, 3b).
Some of these turtles took a very direct route while others
swam along a relatively circuitous path. Table 2 contains
summary statistics for variables of interest associated with
the homing track of each turtle. The effects of these vari-
ables were addressed separately for different subsets of the
homing turtles (see “Detailed analyses for homing turtles”
section). The effects of latitude were examined only for
Table 2 Summary statistics for
29 turtles that were included
in detailed analyses of homing
behaviour
Data values shown as: median (minimum to maximum). For homing segments, sample size (n) is the num-
ber of homing tracks. For day/night segments, sample size (n) is the number of daytime and night-time
track segments analysed for each species, that is, 22 green turtles and seven loggerhead turtles
Variables Chelonia mydas Caretta caretta
Homing segments n = 22 n = 7
No. locations per track 22 (6–149) 46 (9–90)
Displacement distance (km) 17.99 (7.96–28.10) 18.38 (13.65–26.91)
Homing time (days) 1.95 (0.36–21.15) 3.03 (1.06–4.35)
Beeline distance (km) 15.55 (7.69–55.09) 17.72 (10.89–26.51)
Summed track length (km) 26.23 (11.25–191.81) 29.23 (19.69–38.93)
Latitude at release (S°) 27.48 (9.96–27.52) 27.51 (27.50–27.51)
SST at release (°C) 22.8 (17.8–28.9) 23.9 (18.2–26.3)
No. seasons (spring, summer, autumn, winter) 12, 0, 4, 6 0, 0, 5, 2
Travelling speed (km h−1) 0.66 (0.34–1.72) 0.54 (0.26–0.78)
Straightness index 0.60 (0.12–0.94) 0.53 (0.37–0.93)
Day/night segments Day n = 58, night n = 45 Day n = 20, night n = 15
No. locations per day/night track segment
Day 6 (2–13) 8 (2–13)
Night 4 (2–14) 8 (3–16)
Time between first and last fixes (h)
Day 8.09 (1.63–12.53) 9.29 (2.33–11.30)
Night 8.21 (1.58–11.60) 10.89 (4.63–12.24)
Beeline distance (km)
Day 3.55 (0.04–12.87) 1.86 (0.23–6.55)
Night 1.14 (0.02–9.09) 1.74 (0.16–6.07)
Summed track length (km)
Day 5.78 (0.20–19.41) 4.40 (0.61–9.56)
Night 2.51 (0.05–14.61) 3.36 (0.31–9.21)
Travelling speed (km h−1)
Day 0.90 (0.05–2.53) 0.58 (0.05–1.29)
Night 0.37 (0.01–1.61) 0.40 (0.03–0.84)
Straightness index
Day 0.66 (0.08–0.99) 0.51 (0.12–0.90)
Night 0.58 (0.08–0.98) 0.59 (0.16–0.90)
Mar Biol (2016) 163:8
1 3
8 Page 8 of 14
green turtles (homing turtle subset 1). The effects of spe-
cies were examined only for turtles associated with site
1 (homing turtle subset 2). Relocation distance, SST, and
travelling speed/straightness index were included as pos-
sible explanatory variables in both cases. We omitted sea-
son as an explanatory variable in all models because of its
strong correlation with SST. This means that SST may act
as a surrogate variable for other environmental attributes
which change seasonally.
The first homing turtle subset comprised 22 green tur-
tles, for which we tested the effects of latitude and other
relevant variables using the following two global models.
1. Global model: Travelling speed ~ Displacement dis-
tance + SST + Straightness index + Latitude
Latitude did not appear in the best-ranked model and the
model selection process resulted in only one model
being included in the “best subset”. This model had
SST as its only predictor (Table 3). Neither latitude,
displacement distance, nor straightness index provided
any improvement in prediction of travelling speed.
2. Global model: Straightness index ~ Displacement dis-
tance + SST + Travelling speed + Latitude
Four models were included in the “best subset” of models
but the “best subset” included the null model, that is, a
simple estimate of the mean straightness index with no
explanatory variable as predictor (Table 3). This result,
together with likelihood ratio tests, indicates that none
of the variables including latitude had any perceptible
influence on the straightness index.
The second homing turtle subset comprised green turtles
(n = 12) and loggerhead turtles (n = 7) that were released
in the same area (site 1). For this subset, we tested the
effects of species and other relevant variables as expressed
in the following third and fourth global models.
