Access to this full-text is provided by Wiley.
Content available from Disease Markers
This content is subject to copyright. Terms and conditions apply.
Review Article
A Pathophysiologic Approach to Biomarkers in Acute
Respiratory Distress Syndrome
Raiko Blondonnet,1,2 Jean-Michel Constantin,1,2
Vincent Sapin,2,3 and Matthieu Jabaudon1,2
1CHU Clermont-Ferrand, Intensive Care Unit, Department of Perioperative Medicine, Estaing University Hospital,
63000 Clermont-Ferrand, France
2Clermont Universit´
e, Universit´
e d’Auvergne, EA 7281, R2D2, 63000 Clermont-Ferrand, France
3Department of Medical Biochemistry and Molecular Biology, CHU Clermont-Ferrand, 63000 Clermont-Ferrand, France
Correspondence should be addressed to Matthieu Jabaudon; mjabaudon@chu-clermontferrand.fr
Received December ; Accepted January
Academic Editor: George Perry
Copyright © Raiko Blondonnet et al. is is an open access article distributed under the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly
cited.
Acute respiratory distress syndrome (ARDS) is an acute-onset hypoxic condition with radiographic bilateral lung inltration. It is
characterized by an acute exudative phase combining diuse alveolar damage and lung edema followed by a later broproliferative
phase. Despite an improved understanding of ARDS pathobiology, our ability to predict the development of ARDS and risk-stratify
patients with the disease remains limited. Biomarkers may help to identify patients at the highest risk of developing ARDS, assess
response to therapy, predict outcome, and optimize enrollment in clinical trials. Aer a short description of ARDS pathobiology,
here, we review the scientic evidence that supports the value of various ARDS biomarkers with regard to their majorbiological roles
in ARDS-associated lung injury and/or repair. Ongoing research aims at identifying and characterizing novel biomarkers, in order
to highlight relevant mechanistic explorations of lung injury and repair, and to ultimately develop innovative therapeutic approaches
for ARDS patients. is review will focus on the pathophysiologic, diagnostic, and therapeutic implications of biomarkers in ARDS
and on their utility to ultimately improve patient care.
1. Introduction
e acute respiratory distress syndrome (ARDS) is a het-
erogeneous syndrome dened by the association of bilateral
radiographic pulmonary opacities, arterial hypoxemia (par-
tial pressure of arterial oxygen (PaO2) to fraction of inspired
oxygen (FiO2)ratio< with a positive end-expiratory
pressure of cm H2O or more), and exclusion of cardiac
failure as a primary cause []. It is characterized by diuse
alveolar epithelial and lung endothelial injury leading to
increased permeability pulmonary edema and alveolar lling
[]. By denition, ARDS occurs within one week of a known
clinical insult or new or worsening respiratory symptoms, as
a consequence of various risk factors including either direct
(e.g., bacterial or viral pneumonia, gastric aspiration, lung
contusion, toxic inhalation, and near drowning) or indirect
(e.g., sepsis, pancreatitis, severe trauma, massive blood trans-
fusion, and burn) lung injury []. Despite improvements in
intensive care during the last een years, ARDS is still
a frequent (/ inhabitants/year), morbid, and life-
threatening condition, with a mortality rate around % [–
]. In addition, there has been recent recognition of the
clinical and biological heterogeneity within ARDS [–],
thus reecting our incomplete understanding of the biology
of ARDS and hampering the successful clinical translation
of new diagnostic, preventive, and therapeutic strategies
[]. Some investigators have further proposed subdividing
ARDS, for example, on the basis of clinical risk factors
[], by direct versus indirect lung injury [], or by focal
versus nonfocal lung morphology as assessed by CT-scan
[, ]. Characterizing ARDS phenotypes may help to better
understand genetic, genomic, and protein risk factors for
Hindawi Publishing Corporation
Disease Markers
Volume 2016, Article ID 3501373, 20 pages
http://dx.doi.org/10.1155/2016/3501373
Disease Markers
ARDS, predict the syndrome, identify mechanism-dened
subgroups of ARDS, and/or to better target therapy [, ].
e subtype (or phenotype) of a condition is ideally dened
by a distinct functional/pathobiological mechanism, named
endotype, that may explain, at least in part, response to
treatment [].
2. Pathogenesis of ARDS
e pathogenesis of ARDS is characterized by two phases
that may sometimes overlap temporally and spatially []:
exudative and proliferative [] phases. An alveolar-capillary
barrier dysfunction resulting in altered permeability of
epithelial and endothelial alveolar cells characterizes the early
exudative phase. Due to loss of cellular integrity, alveoli
are lled with proteinaceous edema uid that results in
impaired gas exchange. Initially, there is an early exudative
phaseassociatedwithdiusealveolardamage,microvascular
injury with subsequent pulmonary edema, alveolar type
(AT) epithelial cell necrosis, and inux of inammatory cells
which then release active mediators []. During this early
phase, alveolar inammation is mainly mediated by polymor-
phonuclear neutrophils (PMN) [], but recent ndings also
support a key role for monocytes and macrophages [, ].
Other proinammatory mechanisms are also involved, as
the signicant release of proinammatory cytokines by lungs
cells, inammatory cells, and broblasts.
e association of persistent injury and failure to repair
lung damage in a timely manner mainly contributes to the
pathological broproliferative response during which there
are proliferation of broblasts, hyperplasia of AT cells, and
lung repair. e repair of the injured alveolar epithelium
remains incompletely understood; it involves hyperplasia of
AT ( and may be AT) cel ls, mig r ation along t he basem ent
membrane by AT cells to form a new epithelial barrier, and
complex interactions with ECM and other cells including
alveolar macrophages. In the absence of recovery, processes
leading to brosing alveolitis may occur during a brotic
phase, resulting in some cases in marked changes in lung
structure and function [].
3. Biomarkers of ARDS:
A Pathophysiologic Approach
e discovery and validation of biomarkers of myocardial
injury and ventricular overload such as troponin and brain-
natriuretic peptide (BNP) have transformed the diagnosis,
management, and design of clinical trials in conditions such
as myocardial infarction and congestive heart failure [, ].
In a similar way, identication of plasma biomarkers that may
facilitate diagnosis of ARDS could, at least in theory, improve
clinical care, enhance our understanding of pathophysiology,
and be used to enroll more homogeneous groups of patients
in clinical trials of new therapies, increasing the likelihood of
detecting a treatment eect []. Pathophysiologic changes
canprobablybeusedasaframeworktobetterunderstand
various biomarkers that have been studied in ARDS, includ-
ing the cellular injury pathways that are central to lung injury:
endothelial injury, epithelial injury, proinammatory injury,
coagulation, brosis, and apoptosis [].
Among multiple potential applications, biomarkers may
be used to better identify patients with risk factors of ARDS
who are most likely to develop the syndrome. Subsequently,
they may also be useful to improve risk stratication once
ARDS criteria are present. Biomarkers may also play a
pivotalroleinthedesignoffutureclinicaltrialsthrough
the identication of patients at high risk of poor outcome,
thus decreasing the required sample size needed to show
a therapeutic benet []. More recently, biomarkers have
also been proven useful to evaluate the response to therapy
[, ]. Finally, the study of biomarkers in ARDS plays
a fundamental role in understanding the mechanisms and
pathophysiologyunderlyinglunginjury,thusservingasa
solid basis to develop future therapeutic strategies [].
Wewillnowreviewthebiomarkersthathavebeen
investigated in ARDS, with a focus on biomarkers in groups
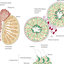
that reect their primary function (Figure and Table ).
3.1. Exudative Phase of ARDS. ARDS is characterized by
an initial exudative phase with diuse alveolar damage
associated with the formation of lung inammatory edema.
Alveolar injury is predominant during this phase, and various
proteins that are specic to lung injury are therefore released
in both the blood and the alveolar compartment, thus serving
as markers of the disease or of its resolution.
3.1.1. Lung Injury
(1) Alveolar Epithelium.ealveolarepithelialliningis
compos e d of AT an d AT epit helia l c ells an d play s a critic a l
role in barrier function, regulating surfactant production,
and in vectorial transport of alveolar uid. During the acute
phase of ARDS, alveolar epithelial cells undergo neutrophil-
mediated damage and eects of proinammatory cytokines
or of hypoxic injury, thus accounting for the clinical syn-
drome []. Lung-secreted proteins may be found in both the
bronchoalveolar (BAL) uid and the systemic blood because
they can move passively across the epithelial barrier into
serum where they may serve as peripheral indicators of
epithelial damage. Several markers of lung epithelial damage
havebeenstudiedasmarkersofARDS,assupportedbythe
fact that they should be more specic to lung injury than
other markers, for example, inammatory cytokines [].
(a) Alveolar Type 1 Cells.eyareoftwotypes.
RAGE. AT epithelial cells cover –% of the alveolar sur-
faceandcontributetobothalveolaruidclearance(AFC)and
barrier integrity. e receptor for advanced glycation end-
products (RAGE) is a transmembrane pattern-recognition
receptor of the immunoglobulin superfamily that is constitu-
tively expressed at low levels in all cells but abundantly in the
lung. RAGE is primarily located on the basal surface of AT
cells [, ]. Activation of RAGE modulates cell signaling,
culminating in a sustained inammatory response through
various intracellular signaling pathways such as cytokines,
reactive oxygen species (ROS), or proteases and leading to
Disease Markers
Exudative
Epithelium damage
Endothelium damage
Inammatory cascade
IL-6, and IL-8
IL-10
Additional markers: HMGB1, LBP, NO, CRP,
Anti-inammatory: IL-1RA, sTNF-RI/II, and
albumin, and LDH
Pulmonary vascular permeability
EF/PL ratio
Lung matrix alteration
Laminin
Elastin/desmosine
MMPs: 2, 3, 8, 9, and 13
PA- 1
Protein C
Coagulation and brinolysis
phase
ATI cells: sRAGE, HTI56
ATII cells: surfactant, KL-6
Club cells: CC16
Ang-1, Ang-2
ICAM-1
Selectins
VEGF
vWF
Epithelial apoptosis
Fibroproliferative phase
Fas/FasL
VEGF
KGF
HGF
N-PCP-III
Epithelial proliferation
Fibroblast proliferation
Endothelial proliferation
Proinflammatory: IL1-𝛽/TNF-𝛼, IL-18,
rombomodulin
Tissue factor
Cell-free hemoglobin
Club cell
Alveolar type I cell
Alveolar type II cell
Red cell
Alveolar
macrophage
Neutroph il
Capillary Interstitium
Protein-rich
edema uid
dierentiation of
alveolar type II cells
Alveolus
Necrotic alveolar
type I cell
Proliferation and
m
Fibroblast
F : Biomarkers of acute respiratory distress syndrome organized by pathways and phases of lung injury (le: early exudative phase;
right: broproliferative phase).
proinammatory activation of nuclear transcription factor
NF-𝜅B[,].RAGEisimplicatedinARDSasanimportant
pathway to innate immunity and alveolar inammation []
and when the soluble form (sRAGE, for soluble RAGE) is
assayedinplasmaorpulmonaryedemauid,asamarker
of alveolar injury [–]. Full-length RAGE is a transmem-
branereceptor,butitcanalsobefoundassolubleisoforms,
generally referred to as soluble RAGE (sRAGE, comprising
the extracellular domain of RAGE and produced through the
cleavage of full-length RAGE by matrix metalloproteinases)
[, ] and endogenous secretory RAGE (esRAGE, pro-
duced aer alternative splicing) []. Full-length RAGE and
its isoforms are abundantly and constitutively expressed in
the lungs in normal conditions [, –], and sRAGE
is now considered as a promising novel marker of AT cell
injury and a key mediator of alveolar inammation [, ,
]. It is shown that sRAGE expression appears enhanced
during the early stage of ARDS. Our team, with others, has
recently reported in both ARDS patients and a mouse model
of ARDS that the extent of sRAGE elevation in plasma and
alveolar uid correlates with markers of severity assessed by
PaO2/FiO2, lung injury, and alveolar uid clearance (AFC)
[–, ]. A role for RAGE pathway in the regulation of
AFC has been recently described for the rst time [] and is
under active investigation by our team and others [, ].
Interestingly, plasma and BAL sRAGE levels are elevated
during ARDS, independently of any associated severe sepsis
[]. In addition, plasma levels of sRAGE are correlated with
diuse damage as assessed by lung CT-scan and are correlated
with the extent of alveolar damage [, ], suggesting that
sRAGE may serve as a useful biomarker of AT cell injury and
lung damage during ARDS. Plasma levels of sRAGE are also
associated with -day and -day mortality in patients with
ARDS [, , ].
Calfee et al. recently compared biomarker levels in
patients with direct versus indirect ARDS enrolled in a single
center study of patients and in a secondary analysis of
ARDS patients drawn from a multicenter randomized con-
trolled trial []: levels of biomarkers of lung epithelial injury
(sRAGE, surfactant protein-D) were signicantly higher in
direct ARDS compared to indirect ARDS.
A recent observational study also supports an ARDS
phenotype based on levels of RAGE ligands and soluble
forms, as elevated sRAGE, high mobility group box- pro-
tein (HMGB), and SA, with decreased esRAGE and
advanced glycation end-products (AGEs), were found to
distinguish patients with ARDS from those without [].
Although these recent ndings warrant further validation in
multicenter studies, monitoring sRAGE levels may be useful
in assessing the response to strategies in ventilator settings
including alveolar recruitment maneuvers in patients with
ARDS [], or in patients without lung injury at risk of post-
operative respiratory complications aer major surgery [].
e predictive value of single-nucleotide polymorphisms in
RAGE gene (AGER) in the development of ARDS in at-risk
patients is currently under study by our team [].
Disease Markers
T : Biomarkers of acute respiratory distress syndrome organized by phases and pathways of lung injury.