3. Global model: Travelling speed ~ Displacement dis-
tance + SST + Straightness index + Species
Two models were included in the “best subset” of models
(Table 3). The best-ranked model used both SST and
species as predictors of travelling speed, and the sec-
ond-best model included only SST. Neither displace-
ment distance nor the straightness index appeared to
affect travelling speed (Table 3).
4. Global model: Straightness index ~ Displacement dis-
tance + SST + Travelling speed + Species
Only one model was included in the “best subset”: the
model included SST as a solo predictor (Table 3).
Neither species, displacement distance nor travelling
speed appeared to influence the straightness index
(Table 3).
Table 3 Detailed analyses for homing turtle tracks: overall movements
Overall TS travelling speed and SI straightness index modelled with gamma and beta GLMs, respectively. Explanatory variables are DD dis-
placement distance, SST sea surface temperature, Lat latitude, Sp species as well as TS or SI. p values, AICc, ∆AICc, and AICc model weights
(ωi) are provided for each best-ranked model
Cm Chelonia mydas, Cc Caretta caretta
Homing turtle subset Global model Best-ranked models pAICc ∆AICc ωi
Subset 1 (Cm n = 22) TS ~ DD + SST + SI + Lat TS ~ SST <0.001 1.8 0.00 1
SI ~ DD + SST + TS + Lat SI ~ Lat 0.069 −1.1 0.00 0.347
SI ~ (Null) NA −0.5 0.61 0.256
SI ~ TS 0.135 0.0 1.07 0.203
SI ~ SST 0.142 0.1 1.16 0.195
Subset 2 (Cm n = 12, Cc n = 7) TS ~ DD + SST + SI + Sp TS ~ SST + Sp 0.002 3.4 0.00 0.514
TS ~ SST 0.002 3.5 0.11 0.486
SI ~ DD + SST + TS + Sp SI ~ SST 0.007 −7.3 0.00 1
Subset 1 + 2 (Cm n = 22, Cc n = 7) TS ~ DD + SST + SI + Sp TS ~ SST + Sp <0.001 −3.7 0.00 0.555
TS ~ SST <0.001 −3.2 0.44 0.445
SI ~ DD + SST + TS + Sp SI ~ SST 0.013 −6.9 0.00 1
Mar Biol (2016) 163:8
1 3
Page 9 of 14 8
5. We re-analysed the data using all qualified homing tur-
tles (subset 1 + subset 2, n = 29), omitting latitude as
a covariate because our results for the first homing data
subset indicated latitude had no effect on travelling
speed or straightness. We used the same global mod-
els (3 and 4 above) that we had applied to our second
homing data subset. The inclusion of additional green
turtles from different sites did not change the results
of the model selection with the second homing subset
(Table 3). That is, cooler SST values were in general
associated with faster travelling speed (Fig. 4a) and
with straighter (less circuitous) routes (Fig. 4b). The
result also indicated that green turtles tended to travel
faster than loggerhead turtles (Fig. 4a).
Day/night movement
Among the 29 homing turtles analysed in detail, there were
large variations in travelling speed and straightness index
by day and by night (Table 2). Day/night effects on homing
behaviour were tested with SST and species as explanatory
variables. Our selection of these two variables was deter-
mined by results of preceding analyses of overall move-
ments. We used data for all qualified homing turtles (subset
1 + subset 2, n = 29) in the following two global models.
6. Global model: Day/night travelling speed ~ SST + Day/
night + Species
Two models were selected in the “best subset” (Table 4).
Day/night was an important variable since it occurred
in both models. Turtles tended to travel faster during
the day than the night (Fig. 5). SST also occurred in
both models as expected. Species was retained in the
best model which had considerable support relative to
the other model: the AICc model weights were more
than double when species was included (Table 4).
Day/night travelling speed decreased approximately
0.06 km h−1 per 1 °C increase, and in general, green
turtles travelled faster (fit = 0.85 km h−1) than logger-
head turtles (fit = 0.60 km h−1).
7. Global model: Day/night straightness index ~ SST + Day/
night + Species
None of the variables was associated with straightness of
day/night segments of homing tracks: the best-ranked
model was the null model (Table 4).
Discussion
This study presented substantial evidence that highly
mobile marine species like Cheloniidae cannot be expected
to remain at new human-selected locations after the ani-
mals have been intentionally displaced from their original
coastal foraging grounds.
Confirmation of homing behaviour
The results provided strong support for our initial hypoth-
esis: most displaced turtles attempted to return home, and
furthermore, most of them succeeded. For our study turtles,
homing ability was not limited by distance of displacement
Fig. 4 a Travelling speed and b straightness index for tracks of hom-
ing turtles that were released at various sea surface temperatures
(SST). Green turtles = filled circle, loggerhead turtles = empty circle.
The solid line is model fit; grey band denotes 95 % confidence inter-
val
Mar Biol (2016) 163:8
1 3
8 Page 10 of 14
(up to 117.4 km) or by captivity duration (up to 514 days).
The successful homing animals included green turtle adults
and juveniles of both sexes and loggerhead turtle adults
of both sexes. In addition, one olive ridley turtle returned
home and the other resettled near its capture area. The sin-
gle hawksbill turtle was not displaced.
A few turtles did not return home according to strict
study criteria, but did not conclusively fail to return.
Most of the non-returned turtles travelled to areas near
their respective capture areas. The single displaced tur-
tle that adopted a resettlement area far (35.1 km) from
its capture location appears to indicate a rare instance of
failure to return home. However, we noted that this tur-
tle was tracked for 120 days, a period shorter than the
median tracking duration (157 days) and there remains
a highly speculative possibility that the turtle could
have completed a homing journey after the cessation of
tracking.
Our results showed no evidence of impaired homing
capacity for rescued turtles that had spent 69–514 days in
rehabilitation centres. Our one apparent failure to home
was not a rescued turtle. Of the five rescued turtles that
were displaced, two returned home and the other three
resettled near home. It was plausible that the near-home
rescued turtles had actually returned to their true original
areas. A rescued turtle may have drifted beyond its home
area while it was in a debilitated state, in which case its
capture location (where it was found and rescued) would
have been outside its true original area.
Potential fitness benefits and costs
Almost all the displaced turtles showed a strong homing
tendency, and all non-displaced turtles remained in their
original areas after release. This finding was consistent with
long-term site fidelity, a widely reported phenomenon in
groups as diverse as Chiropterans (Lewis 1995) and Elas-
mobranchs (Knip et al. 2012) albeit with intra-taxon varia-
tion. The development and persistence of site fidelity would
imply this behaviour is associated with a fitness benefit in
terms of evolutionary adaptation (Parker and Smith 1990).
Details of the potential fitness benefit accruing to Che-
loniidae through their fidelity to foraging areas have not
been determined experimentally. The benefit might be
explained in broad terms by site familiarity. This intuitively
relevant concept has seldom been included in habitat selec-
tion models and remains difficult to measure (Piper 2011).
We surmise that, through long familiarity with a particular
area, sea turtles would discover where to find food effi-
ciently, where to find shelter for resting, where predators
typically occur, and where they can best be evaded. Such
site familiarity could enable individuals to adjust their for-
aging behaviour to balance food acquisition and predation
risk, as has been observed in sea turtles in Western Aus-
tralia (Heithaus et al. 2008). Thus, we infer that each turtle
derives a fitness benefit by remaining faithful to its home
foraging area and conversely, we infer fitness costs will
Table 4 Detailed analyses for homing turtle tracks: day/night movements
Day/night travelling speed (DNTS) and day/night straightness index (DNSI) modelled with gamma and beta GLMMs, respectively
Explanatory variables are SST sea surface temperature, DN day or night, and Sp species. p values, AICc, ∆AICc, AICc model weights (ωi) are
provided for each best-ranked model
Cm Chelonia mydas, Cc Caretta caretta
Homing turtle subset Global model Best-ranked models pAICc ∆AICc ωi
Subset 1 + 2
(Cm n = 22, Cc n = 7)
DNTS ~ SST + DN + Sp DNTS ~ SST + DN + Sp <0.001 147.5 0.00 0.677
DNTS ~ SST + DN <0.001 149.0 1.48 0.323
DNSI ~ SST + DN + Sp DNSI ~ (Null) NA −23.8 0.00 0.458
DNSI ~ Sp 0.191 −23.3 0.41 0.373
DNSI ~ DN 0.716 −21.8 1.99 0.169
Fig. 5 Estimated travelling speed of homing turtles by day and by
night. Filled circle is model fit; error bars denote 95 % confidence
interval
Mar Biol (2016) 163:8
1 3
Page 11 of 14 8
accrue for a displaced turtle. It must necessarily expend
energy in travelling back to its home area after unnatural
displacement, and it may face greater risk and forage less
efficiently while it is in unfamiliar habitat.