Pathophysiologic feature of ARDS Biomarker References
Exudative phase
Epithelium damage
(i) Alveolar type cells RAGE [–]
HTI56 [, ]
(ii) Alveolar type cells Surfactant [, ]
KL- [, ]
(iii) Clara cells CC [, ]
Endothelium damage
Ang-, Ang- [, ]
ICAM- [, –]
Selectins [, ]
VEGF [, ]
vWF [–]
Lung matrix alteration
Laminin [, ]
Elastin/desmosine [, ]
MMPs [–]
Inammatory cascade
(i) Proinammatory
IL-𝛽/TNF-𝛼[, –, ]
IL- [, ]
IL- [–]
IL- [–]
(ii) Anti-inammatory
IL-RA [, ]
sTNF-RI/sTNF-RII [, , ]
IL- [–]
(iii) Additional markers
HMGB [, ]
LBP [, ]
NO [, , ]
CRP []
Albumin []
LDH []
Coagulation and brinolysis
PA- [, , –]
Protein C [, , ]
rombomodulin [, ]
Tissue factor [–]
Cell-free hemoglobin [, , , ]
Pulmonary vascular permeability
EF/PL ratio [, ]
Fibroproliferative phase
Endothelial proliferation
VEGF [, –]
Epithelial proliferation
KGF []
HGF [, , , ]
Epithelial apoptosis
Fas/FasL [, –]
Fibroblast proliferation
N-PCP-III [, , , , ]
Disease Markers
HTI56 . Human type I cell-specic membrane protein (HTI56)
is a -kDa glycosylated lung protein specic to the apical
membrane of human AT cells. HTI56 has biochemical char-
acteristics of an integral membrane protein []. Although
the precise functions of HTI56 remain unknown, HTI56 is
an analog to RTI40, a – kDa integral membrane protein
specic to the apical membrane of rat AT cells []. Patients
with ARDS had higher levels of HTI56 in both lung edema
uid and plasma as compared to patients with hydrostatic
lung edema [], but no study assessing the association
between HTI56 levels and other endpoints in patients with
ARDS (e.g., prognosis) has been published to date.
(b) Alveolar Type 2 Cells.ATcellshaveimportanthomeo-
static functions in the lung, including AFC and production
of alveolar surfactant (involved in lung compliance, keeping
thealveolusopen).ATcellsarealsoknownaskeymediators
of the epithelial repair process [].
Surfactant Proteins. Surfactant has a vital role in maintaining
the integrity of the alveolar-capillary interface. Its essential
function is to decrease surface tension into the alveoli, thus
stabilizing lung volume at low transpulmonary pressures.
Surfactant is composed of approximately % phospholipids,
% other lipids (cholesterol, triacylglycerol, and free fatty
acids), and % proteins. Four surfactant-associated proteins
(SP), designated SP-A, SP-B, SP-C, and SP-D, represent
approximately half of proteins composing surfactant. SP-A
and SP-D easily dissociate from lipids and are hydrosoluble.
eybelongtothelunginnateimmunesystem,thereby
enhancing phagocytosis of bacteria and virus. ey also
exert regulatory eects on AT cells. SP-B and SP-C are
small, extremely hydrophobic proteins that are important in
the formation of the surfactant monolayer in the terminal
airspaces and in the reduction of surface tension, thus
preventing end-expiratory alveolar collapse [].
Early observations in ARDS revealed a loss in surface
tension suggesting a functional loss of the surfactant proteins
[]. In a rst case report of three patients, the ratio
of plasma SP-B/SP-A was inversely associated with both
blood oxygenation and static respiratory system compliance,
suggesting that SP-B breaches the alveolocapillary barrier
more readily than SP-A and may therefore provide a more
sensitive marker of lung injury []. Plasma levels of SP-A
and SP-B are increased in patients with ARDS [] and in at-
risk patients [, ], whereas lower SP-A and SP-B levels
were found in the BAL uid of patients at risk for ARDS
prior to the onset of the clinical syndrome. SP-A and SP-B
levels remained low for as long as days in patients with
sustained ARDS. Interestingly, this decrease in BAL SP-A and
SP-B does not result simply from dilution of alveolar uids by
plasma entering the alveolar spaces, as SP-D levels remained
stable in parallel []. In a cohort of patients, reduced
pulmonary edema uid SP-D and elevated plasma SP-A at
the onset of ARDS were associated with poor prognosis [].
Nevertheless, in a study of patients from the ARDSNet
trial of low versus high end-expiratory pressure in ARDS
(ALVEOLI) as well as in patients enrolled in a randomized
trial of activated protein C for ARDS, plasma SP-D was not
associated with -day mortality or ventilator-free days [].
KL-6. Krebs von den Lungen- (KL-) is a human MUC
mucin that belongs to the high-molecular-weight glyco-
protein family. Aer the cleavage of S-S bond, KL- can
spread into the pulmonary epithelial lining uid. In the
normal lung, this glycoprotein can be predominantly found
in AT cells, and its expression is enhanced during AT
proliferation, regeneration, or injury, thus representing an
attractive biomarker in ARDS. Plasma KL- is elevated in
ARDSpatientsandcorrelateswithlunginjuryandmortality
[, –]. Plasma levels of SP-D and KL- increase over
time in patients with ARDS and may represent biological
markers of ventilator-associated lung injury because their
increase is attenuated by lung-protective ventilation [].
(c) CC16. Clara cell protein (CC) is a . kDa homod-
imeric protein that is abundantly secreted in airways by the
nonciliated bronchiolar Clara cells. Clara cells are devoted
to the protection of the respiratory tract against toxic
inhaled agents, the repair of damaged epithelium, xenobiotics
detoxication, and the secretion of proteins with important
biological activities. CC is highly expressed in the epithelial
lining uid, with antioxidant/inammatory roles, notably by
modulating the production and/or activity of phospholipase-
A, interferon-𝛾, and tumor necrosis factor-𝛼[]. Available
studies found contradictory, inconclusive ndings during
ARDS. Although higher levels of CC are associated with
lung injury and inammation in some experimental and
clinical studies, patients with ARDS had lower plasma and
pulmonaryedemauidlevelsofCCthanpatientswith
acute cardiogenic pulmonary edema, and no correlation was
found between CC and prognosis. So many conicting
ndings do not currently support the association of CC
with the diagnosis or prognosis of ARDS.
(2) Vascular Endothelium.Vascularendothelialinjuryis
characterized by the disruption of cell components leading
to increased microvascular permeability and alveolar edema.
Endothelial injury is mainly driven by the activation of
inammation and coagulation cascades. e activation of
endothelial cells by circulating mediators leads to increased
expression of cell surface molecules that are important
mediators of leukocyte adhesion and contribute to leuko-
cyte accumulation and transmigration []. Activated lym-
phocytes can also release mediators in microvessels that
increase vascular permeability. Along with these leukocyte
signals, inammatory mediators such as tumor necrosis fac-
tor (TNF), thrombin, and vascular endothelial growth factor
(VEGF) disrupt endothelial-cadherin bonds and contribute
to the vascular leak underlying edema formation in ARDS.
Platelets also contribute to endothelial injury through the
releaseofcytokinesandthroughbrinclotting[].
(a) Angiopoietin. Angiogenic agents, along with VEGF and
angiopoietin- (Ang-), play key roles in vascular develop-
ment. VEGF stimulates the generation of new, immature,
Disease Markers
and leaky blood vessels whereas Ang- enhances angiogene-
sis, inducing vascular maturation, and decreases vascular per-
meability []. e most encouraging data result from recent
studies of angiopoietin- (Ang-), an endothelial protein that
has been studied extensively during sepsis []. Ang- has an
important role as it increases endothelial junction instability,
enhances vascular leak, naturally antagonizes Ang-, and, in
the absence of other angiogenic stimuli, induces vascular
regression and endothelial cell apoptosis. Both Ang- and
Ang- are ligands for the tyrosine kinase receptor Tie-
[], and a link between Ang- and inammation has
been reported []. erefore, such vascular growth factors
have been proposed as biomarkers for ARDS []. First,
two single-nucleotide polymorphisms within the Ang- gene
(rs and rs) were associated with the risk
of developing ARDS in trauma patients []. In addition,
Agrawal et al. found in a prospective study of patients
admitted to the intensive care unit (ICU) without ARDS
that higher levels of Ang- were signicantly associated
with increased development of ARDS []. In surgical ICU
patients, levels of Ang- were higher in patients with ARDS
than in those without the syndrome []. A higher Ang-
/Ang- ratio was also an independent predictor of mortality
in ARDS patients [, ], and patients with infection-
relatedARDSwhoseAng-levelsincreasedbetweenday
anddaydoubledtheiroddsofdeath,suggestingthatAng-
kinetics may be particularly valuable by reecting evolving
lung injury []. Finally, in a large study of patients
enrolled in the ARDSNet uid and catheter treatment trial,
baseline plasma levels of Ang- were associated with -day
mortality in patients with noninfectious ARDS, whereas this
association was not found in patients with infection as their
primaryARDSriskfactor.Basedonasecondaryanalysis
of two large studies, Calfee et al. further demonstrated that
indirect lung injury is characterized by a molecular pheno-
type consistent with more severe lung endothelial injury, as
assessed by plasma Ang-, and less severe epithelial injury
[].
(b) ICAM-1. e soluble intercellular adhesion molecule-
(sICAM-) is an inducible glycoprotein expressed on
the surface of vascular endothelial cells and other cells
(e.g., hematopoietic cells, AT cells) []. Under physiologic
conditions, sICAM- is not constitutively expressed or is
expressed at low levels in most tissues. During inammation,
andinresponsetostimulisuchasinterferon-𝛾(IFN-𝛾)or
interleukin- (IL-), levels of sICAM- are upregulated [].
Elevated levels have also been found in both plasma and
lung edema uid from patients with ARDS, as compared to
patients with hydrostatic lung edema []. In a multicenter
study, the increase of sICAM- from baseline to day
was associated with poor clinical outcome [], and in a
prospectivecohortofpatientswithARDS,baselineplasma
levelsofsICAM-werealsoassociatedwithmortality[].
In pediatric patients with ARDS, early elevated plasma levels
of sICAM- were associated with increased risks of death and
of prolonged mechanical ventilation []. In trauma patients,
higher plasma levels of sICAM- at baseline were correlated
with future development of multiple organ dysfunction syn-
drome (MODS) but not with the development of ARDS [–
].
(c) Selectins. Selectins are membrane-associated glycopro-
teins that mediate the adhesion of leukocytes and platelets
to vascular surface. L-selectin is mainly expressed by leuko-
cytes. P-selectin is rapidly redistributed from membranous
secretory granules to the surface of activated platelets and
endothelial cells []. E-selectin is expressed by cytokine-
activated endothelial cells. It has been shown that plasma
levels of such soluble adhesion molecules were markedly
higher in nonsurvivors among critically ill patients and that
they were negatively correlated with lung function (e.g.,
PaO2/FiO2ratio) []. Other studies found that plasma P-
selectin was elevated in patients with ARDS, especially in
those who subsequently died, as compared with patients with
other pulmonary diseases or sepsis but without ARDS [].
Interestingly, patients with ARDS with chronic alcohol con-
sumption had elevated levels of soluble E-selectin in both the
plasma and epithelial uid consistent with altered endothe-
lial and alveolar-capillary function []. More recently, E-
selectin was measured in the plasma levels from individ-
uals admitted to the emergency department and who were
at-risk for developing ARDS, with higher E-selectin levels
beingassociatedwithbothARDSdevelopmentand-day
mortality []. Circulating soluble E-selectin levels were
elevated in pneumonia patients with ARDS, and plasma levels
decreased along with the treatment of pneumonia [].
(d) VEGF. One of the most extensively studied endothelial
markers in ARDS is VEGF, albeit its value as a biomarker
remains unclear. Vascular endothelial growth factors belong
to the platelet-derived growth factor supergene family. ey
play central roles in the regulation of angiogenesis and
lymphangiogenesis []. Alternative splicing of the VEGF
gene (p.) transcript leads to the generation of several
splice variants, or isoforms, with various sizes []. VEGF-A
is a – kDa glycoprotein acting as the major factor impli-
cated in angiogenesis. It binds to two tyrosine kinase (TK)
receptors, named VEGFR- (Flt-) and VEGFR- (KDR/Flk-
), and regulates endothelial cell proliferation, migration, vas-
cular permeability, secretion, and other endothelial functions
[]. e expression of VEGF in ARDS varies, depending
on the degree of epithelial and endothelial damage. Many
lung cells release VEGF, for example, AT cells, neutrophils,
alveolar macrophages, and activated T cells. us, VEGF
is potentially capable of having an eect on both alveolar
epithelial and endothelial barriers. Interestingly, overexpres-
sion of VEGF induces pulmonary edema in animal models
[]. In a single center study, plasma levels of VEGF were
increased in subjects with ARDS, compared to controls, and
elevated plasma VEGF as measured on day was associated
with mortality in patients with ARDS []. Nevertheless,
several studies suggest that plasma and alveolar VEGF may
help to predict the development of ARDS and its recovery.
WhereasplasmaVEGFisincreasedinARDSpatients,VEGF
levelsweredecreasedintheBALuidfromARDSpatients,
Disease Markers
as compared to controls [, , ]. In order to better
understand such dierences between plasma and alveolar
expression of VEGF, Ware et al. conducted a study with the
aim to determine whether changes in alveolar levels of VEGF
were specic to ARDS or not []: the authors found that
alveolar levels of VEGF were decreased in both patients with
ARDS and those with hydrostatic edema. e mechanisms
implicated in this alveolar decrease in VEGF during ARDS
might not depend on the degree of lung injury but rather on
the degree of alveolar ooding [].