Factors influencing homing travel
Sea surface temperature (SST) was the key factor identi-
fied as influencing homing behaviour: in cooler water, the
study turtles travelled faster and followed straighter routes.
Greater speed in cooler water was an unexpected finding
for Cheloniidae. They are ectothermic animals that are
affected by ambient water temperature (Spotila et al. 1997).
Cooler water has been found to slow the metabolic rate of
green turtles (Southwood et al. 2003, 2006) and reduce
their activity. For example, green turtles within the southern
part of our study area were found to make notably longer
resting dives at cooler temperatures than at warmer temper-
atures (Hazel et al. 2009). Similarly, slower travel could be
expected at cooler temperatures, yet our results indicated
the converse. In the scientific literature, we could find no
plausible explanatory principle. Insight regarding this sur-
prising finding might be gained through future research
involving systematic displacement experiments.
Although the straightness of complete homing tracks
was strongly associated with SST, the same association
was not evident when we evaluated day/night effects. This
may reflect imprecise estimates of straightness index for
our day/night track segments. These segments were short,
and thus, each segment contained relatively few locations
(median 4–8 locations used for straightness index of a day/
night segment, Table 2).
Inter-specific differences in travelling speed of the hom-
ing turtles probably reflect differences in swimming ability.
Green turtles generally swim faster than loggerhead turtles
(Heithaus et al. 2002), and our results are consistent with
that observation. In contrast to travelling speed, straight-
ness indices were similar for green turtles and loggerhead
turtles. The similarity in straightness of tracks could sug-
gest both species have similar way-finding ability in coastal
waters.
Way‑finding ability of homing turtles
The present study was not designed to investigate navi-
gational capacity per se, but our results clearly confirmed
the ability of displaced turtles to find the way back to their
original areas. For sea turtles, the underlying mechanisms
for open ocean navigation are understood to involve pre-
dominantly geomagnetic cues at greater distances from the
destination, potentially progressing to a hierarchy of other
cues at closer range, details of which remain to be eluci-
dated (Åkesson et al. 2003; Avens and Lohmann 2003;
Hays et al. 2003a; Benhamou et al. 2011; Lohmann et al.
2013). It seems plausible that a similar hierarchy of cues
guided our study turtles, although they did not undertake
oceanic travel and generally travelled within a few kilome-
tres of the mainland shore.
Our finding that displaced turtles travelled faster dur-
ing the day might imply greater availability of way-find-
ing cues during daylight and hence might suggest that
visual information could be important for way-finding.
This difference is not necessarily related to way-finding;
for example, turtles that are not travelling also appear to
be more active during the day, as reported for foraging
turtles within our study area (Hazel et al. 2009). Fur-
thermore, the findings of Åkesson et al. (2003) suggest
that sea turtles do not use celestial cues for orientation.
Nevertheless, additional insight might be gained if future
studies were to include day/night information when ana-
lysing way-finding and navigational behaviour of sea
turtles.
Premature disruption of tracking
Transmission from a tracking device may cease for diverse
reasons (Hays et al. 2007), and we speculated about the
cause of two transmission failures during homing travel.
For turtle QA12903, the sudden and concurrent changes in
movement pattern and in quality of PTT fixes suggested the
turtle became debilitated or died in the area where move-
ment became localised. We suspect this turtle probably
became stranded on the shore, given the unusually large
proportion of high-quality fixes acquired around the inter-
tidal area during the last period of transmission. The track-
ing period was relatively short for this turtle (54 days), and
there was no apparent sign of degradation in device perfor-
mance prior to cessation. We were unable to confirm turtle
death or investigate possible causes because the site of sus-
pected stranding was inaccessible.
For turtle K89296, signals stopped abruptly after only
31 days, while the turtle was travelling slowly close to
shore. There was no evidence of a change in turtle behav-
iour. Detachment of the tracking device seemed more likely
than an early technical failure. Perhaps, the adhesive bond
had been gradually weakened by the turtle rubbing its cara-
pace on rocky outcrops that were potentially available en
route. A similar explanation might apply for the two non-
displaced turtles that had similarly short tracking dura-
tions (≤31 days) and no apparent change in behaviour.