(e) vWF.Earlystudiesofendothelialmarkersfocusedonvon
Willebrand Factor (vWF), a macromolecular antigen that is
produced predominantly by endothelial cells, and to a lesser
extent by platelets. In the setting of endothelial activation
or injury, vWF is released from preformed stocks into the
circulation[,].VWFhasbeeninvestigatedasabiological
marker of endothelial injury in patients both at-risk for ARDS
and with established ARDS [, ]. In a prospective study
of ICU patients with sepsis, patients with nonpulmonary
sepsis had higher levels of plasma vWF, with good predictive
and prognostic values for ARDS. Indeed, elevated plasma
levels of vWF had a sensitivity of % and a specicity of
% for the prediction of ARDS development in the setting
of nonpulmonary sepsis []. However, subsequent studies
in patients at-risk for ARDS did not conrm these ndings
[, , ]. In another study of patients with ARDS
enrolled in the National Heart, Lung, and Blood Institute
ARDS Network trial of lower tidal volume, nonsurvivors
hadhigherplasmalevelsofvWF,comparedtosurvivors
[]. Higher vWF levels were signicantly associated with
fewer organ failure-free days, suggesting that the degree of
endothelial activation and injury is strongly associated with
outcomes in ARDS; nevertheless, ventilator settings had no
impact on vWF levels in this study [].
( f) Lung Extracellular Matrix. e extracellular matrix (ECM)
forms the region of the lung situated between the alveo-
lar epithelium and the vascular endothelium. ECM plays
a mechanical role as it supports and maintains tissular
structures. ECM also represents a complex and dynamic
meshwork inuencing many biological cell functions such as
development, proliferation, and migration []. Collagens
are the main component of ECM, along with glycoproteins
and proteoglycans including hyaluronic acid.
Laminin. Laminins (LM) are ECM proteins with high molec-
ular weights that deposit in basal membranes. Laminins
areinvolvedincellprocessessuchascellularadhesion,
growth, and dierentiation []. Laminin- (LM-) plays an
importantroleincellmigrationandintheremodelingof
epithelial tissue. LM- is activated through its cleavage by
matrix metalloproteinases (MMPs), thus releasing a soluble
LN 𝛾 NH-terminal fragment (GF) that does not deposit
in the ECM and can therefore be detected in the peripheral
blood. In a small single center study, laminin was measured in
the plasma and lung edema uid from patients with ARDS,
with higher levels found in patients with ARDS as compared
to healthy volunteers []. Interestingly, nonsurvivors had
higher plasma levels of laminin, as measured days aer
ARDS onset, than survivors, and survivors had decreasing
levels of the marker over time, suggesting that its secretion
is suppressed during ARDS recovery.
Elastin/Desmosine. Elastin is another critical protein of the
ECM that gives the lung its elastic recoil ability. In adults,
elastin, which is expressed by lungs and other tissues, is
usually excreted in the urine. During lung epithelial and
endothelial injury, elastin can be broken down by proteases
such as neutrophil elastase []. Elastin breakdown results in
smaller fragments containing desmosine and isodesmosine
[]. In a large study of patients with ARDS, those
ventilated with lower tidal volumes had lower urine desmo-
sine levels, a nding that may reect reduced extracellular
matrix breakdown; however, no correlation with mortality
was found in patients with ARDS [].
MMPs. Matrix metalloproteinases (MMPs) are zinc-depend-
ent endopeptidases that are able to degrade almost all
extracellular matrix components. MMP-, a member of the
leukocyte-derived MMPs, contributes to the degradation,
turnover, and remodeling of the extracellular matrix digest-
ingtypeIcollagen[,].MMPsaremajoractorsin
almost all phases of the inammatory response, and their
function is highly regulated. At the tissue level, most impor-
tant inhibitors are the tissue inhibitors of metalloproteinases
(TIMPs). In fulminant inammation, the inhibitory capacity
of TIMPs may be overwhelmed, leading to excessive tissue
damage and adverse outcome []. Previous studies suggest
that MMPs may have an important role in ARDS, although
this role may be either harmful or benecial [, ]. In a
recent study, despite MMP- levels did not predict outcome in
ARDS patients, higher levels of TIMP- were independently
associated with increased -day mortality in a large group
of critically ill, mechanically ventilated patients []. ese
ndings are in contradiction to those from a study in
pediatric ARDS patients in which higher MMP- and active
MMP- levels, as measured hours aer disease onset, were
associated with longer durations of mechanical ventilation
and fewer ventilator-free days []. Elevated MMP-, MMP-
, and MMP- in the BAL uid from ARDS patients were
associated with patterns of acute inammation but with poor
outcome []. Interestingly, MMP- and MMP- may be
protective against lung injury by cleaving transmembrane
receptor RAGE into sRAGE, thus regulating RAGE activation
by its ligands [, ].
3.1.2. Inammatory Cascades. During ARDS, inammatory
responses can either be related to an ongoing primary
infectious stimulus such as pneumonia or to systemic inam-
mation, such as in sepsis or in pancreatitis []. e inam-
matory cascade involves inammatory cells and the release
of inammatory mediators, as driven by a complex network
of cytokines. A comparison between blood and alveolar
cytokines suggests that most inammatory mediators orig-
inate from the lung []. Alarmins, or damage-associated
molecular patterns (DAMPs), are released by dead cells or
local inammatory cells (e.g., alveolar macrophages). ey
Disease Markers
activate and recruit immune cells via binding to dierent
receptors, such as TLR, IL- receptor (IL-R), or RAGE,
thereby initiating and perpetuating multiple proinamma-
tory pathways [, ].
Regulation of the inammatory response is a complex
process that requires interplay between several immune
mediators []. Both pro- and anti-inammatory biomark-
ers have been studied in ARDS.
(1) Proinammatory Cytokines
(a) IL-1𝛽and TNF-𝛼.IL-𝛽and TNF-𝛼are the most biolog-
ically potent cytokines secreted by activated macrophages in
theearlyphaseofARDS.eycausethereleaseofavariety
of proinammatory chemokines such as monocyte chemo-
tactic protein- (MCP-), macrophage inammatory protein-
𝛼(MIP-𝛼), IL-, and IL- with subsequent recruitment
of inammatory cells into the air spaces, alteration of the
endothelial-epithelial barrier permeability, and impairment
of uid transport leading to alveolar edema []. TNF-𝛼also
promotes lung edema indirectly, through the production of
reactive oxygen species (ROS) and a decreased expression
of epithelial sodium (ENaC) and Na+-K+-ATPase channels
[]. Finally, TNF-𝛼,apotentchemoattractantforbrob-
lasts, is a promoter of lung brosis in experimental studies
[, ]. Interestingly, the ratio of BAL to serum levels
of both TNF-𝛼and IL-𝛽is typically high, suggesting that
such cytokines may originate from the lung in the setting
of ARDS []. Persistent elevation of plasma and BAL IL-
𝛽is associated with worse outcome [, ]. Both TNF-
𝛼and IL-𝛽areelevatedintheplasmaandBALuidfrom
patients at risk of and with ARDS [, ] and associated
with mortality [].
(b) IL-18. Inammasomes are intracellular macromolecular
complexes that serve as platforms for the activation of the
proinammatory enzyme caspase-, which in turn cleaves
pro-IL-𝛽and pro-IL- into IL-𝛽and IL- []. ese
inammasome-activated cytokines play central roles in the
propagation of the acute inammatory response. IL- and
caspase-playcriticalrolesinthedevelopmentoflung
injury, and higher levels of IL- are correlated with disease
severity and mortality in patients with ARDS []. Among
patients with acute respiratory failure, those with ARDS
had signicantly higher serum levels of IL-, and serum IL-
was signicantly higher in nonsurvivors [].
(c) IL-6. IL- is produced by a wide range of cells including
monocytes/macrophages, endothelial cells, broblasts, and
smooth muscle cells in response to stimulation by endotoxin,
IL-𝛽,andTNF-𝛼[]. IL- is one of the most important
mediators of fever and is critical for B-cell dierentiation and
maturation with secretion of immunoglobulins, cytotoxic T
cell dierentiation, macrophage and monocyte function, and
production of acute phase proteins. Although IL- activates
both proinammatory and anti-inammatory mechanisms,
IL- primarily correlates with a proinammatory prole
during the early phase of ARDS. Plasma IL- increases early
in patients at risk of developing ARDS []. IL- is elevated in
both plasma and BAL uid during ARDS [] and correlates
with mortality [, ].
(d) IL-8. IL- is a proinammatory cytokine with a role in
neutrophil/monocyte chemotaxis and neutrophil apoptosis
inhibition. High plasma and BAL levels of IL- are found early
duringARDSandpredictoutcome[].However,previous
studies did not support such ndings [, ]. In a recent
monocenter study of patients, only baseline IL- (among
other biomarkers) was associated with the development of
multiorgan failure, even aer adjustment for other relevant
variables[].Also,severalstudieshaveevaluatedtherole
of the anti-IL- autoantibody/IL- immune complexes in
ARDS, a pathway that could lead to the identication of novel
biomarkers and therapeutic targets [, –].
In a recent study, Calfee et al. used latent class analysis
to integrate both clinical and biological data to identify
two ARDS endotypes in an analysis of , patients from
two ARDSNet trials (ARMA and ALVEOLI) []. A rst
endotype was categorized by more severe inammation, as
assessed by both IL- and IL- levels, and worse clinical
outcomes, whereas a second endotype had less inammation,
lessshock,andbetterclinicaloutcomes.Basedonthedata
from the ALVEOLI trial, a “proinammatory” endotype
was associated with higher mortality and better response to
higher levels of positive end-expiratory pressure [].
(2) Anti-Inammatory Cytokines.einammatoryresponse
is also strongly inuenced by anti-inammatory systems,
including nonspecic (e.g., -macroglobulin, IL-) and
specic (e.g., IL- receptor antagonist (IL-RA) antagonists,
soluble IL- receptor II (sIL-RII), soluble TNF receptor
I (sTNF-RI), and soluble TNF receptor II (sTNF-RII)) of
proinammatory cytokines.
(a) IL-1RA. Circulating IL-RA levels are increased but do
not predict the development of ARDS in at-risk patients
[]. Studies of gene expression in alveolar macrophages
and circulating leukocytes from healthy control subjects and
patients with ARDS revealed that sIL-RII may be valuable as
a biomarker because of increased levels in both the lung and
circulation during ARDS [].
(b) sTNF-RI/sTNF-RII. Soluble TNF-𝛼receptors (sTNF-R)
I and II can bind TNF and compete with its binding to
the cellular receptor, thus reducing its bioavailability. Soluble
TNF-RI and TNF-II are associated with morbidity and
mortality in patients with ARDS [], and a strategy of low
tidal volume ventilation is associated with decreased sTNF-
RI levels []. Trauma-associated ARDS diers clinically and
biologically from ARDS due to other clinical disorders, with
lower levels of sTNF-RI patients with trauma as a primary
cause of ARDS [, ].
(c) IL-10. Interleukin- (IL-) is an anti-inammatory
cytokine that is produced by several cells including B lym-
phocytes, monocytes, and alveolar macrophages []. Aside
from inhibiting the production of IL- and TNF-𝛼,IL-
Disease Markers
upregulates TNF receptors [] and stimulates the produc-
tion of the naturally occurring IL-RA and the releas e of sTNF
receptors []. IL- inhibits the production of proinam-
matory mediators by alveolar macrophages involved during
ARDS []. ARDS patients have lower plasma and BAL
levels of IL- than at-risk patients who did not develop the
syndrome []. Higher baseline IL- levels were associated
with higher morbidity and mortality [].
(3) Additional Markers. Other markers with potential clinical
importance in ARDS-associated inammation have been
identied as putative biomarkers during ARDS.
High mobility group box nuclear protein (HMGB) is a
DNA nuclear binding protein that is secreted by immune cells
including monocytes and macrophages. HMGB increases
early aer severe trauma and correlates with systemic inam-
matory response and development of ARDS []. Alveolar
andplasmalevelsofHMGB(asmeasuredinthearterialor
central venous blood) are elevated in patients with ARDS and
associated with outcome []. In patients with ARDS,
plasma levels of HMGB were also higher in nonsurvivors
and correlated with levels of sRAGE [].
Lipopolysaccharide binding protein (LBP) is an acute
phase protein that is correlated with lung inammation
during ARDS []. More recently, it has also been demon-
stratedthatserumlevelsofLBPwerestronglyassociatedwith
increased mortality and the development of ARDS in patients
with severe sepsis [].
Nitric oxide (NO) is a marker of oxidative stress that
has also been investigated as a marker of ARDS. In patients
with persistent ARDS, higher levels of nitric oxide and
of its end-products (e.g., nitrotyrosine) are associated with
mortality []. In contrast, higher urine NO levels were
strongly associated with better clinical outcomes including
mortality and ventilator-free days in patients enrolled in the
ARDSNet low tidal volume trial []. Extracellular citrulline,
theeectiveprecursorofNO,islowerintheplasmafrom
patients with severe sepsis and lower plasma citrulline is
associated with the presence of ARDS []. Mechanisms
involved in the regulation of lung injury by NO-dependent
pathways remain unknown.
Although C-reactive protein (CRP) is widely considered
asamarkerofsystemicinammation,higherlevelsofCRP
areassociatedwithbetteroutcomeamongpatientswith
ARDS []. Nevertheless, a recent study found that albumin
levels, rather than CRP, may help to predict and monitor the
severity and course of ARDS in febrile critically ill patients
with ARDS or at risk for the syndrome []. In the same
study, levels of lactate dehydrogenase (LDH) predicted -
day mortality but were not correlated with severity [].
3.1.3. Coagulation and Fibrinolysis. During early ARDS, acti-
vation of the inammatory cascades results in the activation
of the coagulation system, which in turn can inuence
inammatory responses by aecting the expression of various
cytokines such as IL-, IL-, and IL-. Activation of coagula-
tion pathways induces migration of inammatory cells into
alveoli through the endothelial and epithelial barriers and
generates thrombin formation. In addition, proinammatory
events may also inhibit brinolysis and induce platelet acti-
vation [].
Extravascular brin deposition, when localized predomi-
nantly in the alveolar compartment, is found in several acute
inammatory lung diseases, and enhanced alveolar procoag-
ulant activity is reported in ARDS patients []. Fibrin depo-
sition may be benecial for gas exchange by sealing leakage
sites when lung capillary endothelial and epithelial barriers
are disrupted. Nevertheless, brin alveolar deposition may
be harmful since it can lead to activation of neutrophils
and broblasts, endothelial injury, loss of surfactant activity
favoring alveolar collapse, impaired alveolar uid clearance,
and thrombotic obstruction of the microcirculation [].
(1) PAI-1. e balance between activation of coagulation
and activation of brinolysis is an important determinant
of the amount and duration of brin deposition during
lung injury. Plasminogen activator (PA) and plasminogen
activator inhibitor- (PAI-) regulate brinolysis through the
conversion of plasminogen to plasmin, a brinolytic enzyme.
PA- is a major endogenous inhibitor of PA. Both PA and PA-
are secreted by various cells including macrophages, brob-
lasts, and lung endothelial and epithelial cells []. During
ARDS, alveolar epithelial cells and activated macrophages
overexpress PAI-, thus contributing to decreased alveolar
brinolytic activity. Nevertheless, the value of PAI- as
a biomarker in ARDS remains controversial. PAI- levels
were higher in patients with ARDS than in patients with
hydrostatic lung edema [], and higher PAI- levels are
associated with mortality and higher durations of mechanical
ventilation in patients with ARDS [, ]. In a large Finnish
study, PAI- levels were not correlated with mortality or
development of ARDS in critically ill patients under mechan-
ical ventilation, but low baseline plasma levels of the soluble
urokinase plasminogen activator receptor (suPAR) were pre-
dictiveofsurvival[].Inasecondaryanalysisoftwolarge
randomized controlled trials, PAI- was associated with lung
injury (as dened as decreased oxygenation index) but not
with mortality []. Nevertheless, in another study of patients
from the ARDSNet ARMA study, higher levels of PAI-
were independently associated with higher mortality and
clinical outcomes, including organ failure []. However,
this association between PAI- levels and the development of
multiorgan failure was not conrmed in a recent study of
ARDS patients [].
(2) Protein C. Protein C system is an important endogenous
regulator of coagulation and brinolysis. Protein C is synthe-
sized by the liver and circulates as an inactive compound.
It is transformed to its active form on cell surface by the
thrombomodulin- (TM-) thrombin complex [, ]. e
endothelial cell protein C receptor (EPCR) is another cell
surfaceproteinthatcanfurtherenhanceproteinactivation
by binding the TM-thrombin complex []. In addition
to suppressing thrombin formation, activated protein C has
anti-inammatory properties such as decreasing the levels
of proinammatory cytokines []. Protein C can improve
endothelial permeability and exert antiapoptotic eects via
Disease Markers
p pathways []. Activated protein C can also inactivate
PAI-, thus promoting brinolysis []. Plasma protein C
was signicantly lower in patients with ARDS as compared
to controls, and it was associated with worse clinical out-
comes, including higher hospital mortality, shorter duration
of unassisted ventilation, and increased risk of multiple organ
failure []. In a larger cohort of patients with early ARDS,
lowplasmalevelsofproteinCwereagainassociatedwith
mortality and adverse clinical outcomes []. Decreased
levels of protein C during ARDS suggest a link between
hypercoagulability and mortality.
(3) rombomodulin. rombomodulin (TM) is a mul-
tidomain transmembrane-bound glycoprotein found on the
surface of endothelial cell. Its main role is to neutralize the
procoagulant eects of thrombin and accelerate activation
of protein C. In addition to its membrane-bound form, TM
also exists as a circulating soluble isoform in the plasma.
In patients with ARDS, levels of soluble thrombomodulin
(sTM) are higher in the pulmonary edema uid than in
plasma [], suggesting an alveolar source, but no correlation
was found between plasma sTM and the development of
ARDS, yet higher levels of sTM were observed in patients
at high risk for ARDS. In patients with established ARDS,
higher plasma and alveolar levels of sTM were correlated
with severity of illness and multiple organ failure []. In
alargeranalysisofpatients,elevatedlevelsofplasma
sTM were associated with increased mortality thus possibly
reecting an increased degree of inammation and both lung
and systemic endothelial damage [].
(4) Tissue Factor (TF). Tissue factor (TF) is a kDa trans-
membrane glycoprotein that is the most potent stimulator of
the extrinsic coagulation cascade. TF initiates the coagulation
cascade by binding and allosterically activating coagulation
factor VIIa. e resulting TF-VIIa complex binds the sub-
strate coagulation factor X via multiple interactions along
an extended interface to produce the TF-VIIa-X complex.
is complex leads eventually to thrombin formation and
brin deposition []. Levels of TF in lung edema uid are
higher than plasma levels in patients with ARDS, supporting
a lung origin for TF in this setting, and both plasma and
alveolar levels of TF are higher in ARDS patients as compared
to patients with hydrostatic edema []. Notably, patients
withsepsis-inducedARDSmayhavehigherlevelsofTFas
compared to patients without ARDS [].
(5) Cell-Free Hb. Levels of cell-free hemoglobin (Hb) are
higher in the air space of ARDS patients as compared
to critically ill patients with hydrostatic lung edema [].
Instillation of red blood cells or cell-free hemoglobin causes
lung injury in rats [] and intra-alveolar hemorrhage is
associated with high levels of intra-alveolar cell-free Hb,
more severe lung injury, and increased lipid peroxidation
in the lung from mice with tissue factor deciency [].
Precise cellular and molecular mechanisms by which cell-
free hemoglobin in the air space could mediate or potenti-
ate ARDS are currently under investigation []. Cell-free
Hbcouldactivatechemokinereleasewithinthelung,and
decompartmentalization of hemoglobin is likely to provide a
signicant proinammatory stimulus in the setting of diuse
alveolar damage and hemorrhage during ARDS [].
3.1.4. EF/PL Protein Ratio. e pathophysiology of ARDS
includes disruption of several physical barriers including
endothelial and epithelial cell layers, the basement mem-
brane, and the extracellular matrix, resulting in increased pul-
monary microvascular permeability. e pulmonary edema
uid-to-plasma protein (EF/PL) ratio is a rapid, safe, and
noninvasive measure of alveolar-capillary membrane per-
meability. e EF/PL ratio was rst proposed as a tool to
determine the etiology of acute pulmonary edema []. More
recently,inalargestudyofcriticallyillpatients,Wareetal.
demonstrated that the EF/PL ratio had an excellent discrimi-
native value in distinguishing ARDS from hydrostatic edema
and was strongly associated with clinical outcomes. Using a
cuto of ., the EF/PL ratio had a sensitivity of % and a
specicity of % for the diagnosis of ARDS [].
3.2. Fibroproliferative Phase of ARDS. In some patients,
important and persistent accumulation of macrophages,
brocytes, broblasts, and myobroblasts in the alveolar
compartment leads to excessive deposition of ECM com-
ponents including bronectin and collagen types I and III,
among other proteins. An imbalance between probrotic and
antibrotic mediators may subsequently drive this bropro-
liferative response []. Growth factors play a major role
in the resolution of ARDS []. Lung endothelial repair is
promoted by vascular endothelial growth factor (VEGF).
A variety of growth factors promote repair of the alveo-
lar epithelium including keratinocyte growth factor (KGF),
hepatocyte growth factor (HGF), broblast growth factor
(FGF), and transforming growth factor-𝛼(TGF-𝛼)[].Two
major pathways with opposite eects involve growth factors
during ARDS: tyrosine kinase receptor mediation (e.g., KGF,
HGF, FGF, and VEGF) and serine-threonine kinase receptors
such as TGF-𝛽,whichtendtohaveopposedeectonthe
upregulation that occurs when the tyrosine kinase receptor
pathway is involved [, , ].
3.2.1. Endothelial Proliferation. Novel evidence points to a
potentialroleofVEGFinpromotingrepairofthealveolar-
capillary membrane during recovery from ARDS, and under-
standingtheroleofVEGFinthisdiseaseprocesscould
be crucial for developing new therapeutic strategies [,
]. In the lung, VEGF is produced primarily by epithelial
cells; it increases microvascular permeability [] but has an
important role also during the repair phase by stimulating
endothelial cell proliferation and survival [, ]. e
levels of VEGF are increased in plasma from patients with
ARDS but are decreased in BAL uid, compared to healthy
controls; subsequently, BAL levels of VEGF increase during
the resolution of lung injury [, , ].
3.2.2. Epithelial Proliferation and Apoptosis. KGF, also known
as FGF-, is a potent mitogenic factor for alveolar epithelial
cells that is primarily produced by broblasts and other
Disease Markers
cellssuchasTlymphocytes.KGFregulatestransepithelial
transport of sodium by stimulating the epithelial channel
Na+-K+-ATPase in alveolar epithelial cells [].
HGF is a nonspecic mitogen secreted by broblasts,
alveolar macrophages, endothelial cells, and epithelial cells.
Several animal and human studies suggest that KGF and HGF
could protect the alveolar space against injury and could
facilitate the repair of alveolar structures aer injury [, ,
].KGFlevelscouldbemeasuredintheBALfrompatients
with ARDS but not in the BAL from those without ARDS; in
addition, BAL KGF was associated with poor prognosis [].
Elevated HGF levels were also associated with outcome [].
In patients with ARDS and patients with hydrostatic
lung edema, HGF and KGF were proven biologically active
in the edema uid of patients with ARDS, and higher levels
ofHGFwereassociatedwithmortalityinthesepatients[].
Apoptosisofalveolarepithelialcellsisamajorphe-
nomenon in the initiation and perpetuation of lung injury
[]. e Fas/FasL system plays an important role in the
regulation of cell life and death through its ability to initiate
apoptosis []. is system combines the cell membrane
surface receptor Fas (CD) and its natural ligand FasL
(CDL). Membrane-bound FasL mediates lymphocyte-
dependent cytotoxicity, clonal deletion of alloreactive T cells,
and activation-induced suicide of T cells []. Its soluble
form (sFasL) results from cleavage of membrane FasL by
MMPs and induces apoptosis in susceptible cells [].
Apoptosis is induced when membrane-bound or soluble FasL
binds to Fas-bearing cells. By contrast, apoptosis is inhibited
when soluble Fas binds to either membrane-bound FasL or
sFasL thus preventing FasL from interacting with membrane-
bound Fas receptors []. In patients with ARDS, sFasL was
detectable in the lung before and aer the onset of clinically
dened ARDS, and nonsurvivors had signicantly higher
BAL levels of sFasL on day as compared with survivors
[]. Both soluble Fas and soluble FasL were associated with
outcome and higher in the lung edema uid from patients
with ARDS, compared to control patients with hydrostatic
pulmonary edema []. Nevertheless, recent ndings sug-
gested limited role for Fas/FasL system and apoptosis in
airway epithelial cell death during ARDS [].
3.2.3. Fibroblast Proliferation. Pulmonary broblasts pro-
duce procollagen III peptide (PCP-III), that is, a precursor
of collagen. e NT part of procollagen III, resulting from
the enzymatic cleavage of procollagen by specic proteases in
the extracellular space, is considered as a marker of collagen
synthesis. Alveolar levels of N-PCP-III are higher in ARDS
patient, as compared with controls []. e elevation of
N-PCP-III in pulmonary edema uid begins within the rst
h of ARDS, that is, during the acute phase of increased
endothelial and epithelial permeability to protein, suggesting
that brosing alveolitis could begin very early in the course of
clinical ARDS []. In another study, high levels of N-PCP-
III were early predictors of poor outcome [, ]. More
recently, Forel et al. measured alveolar N-PCP-III in patients
with nonresolving ARDS, thus identifying patients who had
developed lung broproliferation []. Unfortunately, it
is still unknown whether N-PCP-III measurements could
be useful in selecting patients who would benet from
glucocorticoid therapy, among others, to reduce the ARDS-
associated lung brosis [].
4. Perspectives
4.1. Combining Biomarkers. Despite advances in the identi-
cation of biomarker candidate and better understanding of
ARDS pathogenesis, no single clinical or biological marker
reliably predicts clinical outcomes in ARDS. e combination
of clinical and biological marker is attractive in order to
improve the sensitivity and/or the specicity of the test,
especially through a recent approach aimed at measuring
biological markers that reect endothelial and epithelial
injury, inammation, and coagulation: vWF, SP-D, TNF-R,
IL-, IL-, ICAM-, protein C, and PAI- in patients
enrolled in the the ARDSNet trial of low versus high positive
end-expiratory pressure []. Clinical predictors predicted
mortality with an area under the ROC curve (AUC) of
., whereas a combination of these biomarkers and the
clinical predictors had an AUC of .. e best performing
biomarkers were the neutrophil chemotactic factor IL- and
SP-D, a product of AT cells [], supporting the concept
that acute inammation and alveolar epithelial injury are
important pathogenetic pathways in human ARDS. More
recently,apanelofbiomarkersoflungepithelialinjury
and inammation (SP-D, sRAGE, IL-, CC, and IL-)
provided excellent discrimination for diagnosis of ARDS
in patients with severe sepsis []. erefore, and beyond
their better diagnostic and prognostic values, the use of such
biomarker panels may be useful for selecting patients for
clinical trials that are designed to reduce lung epithelial injury
[]. Nevertheless, whether a therapeutic strategy based on
biomarker measurements would benet patient outcome has
never been investigated.
4.2. Lung Imaging as an ARDS Biomarker. Studies of lung
imaging during ARDS have revealed that adequate ventilator
settingsmayvaryamongpatientswiththesamesyndrome.
In a large study, gas and tissue distribution in the lungs of
ARDS patients were assessed using computed tomography
(CT) and compared to those of healthy volunteers []. Lung
morphology in ARDS is characterized by marked excess of
lung tissue associated with a major decrease in aerated lung
regions and in functional residual capacity. Some patients
with ARDS exhibit preserved aeration of the upper lobes
despitethepresenceofanoverallexcessoflungtissue(“focal”
ARDS), as opposed to other patients with more diuse loss
of aeration and excessive lung tissue (“diuse” or “nonfocal”
ARDS) [].
Lung morphology may inuence the response to pos-
itive end-expiratory pressure (PEEP), recruitment maneu-
vers (RM), prone position, and patient outcome [, ].