Rare events like boat strike or attack by a very large preda-
tor could disrupt tracking, but we remain cautious about
over-interpreting the cessation of tracking. In our study,
the tracking data offered persuasive evidence for morbid-
ity or mortality in only one case, turtle QA12903 described
above. The wide temporal and geographic range of our
Mar Biol (2016) 163:8
1 3
8 Page 12 of 14
study precluded using this single case to derive a quanti-
tative estimate of mortality, as has been done in different
circumstances (Hays et al. 2003b).
Conservation implications
Our findings suggest that displacement and periods in cap-
tivity do not disrupt a turtle’s ability to find its way back
to its original foraging area nor diminish its fidelity to that
area. However, there must be an energetic cost for hom-
ing turtles and there might be heightened risk of harm in
unfamiliar coastal waters. The potential fitness costs of
displacement should not be ignored, despite our strong evi-
dence that the majority of displaced turtles can be expected
to return home.
Confirmed homing ability suggests that moving individ-
ual turtles away from danger could be effective only as a
short-term conservation measure, e.g. rescue from tempo-
rary threats such as oil spills. The relocation of turtles from
their established coastal foraging ground to a new area
cannot be expected to succeed as a long-term conservation
strategy. Priority must rather be placed on protecting their
original habitat.
Acknowledgments This research was funded by the National Envi-
ronmental Research Program (NERP), Department of Environment
and Heritage Protection of Queensland government (EHP), James
Cook University (JCU), Gladstone Port Corporation Limited, GHD
Australia, Healthy Waterways, Beldi consulting, Sea World Gold
Coast Aquarium and Bundaberg Sugar. We are grateful to Reef HQ
Aquarium, Australia Zoo Wildlife Hospital, and Underwater World
Aquarium, for contributing satellite-tracking data of their rescued sea
turtles to this study, and to M. Smith, K. Huff, C. Lacasse, and H.
Campbell for their help in providing access to the data. We thank J.
Limpus, D. Limpus, M. Savige, and numerous volunteers for their
help in capturing and handling turtles, and P. Yates and A. Reside for
their assistance in data analysis. G. Hays and an anonymous reviewer
provided constructive comments that greatly improved an earlier ver-
sion of this paper. T.S. was supported by NERP scholarship and Ito
Foundation for International Education Exchange Scholarship. This
research was conducted under the ethics permits SA212/11/395 of
EHP and, A1229 and A1683 of JCU.
References
Åkesson S, Broderick AC, Glen F, Godley BJ, Luschi P, Papi F, Hays
GC (2003) Navigation by green turtles: which strategy do dis-
placed adults use to find Ascension Island? Oikos 103:363–372.
doi:10.1034/j.1600-0706.2003.12207.x
Avens L, Lohmann KJ (2003) Use of multiple orientation cues by
juvenile loggerhead sea turtles Caretta caretta. J Exp Biol
206:4317–4325. doi:10.1242/jeb.00657
Avens L, Braun-McNeill J, Epperly S, Lohmann KJ (2003) Site
fidelity and homing behavior in juvenile loggerhead sea tur-
tles (Caretta caretta). Mar Biol 143:211–220. doi:10.1007/
s00227-003-1085-9
Bailey H, Shillinger G, Palacios D, Bograd S, Spotila J, Paladino F,
Block B (2008) Identifying and comparing phases of movement
by leatherback turtles using state-space models. J Exp Mar Biol
Ecol 356:128–135. doi:10.1016/j.jembe.2007.12.020
Barham PJ et al (2006) Return to Robben Island of African Pen-
guins that were rehabilitated, relocated or reared in captivity
following the Treasure oil spill of 2000. Ostrich 77:202–209.
doi:10.2989/00306520609485534
Barraquand F, Benhamou S (2008) Animal movements in heterogene-
ous landscapes: identifying profitable places and homogeneous
movement bouts. Ecology 89:3336–3348. doi:10.1890/08-0162.1
Barton K (2015) MuMIn: multi-model inference. R package v. 1.15.1.
http://CRAN.R-project.org/package=MuMIn. Accessed 3 Aug
2015
Batschelet E (1981) Circular statistics in biology. Academic Press,
New York
Beaman R (2010) Project 3DGBR: a high-resolution depth model for
the Great Barrier Reef and Coral Sea. Marine and Tropical Sci-
ences Research Facility (MTSRF) Project 25i1a Final Report:pp.