In a prospective study of nineteen patients with ARDS,
Constantin et al. found that lung morphology at zero end-
expiratory pressure could predict the response to a RM with
continuous positive airway pressure of cm H2Ofor
Disease Markers
seconds. Nonfocal morphology was associated with higher
lung recruitability and PaO2/FiO2was signicantly increased
bytheRM[,].Incontrast,patientswithfocallung
morphology were at risk of signicant hyperination during
the RM, with no improvement of arterial oxygenation.
It has also been hypothesized that the eects of PEEP
may depend on lung morphology. Puybasset et al. assessed
the responses to PEEP among patients with focal or nonfocal
ARDS []. e regional distribution of intrapulmonary
gas and lung tissue inuences the eects of PEEP in ARDS
patients: maximal alveolar recruitment, without evidence
of overdistension, was observed in patients with nonfocal
ARDS. Nevertheless, PEEP induced mild alveolar recruit-
ment in patients with focal ARDS, along with overdistension
of previously aerated lung regions.
Interestingly, phenotyping patients with ARDS based
on their lung morphology might be possible by measuring
plasma sRAGE with commercially available kits, even though
these ndings need further validation [, ]. However,
RAGE pathway is a promising candidate for subphenotyping
patients with ARDS, as it is believed to play a major role
in the mechanisms leading to AFC and their regulation
[]. Recent ndings that support a relationship between
impairedAFCandlungmorphologymaythereforellagap
in the full recognition of an ARDS phenotype based on lung
morphologythatcouldbelinkedtoanendotypeofimpaired
AFC and activated RAGE pathway [, , , , , ].
4.3. Biomarkers in ARDS: Can ey Improve Patient Care?
Biomarkers are broadly used in critically ill patients,
especially during inammatory and/or infectious diseases.
Biomarkers have been commonly dened as characteristics
that are objectively measured and evaluated as indicators
of normal biological processes, pathogenic processes, or
pharmacologic responses to therapeutic interventions [,
].Biomarkers provide a powerful approach to understand
a disease with multiple applications in observational and
analytic epidemiology, randomized clinical trials, screening,
and diagnosis or prognosis [].
Nevertheless, there are important technical attributes for
a relevant biomarker. First, the marker must be present in
peripheral body tissue and/or uid (e.g., blood, urine, saliva,
breath, or cerebrospinal uid); second, it must be easy to
detect or quantify in assays that are both aordable and
robust; and, third, its regulation should be associated as
specically as possible with damage of a particular tissue,
preferably in a quantiable manner. Prior to the widespread
use of a marker of interest, it is essential that validation and
conrmation of candidate biomarkers by robust statistical
methods are performed during biomarker discovery [].
Sensitivity and specicity are common quality parameters
for biomarkers. Sensitivity describes the probability of a
positive test in cases and specicity describes probability of
negative test in controls. An association between sensitivity
and specicity is represented in the ROC curve by graphing
sensitivity versus −specicity. Area under the ROC curve
(AUROC) is therefore a measure of performance of a marker.
ere is no absolute cuto value of AUROC for robustness of
a marker, but a minimum of . is required and values greater
than . are good particularly in a heterogeneous critically ill
patient population [, ].
To summarize, an ideal biomarker should indicate a
clear relationship with the pathophysiologic event, needs
to be reliable, reproducible, disease specic, and sensitive,
and should be sampled by simple methods and relatively
inexpensive, with little or no diurnal variation. During
ARDS, no single marker has been validated with all these
criteria to date, yet we believe that sRAGE may fulll all
prerequisitesofabiomarkerofARDS.First,plasmasRAGE
has good diagnostic and prognostic values [, , ,
, ]. Second, it is very well correlated with lung injury
severity and specic pathophysiologic features of ARDS, for
example, alveolar uid clearance and lung morphology [,
–, , ]. Previous studies of the predictive value of
early levels of sRAGE for the development of ARDS in a
general population of patients admitted to the emergency
department were negative [], but current research focuses
on both the kinetics of sRAGE and esRAGE and RAGE gene
polymorphisms as predictors of the development of ARDS
in at-risk critically ill patients []. Finally, there is some
evidence suggesting that monitoring sRAGE could inform,
at least partially, on therapeutic responses in patients with or
without ARDS [, , ]. If these data are conrmed by
future studies, such ndings would denitely help to reinforce
sRAGE a real biomarker of ARDS.
5. Conclusion
Biomarker research provides an important translational link
to our understanding of lung pathobiology. rough the
identication and testing of candidate biomarkers, we have
gained insight into the pathogenic importance of endothelial
and epithelial injury and have started to unravel the complex
pathways that contribute to endothelial and epithelial cell
dysfunction, inammation, brosis, and apoptosis in ARDS.
In addition, biomarker studies may help us to explore the
cellular and molecular mechanisms of various therapeutic
strategies for ARDS, and to better understand the potential
proinammatory eects of mechanical ventilation. Given
the clinical heterogeneity of patients with ARDS and the
complexity of the underlying pathobiology, it is unlikely that
a single biomarker will emerge for ARDS, as cardiac-specic
troponin did for myocardial infarction, but the develop-
ment of small biomarker panels reecting each important
lung injury pathway would provide valuable predictive and
prognostic information for both clinicians and investigators.
While biomarkers are currently not recommended for use
in clinical practice in ARDS, biomarker discovery may
hold signicant promise in order to develop and apply
targeted therapies, and to identify candidates for enrollment
in patient-tailored clinical trials of novel therapies for ARDS.
Disclosure
e funders had no inuence on the study design, conduct,
andanalysisoronthepreparationofthispaper.
Disease Markers
Conflict of Interests
No conict of interests, other sources of nancial support,
corporate involvement, patent holdings, and so forth are to
be declared for all authors.
Authors’ Contribution
Raiko Blondonnet was involved in the conception and design
of the review, in writing the paper, and in its revision prior
to submission. Jean-Michel Constantin was involved in the
conception and design of the review, in writing the paper,
and in its revision prior to submission. Vincent Sapin was
involved in the conception and design of the review, in
writing the paper, and in its revision prior to submission.
Matthieu Jabaudon takes responsibility for the content of
the paper and was involved in the conception and design of
the review, in writing the paper, and in its revision prior to
submission.
Acknowledgments
is work was supported by grants from the Auvergne
Regional Council (“Programme Nouveau Chercheur de la
R´
egion Auvergne”),thefrenchAgence Nationale de la
Recherche, and the Direction G´
en´
eraledel’OredeSoins
(“Programme de Recherche Translationnelle en Sant´
e”ANR-
-PRTS-).
References
[] V.M.Ranieri,G.D.Rubenfeld,P.T.ompsonetal.,“Acuteres-
piratory distress syndrome: the Berlin denition,” e Journal
of the American Medical Association,vol.,no.,pp.–
, .
[] L.B.WareandM.A.Matthay,“eacuterespiratorydistress
syndrome,” e New England Journal of Medicine,vol.,no.
, pp. –, .
[] C. Brun-Buisson, C. Minelli, G. Bertolini et al., “Epidemiology
and outcome of acute lung injury in European intensive care
units,” IntensiveCareMedicine,vol.,no.,pp.–,.
[] G.D.Rubenfeld,E.Caldwell,E.Peabodyetal.,“Incidenceand
outcomes of acute lung injury,” e New England Journal of
Medicine,vol.,no.,pp.–,.
[]D.W.Dowdy,M.P.Eid,C.R.Dennisonetal.,“Qualityof
life aer acute respiratory distress syndrome: a meta-analysis,”
Intensive Care Medicine, vol. , no. , pp. –, .
[] C.S.Calfee,M.D.Eisner,L.B.Wareetal.,“Trauma-associated
lung injury diers clinically and biologically from acute lung
injury due to other clinical disorders,” Critical Care Medicine,
vol.,no.,pp.–,.
[] C.S.Calfee,D.R.Janz,G.R.Bernardetal.,“Distinctmolecular
phenotypes of direct versus indirect ARDS in single and multi-
center studies,” Chest,vol.,no.,pp.–,.
[] P. Tejera, N. J. Meyer, F. Chen et al., “Distinct and replicable
genetic risk factors for acute respiratory distress syndrome
of pulmonary or extrapulmonary origin,” Journal of Medical
Genetics,vol.,no.,pp.–,.
[]M.A.Matthay,G.A.Zimmerman,C.Esmonetal.,“Future
research directions in acute lung injury: summary of a National
Heart, Lung, and Blood Institute Working Group,” American
Journal of Respiratory and Critical Care Medicine,vol.,no.
, pp. – , .
[] J. P. Reilly, S. Bellamy, M. G. S. Shashaty et al., “Heterogeneous
phenotypes of acute respiratory distress syndrome aer major
trauma,” Annals of the American oracic Society,vol.,no.,
pp. –, .
[] L. Puybasset, P. Cluzel, N. Chao et al., “A computed tomography
scan assessment of regional lung volume in acute lung injury,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
[] J.-M. Constantin, S. Grasso, G. Chanques et al., “Lung morphol-
ogy predicts response to recruitment maneuver in patients with
acute respiratory distress syndrome,” Critical Care Medicine,
vol. , no. , pp. –, .
[] C.S.Calfee,K.Delucchi,P.E.Parsons,B.T.ompson,L.B.
Ware, and M. A. Matthay, “Subphenotypes in acute respiratory
distress syndrome: latent class analysis of data from two
randomised controlled trials,” e Lancet Respiratory Medicine,
vol. , no. , pp. –, .
[] R.Marshall,G.Bellingan,andG.Laurent,“eacuterespira-
tory distress syndrome: brosis in the fast lane,” orax,vol.,
no.,pp.–,.
[]R.B.Henderson,J.A.R.Hobbs,M.Mathies,andN.Hogg,
“Rapid recruitment of inammatory monocytes is independent
of neutrophil migration,” Blood,vol.,no.,pp.–,
.
[] H. M. Marriott and D. H. Dockrell, “e role of the macrophage
in lung disease mediated by bacteria,” Experimental Lung
Research,vol.,no.,pp.–,.
[] L. J. M. Cross and M. A. Matthay, “Biomarkers in acute lung
injury: insights into the pathogenesis of acute lung injury,”
Critical Care Clinics,vol.,no.,pp.–,.
[] H. K. Gaggin and J. L. Januzzi, “Biomarkers and diagnostics in
heart failure,” Biochimica et Biophysica Acta (BBA)—Molecular
Basis of Disease, vol. , no. , pp. –, .
[] E. Christenson and R. H. Christenson, “e role of cardiac
biomarkers in the diagnosis and management of patients
presenting with suspected acute coronary syndrome,” Annals of
Laboratory Medicine,vol.,no.,pp.–,.
[] L. B. Ware, T. Koyama, Z. Zhao et al., “Biomarkers of lung
epithelial injury and inammation distinguish severe sepsis
patients with acute respiratory distress syndrome,” Critical Care,
vol. , no. , article R, .
[] M. A. Matthay, L. B. Ware, and G. A. Zimmerman, “e
acute respiratory distress syndrome,” e Journal of Clinical
Investigation,vol.,no.,pp.–,.
[] M. Shirasawa, N. Fujiwara, S. Hirabayashi et al., “Receptor for
advanced glycation end-products is a marker of type I lung
alveolar cells,” Genes to Cells,vol.,no.,pp.–,.
[] M. Neeper, A. M. Schmidt, J. Brett et al., “Cloning and
expression of a cell surface receptor for advanced glycosylation
end products of proteins,” e Journal of Biological Chemistry,
vol. , no. , pp. –, .
[] M. Jabaudon, E. Futier, L. Roszyk, V. Sapin, B. Pereira, and
J. Constantin, “Association between intraoperative ventilator
settings and plasma levels of soluble receptor for advanced
glycation end-products in patients without pre-existing lung
injury,” Respirology, vol. , no. , pp. –, .
[] L. G. Dobbs, R. F. Gonzalez, L. Allen, and D. K. Froh, “HTI,
an integral membrane protein specic to human alveolar type
Disease Markers
Icells,”Journal of Histochemistry & Cytochemistry,vol.,pp.
–, .
[] L. G. Dobbs, M. C. Williams, and R. Gonzalez, “Monoclonal
antibodies specic to apical surfaces of rat alveolar type I cells
bind to surfaces of cultured, but not freshly isolated, type II
cells,” Molecular Cell Research, vol. , no. , pp. –, .
[] I. W. Cheng, L. B. Ware, K. E. Greene, T. J. Nuckton, M. D.
Eisner, and M. A. Matthay, “Prognostic value of surfactant
proteins A and D in patients with acute lung injury,” Critical
Care Medicine,vol.,no.,pp.–,.
[] A. Agrawal, H. Zhuo, S. Brady et al., “Pathogenetic and
predictive value of biomarkers in patients with ALI and lower
severity of illness: results from two clinical trials,” American
Journal of Physiology—Lung Cellular and Molecular Physiology,
vol. , no. , pp. L–L, .
[] H. Sato, M. E. J. Callister, S. Mumby et al., “KL- levels are
elevated in plasma from patients with acute respiratory distress
syndrome,” European Respiratory Journal,vol.,no.,pp.–
, .
[] R. M. Determann, A. A. N. M. Royakkers, J. J. Haitsma et al.,
“Plasma levels of surfactant protein D and KL- for evaluation
of lung injury in critically ill mechanically ventilated patients,”
BMC Pulmonary Medicine,vol.,article,.
[] F. Broeckaert and A. Bernard, “Clara cell secretory protein
(CC): characteristics and perspectives as lung peripheral
biomarker,” Clinical and Experimental Allergy,vol.,no.,pp.
–, .
[] R. Cartin-Ceba, R. D. Hubmayr, R. Qin et al., “Predictive value
of plasma biomarkers for mortality and organ failure devel-
opment in patients with acute respiratory distress syndrome,”
Journal of Critical Care,vol.,no.,pp..e–.e,.
[] L. Eklund and P. Saharinen, “Angiopoietin signaling in the
vasculature,” ExperimentalCell Research,vol.,no.,pp.–
, .
[] C. S. Calfee, D. Gallagher, J. Abbott, B. T. ompson, and
M. A. Matthay, “Plasma angiopoietin- in clinical acute lung
injury: prognostic and pathogenetic signicance,” Critical Care
Medicine,vol.,no.,pp.–,.
[] M. L. Dustin, R. Rothlein, A. K. Bhan, C. A. Dinarello, and T.
A. Springer, “Induction by IL and interferon-gamma: tissue
distribution, biochemistry, and function of a natural adherence
molecule (ICAM-),” e Journal of Immunology,vol.,no.,
pp.–,.
[] R. P. McEver, “Selectins: lectins that initiate cell adhesion under
ow,” Current Opinion in Cell Biology,vol.,no.,pp.–,
.
[] D. Osaka, Y. Shibata, K. Kanouchi et al., “Soluble endothelial
selectin in acute lung injury complicated by severe pneumonia,”
International Journal of Medical Sciences,vol.,no.,pp.–
, .
[] M. Shibuya, “Vascular endothelial growth factor and its receptor
system: physiological functions in angiogenesis and pathologi-
cal roles in various diseases,” JournalofBiochemistry,vol.,
no. , pp. –, .
[] L.B.Ware,R.J.Kaner,R.G.Crystaletal.,“VEGFlevelsinthe
alveolar compartment do not distinguish between ARDS and
hydrostatic pulmonary oedama,” European Respiratory Journal,
vol.,no.,pp.–,.
[] L. B. Ware, M. D. Eisner, B. T. ompson, P. E. Parsons, and M.
A. Matthay, “Signicance of von Willebrand factor in septic and
nonseptic patients with acute lung injury,” American Journal of
Respiratory and Critical Care Medicine,vol.,no.,pp.–
, .
[] A. J. Reininger, “Function of von Willebrand factor in
haemostasis and thrombosis,” Haemophilia,vol.,supplement
, pp. –, .
[] M. S. Bajaj and S. M. Tricomi, “Plasma levels of the three
endothelial-specic proteins von Willebrand factor, tissue fac-
tor pathway inhibitor, and thrombomodulin do not predict the
development of acute respiratory distress syndrome,” Intensive
Care Medicine,vol.,no.,pp.–,.
[] J. Tzu and M. P. Marinkovich, “Bridging structure with function:
structural, regulatory, and developmental role of laminins,”
International Journal of Biochemistry and Cell Biology,vol.,
no. , pp. –, .
[] K. Torii, K.-I. Iida, Y. Miyazaki et al., “Higher concentrations
of matrix metalloproteinases in bronchoalveolar lavage uid of
patients with adult respiratory distress syndrome,” American
Journal of Respiratory and Critical Care Medicine,vol.,no.
,pp.–,.
[] E. G. Cleary and M. A. Gibson, “Elastic tissue, elastin and
elastin associated microbrils,” in Extracellular Matrix,vol.,
pp. –, Harwood Academic Publishers, Amsterdam, e
Netherlands, .
[] D. E. McClintock, B. Starcher, M. D. Eisner et al., “Higher urine
desmosine levels are associated with mortality in patients with
acute lung injury,” e American Journal of Physiology—Lung
Cellular and Molec ular Physiology,vol.,no.,pp.L–L,
.
[] J. H¨
astbacka, R. Linko, T. Tervahartiala et al., “Serum MMP-
and TIMP- in critically ill patients with acute respiratory
failure: TIMP- is associated with increased -day mortality,”
Anesthesia & Analgesia, vol. , no. , pp. –, .
[] A. H. Hergrueter, K. Nguyen, and C. A. Owen, “Matrix met-
alloproteinases: all the RAGE in the acute respiratory distress
syndrome,” e American Journal of Physiology—Lung Cellular
and Molecular Physiology,vol.,no.,pp.L–L,.
[] N. Yamakawa, T. Uchida, M. A. Matthay, and K. Makita,
“Proteolytic release of the receptor for advanced glycation end
products from in vitro and in situ alveolar epithelial cells,” e
American Journal of Physiology— Lung Cellular and Molecular
Physiology,vol.,no.,pp.L–L,.
[] T. Strowig, J. Henao-Mejia, E. Elinav, and R. Flavell, “Inam-
masomes in health and disease,” Nature,vol.,no.,pp.
–, .
[] H. Makabe, M. Kojika, G. Takahashi et al., “Interleukin- levels
reect the long-term prognosis of acute lung injury and acute
respiratory distress syndrome,” Journal of Anesthesia,vol.,no.
,pp.–,.
[] J. Cohen, “e immunopathogenesis of sepsis,” Nature,vol.,
no.,pp.–,.
[] D. Bouros, M. G. Alexandrakis, K. M. Antoniou et al., “e
clinical signicance of serum and bronchoalveolar lavage
inammatory cytokines in patients at risk for Acute Respirator y
Distress Syndrome,” BMC Pulmonary Medicine,vol.,article,
.
[] P. E. Parsons, M. A. Matthay, L. B. Ware, and M. D. Eisner,
“Elevated plasma levels of soluble TNF receptors are associated
with morbidity and mortality in patients with acute lung
injury,” e American Journal of Physiology—Lung Cellular and
Molecular Physiology, vol. , no. , pp. L–L, .
[] G.U.Meduri,S.Headley,G.Kohleretal.,“Persistentelevation
of inammatory cytokines predicts a poor outcome in ARDS:
Disease Markers
plasma IL-𝛽and IL- levels are consistent and ecient predic-
tors of outcome over time,” Chest, vol. , no. , pp. –,
.
[] P. E. Parsons, M. Moss, J. L. Vannice, E. E. Moore, F. A.
Moore, and J. E. Repine, “Circulating IL-ra and IL- levels
are increased but do not predict the development of acute
respiratory distress syndrome in at-risk patients,” American
JournalofRespiratoryandCriticalCareMedicine,vol.,no.
, pp. –, .
[]M.A.Kovach,K.A.Stringer,R.Buntingetal.,“Microarray
analysis identies IL- receptor type as a novel candidate
biomarker in patients with acute respiratory distress syndrome,”
Respiratory Research,vol.,article,.
[] R. G. Brower, P. N. Lanken, N. MacIntyre et al., “Higher versus
lower positive end-expiratory pressures in patients with the
acute respiratory distress syndrome,” e New England Journal
of Medicine, vol. , no. , pp. –, .
[] P. E. Parsons, M. D. Eisner, B. T. ompson et al., “Lower tidal
volume ventilation and plasma cytokine markers of inamma-
tion in patients with acute lung injury,” Critical Care Medicine,
vol.,no.,pp.–,.
[] D. F. Fiorentino, A. Zlotnik, T. R. Mosmann, M. Howard, and
A. O’Garra, “IL- inhibits cytokine production by activated
macrophages,” JournalofImmunology,vol.,no.,pp.–
, .
[] L. Armstrong and A. B. Millar, “Relative production of tumour
necrosis factor and interleukin in adult respiratory distress
syndrome,” orax,vol.,no.,pp.–,.
[] M. J. Cohen, K. Brohi, C. S. Calfee et al., “Early release of
high mobility group box nuclear protein aer severe trauma
in humans: role of injury severity and tissue hypoperfusion,”
Critical Care,vol.,no.,articleR,.
[] T.Nakamura,E.Sato,N.Fujiwara,Y.Kawagoe,S.Maeda,andS.-
I. Yamagishi, “Increased levels of soluble receptor for advanced
glycation end products (sRAGE) and high mobility group box
(HMGB) are associated with death in patients with acute
respiratory distress syndrome,” Clinical Biochemistry,vol.,
no. -, pp. –, .
[] T. R. Martin, G. D. Rubenfeld, J. T. Ruzinski et al., “Relationship
between soluble CD, lipopolysaccharide binding protein,
and the alveolar inammatory response in patients with acute
respiratory distress syndrome,” American Journal of Respiratory
and Critical Care Medicine,vol.,no.,pp.–,.
[] J.Villar,L.P
´
erez-M´
endez, E. Espinosa et al., “Serum lipopol-
ysaccharide binding protein levels predict severity of lung
injury and mortality in patients with severe sepsis,” PLoS ONE,
vol. , no. , Article ID e, .
[] C. Sittipunt, K. P. Steinberg, J. T. Ruzinski et al., “Nitric oxide
and nitrotyrosine in the lungs of patients with acute respiratory
distress syndrome,” AmericanJournalofRespiratoryandCritical
Care Medicine,vol.,no.,pp.–,.
[] L.B.Ware,J.A.Magarik,N.Wickershametal.,“Lowplasma
citrulline levels are associated with acute respiratory distress
syndrome in patients with severe sepsis,” Critical Care,vol.,
article R, .
[]E.K.Bajwa,U.A.Khan,J.L.Januzzi,M.N.Gong,B.T.
ompson, and D. C. Christiani, “Plasma C-reactive protein
levelsareassociatedwithimprovedoutcomeinARDS,”Chest,
vol. , no. , pp. –, .
[] S. H. Hoeboer, H. M. Oudemans-van Straaten, and A. B. J.
Groeneveld, “Albumin rather than C-reactive protein may be
valuable in predicting and monitoring the severity and course
of acute respiratory distress syndrome in critically ill patients
with or at risk for the syndrome aer new onset fever,” BMC
Pulmonary Medicine, vol. , article , .
[] I. R. Doyle, C. Hermans, A. Bernard, T. E. Nicholas, and
A. D. Bersten, “Clearance of clara cell secretory protein
(CC) and surfactant proteins A and B from blood in acute
respiratory failure,” American Journal of Respirator yand Critical
Care Medicine,vol.,no.,pp.–,.
[] L. B. Ware, J. A. Bastarache, and L. Wang, “Coagulation
and brinolysis in human acute lung injury—new therapeutic
targets?” Keio Journal of Medicine,vol.,no.,pp.–,
.
[]P.Prabhakaran,L.B.Ware,K.E.White,M.T.Cross,M.A.
Matthay, and M. A. Olman, “Elevated levels of plasminogen
activator inhibitor- in pulmonary edema uid are associated
with mortality in acute lung injury,” American Journal of
Physiology—Lung Cellular and Molecular Physiology,vol.,
no. , pp. L–L, .
[]L.B.Ware,X.Fang,andM.A.Matthay,“ProteinCand
thrombomodulin in human acute lung injury,” e American
Journal of Physiology—Lung Cellular and Molecular Physiology,
vol. , no. , pp. L–L, .
[] A. Sapru, C. S. Calfee, K. D. Liu et al., “Plasma soluble
thrombomodulin levels are associated with mortality in the
acute respiratory distress syndrome,” Intensive Care Medicine,
vol. , pp. –, .
[] A.Koutsi,A.Papapanagiotou,andA.G.Papavassiliou,“rom-
bomodulin: from haemostasis to inammation and tumouri-
genesis,” InternationalJournalofBiochemistryandCellBiology,
vol. , no. , pp. –, .
[] E. M. Conway, “rombomodul in and its role in inammation,”
Seminars in Immunopathology,vol.,no.,pp.–,.
[] W. Ruf and M. Riewald, “Tissue factor-dependent coagulation
protease signaling in acute lung injury,” Critical Care Medicine,
vol. , no. , pp. S–S, .
[] M. Xue, Z. Sun, M. Shao et al., “Diagnostic and prognostic utility
of tissue factor for severe sepsis and sepsis-induced acute lung
injury,” Journal of Translational Medicine,vol.,article,
.
[] J.A.Bastarache,S.C.Sebag,J.K.Cluneetal.,“Lowlevelsof
tissue factor lead to alveolar haemorrhage, potentiating murine
acute lung injury and oxidative stress,” orax,vol.,no.,
pp. –, .
[]S.Mumby,L.Ramakrishnan,T.W.Evans,M.J.D.Griths,
and G. J. Quinlan, “Methemoglobin-induced signaling and
chemokine responses in human alveolar epithelial cells,” e
American Journal of Physiology—Lung Cellular and Molecular
Physiology,vol.,no.,pp.L–L,.
[] A. Fein, R. F. Grossman, J. G. Jones et al., “e value of edema
uid protein measurement in patients with pulmonary edema,”
e American Journal of Medicine,vol.,no.,pp.–,.
[]L.B.Ware,R.D.Fremont,J.A.Bastarache,C.S.Calfee,
and M. A. Matthay, “Determining the aetiology of pulmonary
oedema by the oedema uid-to-plasma protein ratio,” European
Respiratory Journal,vol.,no.,pp.–,.
[] D.R.ickett,L.Armstrong,S.J.Christie,andA.B.Millar,
“Vascular endothelial growth factor may contribute to increased
vascular permeability in acute respiratory distress syndrome,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
Disease Markers
[] Y. Abadie, F. Bregeon, L. Papazian et al., “Decreased VEGF
concentration in lung tissue and vascular injury during ARDS,”
European Respiratory Journal,vol.,no.,pp.–,.
[] T. J. Desai and W. V. Cardoso, “Growth factors in lung develop-
ment and disease: friends or foe?” Respiratory Research,vol.,
article , .
[] B.Maitre,S.Boussat,D.Jeanetal.,“Vascularendothelialgrowth
factor synthesis in the acute phase of experimental and clinical
lung injury,” European Respiratory Journal,vol.,no.,pp.–
, .
[] Z. Borok, S. I. Danto, L. L. Dimen, X.-L. Zhang, and R.
L. Lubman, “Na+-K+-ATPase expression in alveolar epithelial
cells: upregulation of active ion transport by KGF,” American
Journal of Physiology—Lung Cellular and Molecular Physiology,
vol. , no. , pp. L–L, .
[]L.B.WareandM.A.Matthay,“Keratinocyteandhepatocyte
growth factors in the lung: Roles in lung development, inam-
mation, and repair,” e American Journal of Physiology—Lung
Cellular and Molecular Physiology,vol.,no.,pp.L–
L, .
[]V.Galani,E.Tatsaki,M.Baietal.,“eroleofapoptosisin
the pathophysiology of Acute Respiratory Distress Syndrome
(ARDS): an up-to-date cell-specic review,” Pathology Research
and Practice,vol.,no.,pp.–,.