13 plus Appendix 11
Becker RA, Chambers JM, Wilks AR (1988) The new S language: a
programming environment for data analysis and graphics. Wads-
worth and Brooks/Cole Advanced Books & Software, Monterey,
CA
Benhamou S (2011) Dynamic approach to space and habitat use based
on biased random bridges. PLoS One 6:e14592. doi:10.1371/
journal.pone.0014592
Benhamou S, Sudre J, Bourjea J, Ciccione S, De Santis A, Luschi P
(2011) The role of geomagnetic cues in green turtle open sea nav-
igation. PLoS One 6:e26672. doi:10.1371/journal.pone.0026672
Bureau of Meteorology (2015) Climate glossary. Commonwealth of
Australia. http://www.bom.gov.au/climate/glossary/seasons.
shtml. Accessed 15 July 2015
Burnham KP, Anderson DR (2002) Model selection and multi-model
inference: a practical information-theoretic approach, 2nd edn.
Springer, New York
Calenge C (2006) The package “adehabitat” for the R software: a tool
for the analysis of space and habitat use by animals. Ecol Model
197:516–519. doi:10.1016/j.ecolmodel.2006.03.017
Calenge C (2015a) adehabitatHR: home range estimation. R package
v. 0.4.14. http://CRAN.R-project.org/package=adehabitatHR.
Accessed 19 Sept 2015
Calenge C (2015b) adehabitatLT: analysis of animal movements.
R package v. 0.3.20. http://CRAN.R-project.org/package=
adehabitatLT. Accessed 19 Sept 2015
CLS (2011) Argos user’s manual. CLS, Ramonville Saint-Agne
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw
34:1–24
Daniell JJ (2008) Development of a bathymetric grid for the Gulf
of Papua and adjacent areas: a note describing its develop-
ment. J Geophys Res (Earth Surf) 113:F01S15. doi:10.1029/20
06JF000673
Fischer J, Lindenmayer DB (2000) An assessment of the pub-
lished results of animal relocations. Biol Conserv 96:1–11.
doi:10.1016/S0006-3207(00)00048-3
Fournier DA et al (2011) AD model builder: using automatic differen-
tiation for statistical inference of highly parameterized complex
nonlinear models. Optim Methods Softw 27:233–249. doi:10.10
80/10556788.2011.597854
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd
edn. Sage, Beverley Hills, CA
Godley BJ, Blumenthal JM, Broderick AC, Coyne MS, Godfrey MH,
Hawkes LA, Witt MJ (2008) Satellite tracking of sea turtles:
where have we been and where do we go next? Endanger Spec
Res 4:3–22. doi:10.3354/esr00060
Griffith B, Scott JM, Carpenter JW, Reed C (1989) Translocation as a
species conservation tool: status and strategy. Science 245:477–
480. doi:10.1126/science.245.4917.477
Mar Biol (2016) 163:8
1 3
Page 13 of 14 8
Hays GC, Åkesson S, Godley BJ, Luschi P, Santidrian P (2001) The
implications of location accuracy for the interpretation of sat-
ellite-tracking data. Anim Behav 61:1035–1040. doi:10.1006/
anbe.2001.1685
Hays GC, Åkesson S, Broderick AC, Glen F, Godley BJ, Papi F,
Luschi P (2003a) Island-finding ability of marine turtles. Proc R
Soc Lond B Biol Sci 270:S5–S7. doi:10.1098/rsbl.2003.0022
Hays GC, Broderick AC, Godley BJ, Luschi P, Nichols WJ (2003b)
Satellite telemetry suggests high levels of fishing-induced
mortality in marine turtles. Mar Ecol Prog Ser 262:305–309.
doi:10.3354/meps262305
Hays GC, Bradshaw CJA, James MC, Lovell P, Sims DW (2007)
Why do Argos satellite tags deployed on marine animals stop
transmitting? J Exp Mar Biol Ecol 349:52–60. doi:10.1016/j.