[] R.C.Pires-Neto,M.M.B.Morales,T.Lancasetal.,“Expression
of acute-phase cytokines, surfactant proteins, and epithelial
apoptosis in small airways of human acute respiratory distress
syndrome,” Journal of Critical Care, vol. , no. , pp. .e–
.e, .
[] D. Talmor, T. Sarge, A. Legedza et al., “Cytokine release
following recruitment maneuvers,” Chest,vol.,no.,pp.
–, .
[]J.M.Walter,J.Wilson,andL.B.Ware,“Biomarkersinacute
respiratory distress syndrome: from pathobiology to improving
patient care,” Expert Review of Respiratory Medicine,vol.,no.
, pp. –, .
[] C. S. Calfee, K. Delucchi, P. E. Parsons, B. T. ompson, L. B.
Ware, and M. A. Matthay, “Latent class models identify two
subphenotypes in respiratory distress syndrome with dieren-
tial response to positive end-expiratory pressure,” Annals of the
American oracic Society,vol.,supplement,p.S,.
[] V. Newman, R. F. Gonzalez, M. A. Matthay, and L. G. Dobbs, “A
novel alveolar type I cell-specic biochemical marker of human
acute lung injury,” American Journal of Respiratory and Critical
Care Medicine,vol.,no.,pp.–,.
[] A. M. Schmidt, S. D. Yan, S. F.Yan, and D. M. Stern, “e biology
of the receptor for advanced glycation end products and its
ligands,” Biochimica et Biophysica Acta (BBA)—Molecular Cell
Research, vol. , no. -, pp. –, .
[] T. Uchida, M. Shirasawa, L. B. Ware et al., “Receptor for
advanced glycation end-products is a marker of type I cell
injury in acute lung injury,” American Journal of Respiratory and
Critical Care Medicine,vol.,no.,pp.–,.
[] M.T.Kuipers,T.vanderPoll,M.J.Schultz,andC.W.Wieland,
“Bench-to-bedside review: damage-associated molecular pat-
terns in the onset of ventilator-induced lung injury,” Critical
Care,vol.,no.,article,.
[] T. Uchida, M. Shirasawa, L. B. Ware et al., “Receptor for
advanced glycation end-products is a marker of type I cell
injury in acute lung injury,” American Journal of Respiratory and
Critical Care Medicine,vol.,no.,pp.–,.
[] C. S. Calfee, L. B. Ware, M. D. Eisner et al., “Plasma receptor for
advanced glycation end products and clinical outcomes in acute
lung injury,” orax, vol. , no. , pp. –, .
[] M.Jabaudon,E.Futier,L.Roszyketal.,“Solubleformofthe
receptor for advanced glycation end products is a marker of
acute lung injury but not of severes epsis in critically ill patients,”
Critical Care Medicine, vol. , no. , pp. –, .
[] M. Jabaudon, R. Blondonnet, L. Roszyk et al., “Soluble RAGE
predicts impaired alveolar uid clearance in acute respiratory
distress syndrome,” AmericanJournalofRespiratoryandCritical
Care Medicine,vol.,no.,.
[] M. D. Johnson, J. H. Widdicombe, L. Allen, P. Barbry, and L.
G. Dobbs, “Alveolar epithelial I cells contain transport proteins
and transport sodium, supporting an active role for type I cells
in regulation of lung liquid homeostasis,” Proceedings of the
National Academy of Sciences of the United States of America,
vol. , no. , pp. –, .
[] L. E. Hanford, J. J. Enghild, Z. Valnickova et al., “Purication
and characterization of mouse soluble receptor for advanced
glycation end products (sRAGE),” e Journal of Biological
Chemistry,vol.,no.,pp.–,.
[] C.Cheng,K.Tsuneyama,R.Kominamietal.,“Expressionpro-
ling of endogenous secretory receptor for advanced glycation
end products in human organs,” Modern Pathology,vol.,no.
, pp. –, .
[] J. Brett, A. M. Schmidt, S. D. Yan et al., “Survey of the
distribution of a newly characterized receptor for advanced
glycation end products in tissues,” e American Journal of
Patholog y,vol.,no.,pp.–,.
[]L.E.Hanford,C.L.Fattman,L.M.Schaefer,J.J.Enghild,
Z. Valnickova, and T. D. Oury, “Regulation of receptor for
advanced glycation end products during bleomycin-induced
lung injury,” American Journal of Respiratory Cell and Molecular
Biology,vol.,no.,pp.S–S,.
[]J.M.Englert,L.E.Hanford,N.Kaminskietal.,“Arolefor
the receptor for advanced glycation end products in idiopathic
pulmonary brosis,” e American Journal of Pathology,vol.,
no.,pp.–,.
[] A. M. Schmidt, S. D. Yan, S. F. Yan, and D. M. Stern, “e
multiligand receptor RAGE as a progression factor amplifying
immune and inammatory responses,” e Journal of Clinical
Investigation,vol.,no.,pp.–,.
[] M. Jabaudon, R. Blondonnet, L. Roszyk et al., “Soluble forms
and ligands of the re ceptor for advanced glycation end-products
in patients with acute respiratory distress syndrome: an obser-
vational prospective study,” PLoS ONE,vol.,no.,ArticleID
e, .
[] C. A. Downs, L. H. Kreiner, N. M. Johnson, L. A. Brown, and
M. N. Helms, “Receptor for advanced glycation end-products
regulates lung uid balance via protein kinase C-gp91phox
signaling to epithelial sodium channels,” American Journal of
Respiratory Cell and Molecular Biology,vol.,no.,pp.–,
.
[] R. Briot, J. A. Frank, T. Uchida, J. W. Lee, C. S. Calfee, and M. A.
Matthay, “Elevated levels of the receptor for advanced glycation
end products, a marker of alveolar epithelial type i cell injury,
predict impaired alveolar uid clearance in isolated perfused
human lungs,” Chest,vol.,no.,pp.–,.
[] J.-M. Constantin, “Impact of Inammation Biomarkers
on the Acute Respiratory Distress Syndrome (ARDS)
Denition,” (NCT), https://clinicaltrials.gov/ct/show/
NCT?term=constantin+clermont&rank=.
Disease Markers
[] M. Jabaudon, N. Hamroun, L. Roszyk et al., “Eects of a recruit-
ment maneuver on plasma levels of soluble RAGE in patients
with diuse acute respiratory distress syndrome: a prospective
randomized crossover study,” Intensive Care Medicine,vol.,
no.,pp.–,.
[] M. Jabaudon, “Predictive Values of Plasma Soluble RAGE Levels
and RAGE Polymorphisms for the Onset of Acute Respira-
tory Distress Syndrome in Critically Ill Patients (PrediRAGE
Study),” in: ClinicalTrials.gov, , http://clinicaltrials.gov/
show/NCT.
[] J. Bhattacharya and M. A. Matthay, “Regulation and repair of the
alveolar-capillary barrier in acute lung injury,” Annual Review of
Physiology,vol.,pp.–,.
[] I. Frerking, A. G¨
unther,W.Seeger,andU.Pison,“Pulmonary
surfactant: functions, abnormalities and therapeutic options,”
Intensive Care Medicine,vol.,no.,pp.–,.
[]D.G.Ashbaugh,D.B.Bigelow,T.L.Petty,andB.E.Levine,
“Acute respiratory distress in adults,” e Lancet, vol. , no. ,
pp.–,.
[] I. R. Doyle, A. D. Bersten, and T. E. Nicholas, “Surfactant
proteins-A and -B are elevated in plasma of patients with acute
respiratory failure,” American Journal of Respirator yand Critical
Care Medicine,vol.,no.,pp.–,.
[]K.E.Greene,S.Ye,R.J.Mason,andP.E.Parsons,“Serum
surfactant protein-A levels predict development of ARDS in at-
risk patients,” Chest, vol. , supplement , pp. S–S, .
[]A.D.Bersten,T.Hunt,T.E.Nicholas,andI.R.Doyle,
“Elevated plasma surfactant protein-B predicts development
of acute respiratory distress syndrome in patients with acute
respiratory failure,” American Journal of Respirator yand Critical
Care Medicine,vol.,no.,pp.–,.
[] A. Ishizaka, T. Matsuda, K. H. Albertine et al., “Elevation of KL-
, a lung epithelial cell marker, in plasma and epithelial lining
uid in acute respiratory distress syndrome,” e American
Journal of Physiology—Lung Cellular and Molecular Physiology,
vol. , no. , pp. L–L, .
[] N. Nathani, G. D. Perkins, W. Tunniclie, N. Murphy, M. Manji,
andD.R.ickett,“KerbsvonLungrenantigenisamarkerof
alveolar inammation but not of infection in patients with acute
respiratory distress syndrome,” Critical Care,vol.,articleR,
.
[] T.Kondo,N.Hattori,N.Ishikawaetal.,“KL-concentration
in pulmonary epithelial lining uid is a useful prognostic
indicator in patients with acute respiratory distress syndrome,”
Respiratory Research, vol. , article , .
[] R.Lucas,A.D.Verin,S.M.Black,andJ.D.Catravas,“Regulators
of endothelial and epithelial barrier integrity and function in
acute lung injury,” Biochemical Pharmacology,vol.,no.,pp.
–, .
[] A. Binnie, J. L. Tsang, and C. C. dos Santos, “Biomarkers
in acute respiratory distress syndrome,” Current Opinion in
Critical Care,vol.,no.,pp.–,.
[] S.Tsigkos,M.Koutsilieris,andA.Papapetropoulos,“Angiopoi-
etins in angiogenesis and beyond,” Expert Opinion on Investiga-
tional Drugs,vol.,no.,pp.–,.
[] U. Fiedler, Y. Reiss, M. Scharpfenecker et al., “Angiopoietin-
sensitizes endothelial cells to TNF-𝛼and has a crucial role in
the induction of inammation,” Nature Medicine,vol.,no.,
pp. –, .
[] V. Bhandari and J. A. Elias, “e role of angiopoietin in
hyperoxia-induced acute lung injury,” Cell Cycle,vol.,no.,
pp.–,.
[] N. J. Meyer, M. Li, R. Feng et al., “ANGPT genetic variant
is associated with trauma-associated acute lung injury and
altered plasma angiopoietin- isoform ratio,” American Journal
of Respiratory and Critical Care Medicine,vol.,no.,pp.
–, .
[] A. Agrawal, M. A. Matthay, K. N. Kangelaris et al., “Plasma
angiopoietin- predicts the onset of acute lung injury in criti-
cally ill patients,” American Journal of Respiratory and Critical
Care Medicine,vol.,no.,pp.–,.
[] T. Wada, S. Jesmin, S. Gando et al., “e role of angiogenic
factors and their soluble receptors in acute lung injury (ALI)/
acute respiratory distress syndrome (ARDS) associated with
critical illness,” Journal of Inammation,vol.,article,.
[] T. Ong, D. E. McClintock, R. H. Kallet, L. B. Ware, M.
A. Matthay, and K. D. Liu, “Ratio of angiopoietin- to
angiopoietin- as a predictor of mortality in acute lung injury
patients,” Critical Care Medicine,vol.,no.,pp.–,
.
[] K. A. Roebuck and A. Finnegan, “Regulation of intercellular
adhesion molecule- (CD) gene expression,” Journal of Leu ko-
cyte Biology, vol. , no. , pp. –, .
[] E. R. Conner, L. B. Ware, G. Modin, and M. A. Matthay,
“Elevated pulmonary edema uid concentrations of soluble
intercellular adhesion molecule- in patients with acute lung
injury: biological and clinical signicance,” Chest,vol.,pp.
S–S, .
[] C.S.Calfee,M.D.Eisner,P.E.Parsonsetal.,“Solubleinter-
cellular adhesion molecule- and clinical outcomes in patients
with acute lung injury,” IntensiveCareMedicine,vol.,no.,
pp. –, .
[] P.Agouridakis,D.Kyriakou,M.G.Alexandrakisetal.,“e
predictive role of serum and bronchoalveolar lavage cytokines
and adhesion molecules for acute respiratory distress syndrome
development and outcome,” Respiratory Research,vol.,article
, .
[] H. R. Flori, L. B. Ware, D. Glidden, and M. A. Matthay, “Early
elevation of plasma soluble intercellular adhesion molecule- in
pediatric acute lung injury identies patients at increased risk of
death and prolonged mechanical ventilation,” Pediatric Critical
Care Medicine,vol.,no.,pp.–,.
[] A. Sousa, F. Raposo, S. Fonseca et al., “Measurement of
cytokines and adhesion molecules in the rst hours aer
severe trauma: association with severity and outcome,” Disease
Markers,vol.,ArticleID,pages,.
[] S. Gando, T. Kameue, N. Matsuda et al., “Combined activation
of coagulation and inammation has an important role in
multiple organ dysfunction and poor outcome aer severe
trauma,” rombosis and Haemostasis,vol.,no.,pp.–
, .
[] K. Xing, S. Murthy, W. C. Liles, and J. M. Singh, “Clinical utility
of biomarkers of endothelial activation in sepsis-a systematic
review,” Critical Care,vol.,articleR,.
[] J.Boldt,M.Wollbr
¨
uck, D. Kuhn, L. C. Linke, and G. Hempel-
mann, “Do plasma levels of circulating soluble adhesion
molecules dier between surviving and nonsurviving critically
ill patients?” Chest,vol.,no.,pp.–,.
[] F. Sakamaki, A. Ishizaka, M. Handa et al., “Soluble form of P-
selectin in plasma is elevated in acute lung injury,” American
JournalofRespiratoryandCriticalCareMedicine,vol.,no.,
pp.–,.
[] E.L.Burnham,M.Moss,F.Harris,andL.A.S.Brown,“Elevated
plasma and lung endothelial selectin levels in patients with
Disease Markers
acute respiratory distress syndrome and a history of chronic
alcohol abuse,” Critical Care Medicine,vol.,no.,pp.–
, .
[] K. Okajima, N. Harada, G. Sakurai et al., “Rapid assay for plasma
soluble E-selectin predicts the development of acute respiratory
distress syndrome in patients with systemic inammatory
response syndrome,” Tra nsla tion al Res e arc h,vol.,no.,pp.
–, .