jembe.2007.04.016
Hazel J (2009) Evaluation of fast-acquisition GPS in stationary tests
and fine-scale tracking of green turtles. J Exp Mar Biol Ecol
374:58–68. doi:10.1016/j.jembe.2009.04.009
Hazel J, Lawler IR, Hamann M (2009) Diving at the shallow end:
green turtle behaviour in near-shore foraging habitat. J Exp Mar
Biol Ecol 371:84–92. doi:10.1016/j.jembe.2009.01.007
Hazen E et al (2012) Ontogeny in marine tagging and tracking sci-
ence: technologies and data gaps. Mar Ecol Prog Ser 457:221–
240. doi:10.3354/meps09857
Heithaus MR, Frid A, Dill LM (2002) Shark-inflicted injury frequen-
cies, escape ability, and habitat use of green and loggerhead tur-
tles. Mar Biol 140:229–236. doi:10.1007/s00227-001-0712-6
Heithaus MR, Wirsing AJ, Thomson JA, Burkholder DA (2008)
A review of lethal and non-lethal effects of predators on adult
marine turtles. J Exp Mar Biol Ecol 356:43–51. doi:10.1016/j.
jembe.2007.12.013
Hoenner X, Whiting SD, Hindell MA, McMahon CR (2012) Enhanc-
ing the use of argos satellite data for home range and long dis-
tance migration studies of marine animals. PLoS One 7:e40713.
doi:10.1371/journal.pone.0040713
IUCN/SSC (2013) Guidelines for reintroductions and other conserva-
tion translocations. Version 1.0. IUCN Species Survival Com-
mission, Gland
Jonsen ID, Myers RA, James MC (2006) Robust hierarchi-
cal state-space models reveal diel variation in travel rates of
migrating leatherback turtles. J Anim Ecol 75:1046–1057.
doi:10.1111/j.1365-2656.2006.01129.x
Jonsen ID et al (2013) State-space models for bio-loggers: a meth-
odological road map. Deep Sea Res Part II Top Stud Oceanogr
88–89:34–46. doi:10.1016/j.dsr2.2012.07.008
Knip D, Heupel M, Simpfendorfer C (2012) To roam or to home: site
fidelity in a tropical coastal shark. Mar Biol 159:1647–1657.
doi:10.1007/s00227-012-1950-5
Lewis SE (1995) Roost fidelity of bats: a review. J Mammal 76:481–
496. doi:10.2307/1382357
Limpus CJ (1978) The reef. In: Lavery HJ (ed) Exploration north:
Australia’s wildlife from desert to reef. Richmond Hill Press,
Richmond, VIC, pp 187–222
Limpus CJ (1992) The hawksbill turtle, Eretmochelys imbricata, in
Queensland: population structure within a southern Great Bar-
rier Reef feeding ground. Wildl Res 19:489–506. doi:10.1071/
wr9920489
Limpus CJ (2008) A biological review of Australian marine turtle
species. The State of Queensland, Environmental Protection
Agency, Brisbane
Limpus CJ, Limpus DJ (2003) Biology of the loggerhead turtle in
western south Pacific Ocean foraging areas. In: Bolten AB, With-
erington BE (eds) Loggerhead sea turtles. Smithsonian Institu-
tion, Washington, DC, pp 93–113
Limpus CJ, Reed PC (1985) The green turtles, Chelonia mydas, in
Queensland: a preliminary description of the population structure
in a coral reef feeding ground. In: Grigg G, Shine R, Ehmann H
(eds) Biology of Australasian frogs and reptiles. Surrey Beatty
in association with The Royal Zoological Society of New South
Wales, New South Wales, pp 47–52
Limpus CJ, Limpus DJ, Arthur KE, Parmenter CJ (2005) Monitoring
green turtle population dynamics in Shoalwater Bay: 2000–2004.
Great Barrier Reef Marine Park Authority, Queensland
Lohmann KJ, Luschi P, Hays GC (2008) Goal navigation and
island-finding in sea turtles. J Exp Mar Biol Ecol 356:83–95.
doi:10.1016/j.jembe.2007.12.017
Lohmann KJ, Lohmann CMF, Brothers JR, Putman NF (2013) Natal
homing and imprinting in sea turtles. In: Wyneken J, Lohmann
KJ, Musick JA (eds) The biology of sea turtles, vol 3. CRC
Press, Boca Raton, FL, pp 59–78. doi:10.1201/b13895-4
Luschi P, Papi F, Liew HC, Chan EH, Bonadonna F (1996) Long-dis-
tance migration and homing after displacement in the green tur-
tle (Chelonia mydas): a satellite tracking study. J Comp Physiol
178:447–452
Luschi P, Åkesson S, Broderick A, Glen F, Godley B, Papi F, Hays G
(2001) Testing the navigational abilities of ocean migrants: dis-
placement experiments on green sea turtles (Chelonia mydas).
Behav Ecol Sociobiol 50:528–534
Miller JD (1997) Reproduction in sea turtles. In: Lutz PL, Musick JA
(eds) The biology of sea turtles, vol 1. CRC Press, Boca Raton,
FL, pp 51–81
Morreale SJ, Standora EA (2005) Western north Atlantic waters: cru-
cial developmental habitat for Kemp’s ridley and loggerhead sea
turtles. Chelonian Conserv Biol 4:872–882
Musick JA, Limpus CJ (1997) Habitat utilization and migration in
juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of
sea turtles, vol 1. CRC Press, Boca Raton, FL, pp 137–163
NASA Earth Observations (2014) Sea surface temperature. EOS Pro-
ject Science Office, NASA Goddard Space Flight Center. http://
neo.sci.gsfc.nasa.gov. Accessed 6 Nov 2014
Parker GA, Smith JM (1990) Optimality theory in evolutionary biol-
ogy. Nature 348:27–33. doi:10.1038/348027a0
Piper W (2011) Making habitat selection more “familiar”: a
review. Behav Ecol Sociobiol 65:1329–1351. doi:10.1007/
s00265-011-1195-1
Plotkin P (2003) Adult migrations and habitat use. In: Lutz PL,
Musick JA, Wyneken J (eds) The biology of sea turtles, vol 2.
CRC Press, Boca Raton, FL, pp 225–241
R Core Team (2015) R: a language and environment for statistical
computing (v. 3.1.2). R Foundation for Statistical Computing,
Vienna
Sefick S (2015) Stream metabolism: a package for calculating single
station metabolism from diurnal oxygen curves. R package v.
1.1.1. http://CRAN.R-project.org/package=StreamMetabolism.
Accessed 3 Aug 2015
Shimada T, Jones R, Limpus C, Hamann M (2012) Improving data
retention and home range estimates by data-driven screening.
Mar Ecol Prog Ser 457:171–180. doi:10.3354/meps09747
Shimada T, Aoki S, Kameda K, Hazel J, Reich K, Kamezaki N (2014)
Site fidelity, ontogenetic shift and diet composition of green tur-
tles Chelonia mydas in Japan inferred from stable isotope analy-
sis. Endanger Spec Res 25:151–164. doi:10.3354/esr00616
Skaug H, Fournier D, Bolker B, Magnusson A, Nielsen A (2015) glm-
mADMB: generalized linear mixed models using AD model
builder. R package v. 0.8.1. http://glmmadmb.r-forge.r-project.
org. Accessed 3 Aug 2015
Southwood AL, Reina RD, Jones VS, Jones DR (2003) Seasonal div-
ing patterns and body temperatures of juvenile green turtles at
Heron Island, Australia. Can J Zool 81:1014–1024. doi:10.1139/
Z03-081
Southwood AL, Reina RD, Jones VS, Speakman JR, Jones DR (2006)
Seasonal metabolism of juvenile green turtles (Chelonia mydas)
Mar Biol (2016) 163:8
1 3
8 Page 14 of 14
at Heron Island, Australia. Can J Zool 84:125–135. doi:10.1139/
z05-185
Spotila JR, O’Connor MP, Paladino FV (1997) Thermal biology. In:
Lutz PL, Musick JA (eds) The biology of sea turtles, vol 1. CRC
Press, Boca Raton, FL, pp 297–314
Stamps JA, Swaisgood RR (2007) Someplace like home: experience,
habitat selection and conservation biology. Appl Anim Behav Sci
102:392–409. doi:10.1016/j.applanim.2006.05.038
Zeileis A, Hothorn T (2002) Diagnostic checking in regression rela-
tionships. R News 2:7–10
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data explora-
tion to avoid common statistical problems. Methods Ecol Evol
1:3–14. doi:10.1111/j.2041-210X.2009.00001.x
A preview of this full-text is provided by Springer Nature.
Content available from Marine Biology
This content is subject to copyright. Terms and conditions apply.