[] A.R.L.MedfordandA.B.Millar,“Vascularendothelialgrowth
factor (VEGF) in acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS): paradox or paradigm?” orax,vol.
,no.,pp.–,.
[] M. Shibuya, “Dierential roles of vascular endothelial growth
factor receptor- and receptor- in angiogenesis,” Journal of
Biochemistry and Molecular Biology,vol.,no.,pp.–,
.
[] R.J.Kaner,J.V.Ladetto,R.Singh,N.Fukuda,M.A.Matthay,
and R. G. Crystal, “Lung overexpression of the vascular
endothelial growth factor gene induces pulmonary edema,”
American Journal of Respiratory Cell and Molecular Biology,vol.
, no. , pp. –, .
[] L. Azamrei, S. Gurzu, R. Solomon et al., “Vascular endothelial
growth factor: a possible mediator of endothelial activation in
acute respiratory distress syndrome,” Minerva Anestesiologica,
vol.,no.,pp.–,.
[]D.R.ickett,L.Armstrong,andA.B.Millar,“Arolefor
vascular endothelial growth factor in acute and resolving lung
injury,” American Journal of Respiratory and Critical Care
Medicine,vol.,no.,pp.–,.
[] A. C. A. Carvalho, S. M. Bellman, V. J. Saullo, D. Quinn, and W.
M. Zapol, “Altered factor VIII in acute respiratory failure,” e
New England Journal of Medicine, vol. , no. , pp. –,
.
[] D. B. Rubin, J. P. Wiener-Kronish, J. F. Murray et al., “Elevated
vonWillebrandfactorantigenisanearlyplasmapredictorof
acute lung injury in nonpulmonary sepsis syndrome,” Journal
of Clinical Investigation,vol.,no.,pp.–,.
[] M. Moss, L. Ackerson, M. K. Gillespie, F. A. Moore, E. E.
Moore, and P. E. Parsons, “Von willebrand factor antigen levels
are not predictive for the adult respiratory distress syndrome,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
[] A. K. Sabharwal, S. P. Bajaj, A. Ameri et al., “Tissue factor
pathway inhibitor and von Willebrand factor antigen levels in
adult respiratory distress syndrome and in a primate model
of sepsis,” American Journal of Respiratory and Critical Care
Medicine,vol.,no.I,pp.–,.
[] P. R. M. Rocco, C. Dos Santos, and P. Pelosi, “Lung parenchyma
remodeling in acute respiratory distress syndrome,” Minerva
Anestesiologica,vol.,no.,pp.–,.
[] B. C. Starcher, “Lung elastin and matrix,” Chest,vol.,no.,
supplement , pp. S–S, .
[] G. M. Albaiceta, A. Gutierrez-Fern´
andez, E. Garc´
ıa-Prieto et al.,
“Absence or inhibition of matrix metalloproteinase- decreases
ventilator-induced lung injury,” American Journal of Respirator y
Cell and Molecular Biology,vol.,no.,pp.–,.
[] S. E. G. Fligiel, T. Standiford, H. M. Fligiel et al., “Matrix
metalloproteinases and matrix metalloproteinase inhibitors in
acute lung injury,” Human Pathology,vol.,no.,pp.–,
.
[] M. Y. F. Kong, Y. Li, R. Oster, A. Gaggar, and J. P. Clancy,
“Early elevation of matrix metalloproteinase- and - in pedi-
atric ARDS is associated with an increased risk of prolonged
mechanical ventilation,” PLoS ONE,vol.,no.,ArticleID
e, .
[] A. Gonz´
alez-L´
opez and G. M. Albaiceta, “Repair aer acute
lung injury: molecular mechanisms and therapeutic opportu-
nities,” Critical Care,vol.,no.,article,.
[] M. Bhatia and S. Moochhala, “Role of inammatory mediators
in the pathophysiology of acute respiratory distress syndrome,”
Journal of Patholog y,vol.,no.,pp.–,.
[]L.A.DadaandJ.I.Sznajder,“Hypoxicinhibitionofalveolar
uid reabsorption,” in Hypoxia and the Circulation, pp. –,
Springer US, .
[]A.E.PostlethwaiteandJ.M.Seyer,“Stimulationofbroblast
chemotaxis by human recombinant tumor necrosis factor 𝛼
(TNF-𝛼)andasyntheticTNF-𝛼- peptide,” e Journal of
Experimental Medicine,vol.,no.,pp.–,.
[] P. F. Piguet, M. A. Collart, G. E. Grau, A.-P. Sappino, and P. Vas-
salli, “Requirement of tumour necrosis factor for development
of silica-induced pulmonary brosis,” Nature,vol.,no.,
pp. –, .
[] W.Y.Park,R.B.Goodman,K.P.Steinbergetal.,“Cytokine
balance in the lungs of patients with acute respiratory distress
syndrome,” American Journal of Respiratory and Critical Care
Medicine,vol.,no.,pp.–,.
[] J. Pugin, B. Ricou, K. P. Steinberg, P. M. Suter, and T. R. Martin,
“Proinammatory activity in bronchoalveolar lavage uids
from patients with ARDS, a prominent role for interleukin-,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
[]G.U.Meduri,G.Kohler,S.Headley,E.Tolley,F.Stentz,
and A. Postlethwaite, “Inammatory cytokines in the BAL of
patients with ARDS. Persistent elevation over time predicts
poor outcome,” Chest,vol.,no.,pp.–,.
[] R.Roten,M.Markert,F.Feihl,M.-D.Schaller,M.-C.Tagan,and
C. Perret, “Plasma levels of tumor necrosis factor in the adult
respiratory distress syndrome,” American Review of Respiratory
Disease,vol.,no.,pp.–,.
[] T. Dolinay, Y. S. Kim, J. Howrylak et al., “Inammasome-
regulated cytokines are critical mediators of acute lung injury,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
[] D. McClintock, H. Zhuo, N. Wickersham, M. A. Matthay, and
L. B. Ware, “Biomarkers of inammation, coagulation and
brinolysis predict mortality in acute lung injury,” Critical Care,
vol. , no. , article R, .
[] R. P. Baughman, K. L. Gunther, M. C. Rashkin, D. A. Keeton,
and E. N. Pattishall, “Changes in the inammatory response of
the lung during acute respiratory distress syndrome: prognostic
indicators,” American Journal of Respiratory and Critical Care
Medicine,vol.,no.,pp.–,.
[] H. Sch¨
utte,J.Lohmeyer,S.Rosseauetal.,“Bronchoalveolar
and systemic cytokine proles in patients with ARDS, severe
pneumonia and cardiogenic pulmonary oedema,” European
Respiratory Journal, vol. , no. , pp. –, .
[] A. Kurdowska, E. J. Miller, J. M. Noble et al., “Anti-IL-
autoantibodies in alveolar uid from patients with the adult
respiratory distress syndrome,” e Journal of Immunology,vol.
, no. , pp. –, .
[]A.Kurdowska,J.M.Noble,I.S.Grant,C.R.Robertson,C.
Haslett, and S. C. Donnelly, “Anti-interleukin- autoantibodies
Disease Markers
in patients at risk for acute respiratory distress syndrome,”
Critical Care Medicine,vol.,no.,pp.–,.
[]A.Krupa,H.Kato,M.A.Matthay,andA.K.Kurdowska,
“Proinammatory activity of anti-IL- autoantibody:IL- com-
plexes in alveolar edema uid from patients with acute lung
injury,” e American Journal of Physiology—Lung Cellular and
Molecular Physiology, vol. , no. , pp. L–L, .
[] R. Fudala, A. Krupa, M. A. Matthay, T. C. Allen, and A. K.
Kurdowska, “Anti-IL- autoantibody:IL- immune complexes
suppress spontaneous apoptosis of neutrophils,” American Jour-
nal of Physiology—Lung Cellular and Molecular Physiology,vol.
, no. , pp. L–L, .
[] M.A.MatthayandL.B.Ware,“Resolutionofalveolaredemain
acute respiratory distress syndrome. Physiology and biology,”
American Journal of Respiratory and Critical Care Medicine,vol.
, no. , pp. –, .
[] P. Ralph, I. Nakoinz, A. Sampson-Johannes et al., “IL-, T
lymphocyte inhibitor of human blood cell production of IL-
and tumor necrosis factor,” e Journal of Immunology,vol.,
no. , pp. –, .
[] M. Seitz, P. Loetscher, B. Dewald, H. Towbin, H. Gallati, and
M. Baggiolini, “Interleukin- dierentially regulates cytokine
inhibitor and chemokine release from blood mononuclear cells
and broblasts,” EuropeanJournalofImmunology,vol.,no.,
pp. –, .
[] C.-J.Lo,M.Fu,andH.G.Cryer,“Interleukininhibitsalveolar
macrophage production of inammatory mediators involved
in adult respiratory distress syndrome,” Journal of Surgical
Research, vol. , no. , pp. –, .
[]A.Sapru,J.L.Wiemels,J.S.Witte,L.B.Ware,andM.A.
Matthay, “Acute lung injury and the coagulation pathway:
potential role of gene polymorphisms in the protein C and
brinolytic pathways,” IntensiveCareMedicine,vol.,no.,pp.
–, .
[] V. Jalkanen, R. Yang, R. Linko et al., “SuPAR and PAI- in
critically ill, mechanically ventilated patients,” Intensive Care
Medicine,vol.,no.,pp.–,.
[] L. B. Ware, M. A. Matthay, P. E. Parsons et al., “Pathogenetic and
prognostic signicance of alteredcoagulation and brinolysis in
acute lung injury/acute respiratory distress syndrome,” Critical
Care Medicine,vol.,no.,pp.–,.
[] C.T.Esmon,“InammationandtheactivatedproteinCantico-
agulant pathway,” Seminars in rombosis and Hemostasis,vol.
, supplement , pp. –, .
[] M. F. Nold, C. A. Nold-Petry, D. Fischer et al., “Activated
protein C downregulates p mitogen-activated protein kinase
and improves clinical parameters in an in-vivo model of septic
shock,” rombosis and Haemostasis,vol.,no.,pp.–,
.
[] T.Cheng,D.Liu,J.H.Grinetal.,“ActivatedproteinCblocks
p-mediated apoptosis in ischemic human brain endothelium
and is neuroprotective,” Nature Medicine,vol.,no.,pp.–
, .
[] S.C.Christiaans,B.M.Wagener,C.T.Esmon,andJ.F.Pittet,
“Protein C and acute inammation: a clinical and biological
perspective,” e American Journal of Physiology—Lung Cellular
and Molecular Physiology,vol.,no.,pp.L–L,.
[] J.A.Bastarache,L.Wang,T.Geiseretal.,“ealveolarepithe-
lium can initiate the extrinsic coagulation cascade through
expression of tissue factor,” orax,vol.,no.,pp.–,
.
[] A.J.Ghio,J.H.Richards,K.M.Crissman,andJ.D.Carter,“Iron
disequilibrium in the rat lung aer instilled blood,” Chest,vol.
,no.,pp.–,.
[] J. A. Bastarache, J. L. Wynn, and L. B. Ware, “Fanning
the re: can methemoglobin enhance neutrophil activation?”
EBioMedicine,vol.,no.,pp.–,.
[] E.L.Burnham,W.J.Janssen,D.W.H.Riches,M.Moss,and
G. P. Downey, “e broproliferative response in acute respira-
tory distress syndrome: mechanisms and clinical signicance,”
European Respiratory Journal,vol.,no.,pp.–,.
[] T. Geiser, K. Atabai, P.-H. Jarreau, L. B. Ware, J. Pugin, and M.
A. Matthay, “Pulmonary edema uid from patients with acute
lung injury augments in vitro alveolar epithelial repair by an IL-
𝛽-dependent mechanism,” Amer ican Journal of Respiratory and
Critical Care Medicine, vol. , no. , pp. –, .
[] K.Atabai,M.Ishigaki,T.Geiser,I.Ueki,M.A.Matthay,andL.B.
Ware, “Keratinocyte growth factor can enhance alveolar epithe-
lial repair by nonmitogenic mechanisms,” American Journal of
Physiology—Lung Cellular and Molecular Physiology,vol.,
no. , pp. L–L, .
[] M. Mura, C. C. dos Santos, D. Stewart, and M. Liu, “Vascular
endothelial growth factor and related molecules in acute lung
injury,” Journal of Applied Physiology,vol.,no.,pp.–
, .
[]C.Quesnel,S.Marchand-Adam,A.Fabreetal.,“Regulation
of hepatocyte growth factor secretion by broblasts in patients
with acute lung injury,” American Journal of Physiology—Lung
Cellular and Molecular Physiology,vol.,no.,pp.L–
L, .
[]S.Fan,Y.X.Ma,J.-A.Wangetal.,“ecytokinehepatocyte
growth factor/scatter factor inhibits apoptosis and enhances
DNA repair by a common mechanism involving signaling
through phosphatidyl inositol kinase,” Oncogene,vol.,no.
, pp. –, .
[] J.-B. Stern, L. Fierobe, C. Paugam et al., “Keratinocyte growth
factor and hepatocyte growth factor in bronchoalveolar lavage
uid in acute respiratory distress syndrome patients,” Critical
Care Medicine,vol.,no.,pp.–,.
[] G. M. Verghese, K. McCormick-Shannon, R. J. Mason, and M.
A. Matthay, “Hepatocyte growth factor and keratinocyte growth
factor in the pulmonary edema uid of patients with acute lung
injury: biologic and clinical signicance,” American Journal of
Respiratory and Critical Care Medicine,vol.,no.,pp.–
, .
[] M. A. Rahman, K. Sundaram, S. Mitra, M. A. Gavrilin, and
M. D. Wewers, “Receptor interacting protein- plays a critical
role in human lung epithelial cells survival in response to Fas-
induced cell-death,” PLoS ONE,vol.,no.,ArticleIDe,
.
[]A.D.Lopez,S.Avasarala,S.Grewal,A.K.Murali,andL.
London, “Dierential role of the Fas/Fas ligand apoptotic
pathway in inammation and lung brosis associated with
reovirus /L-induced bronchiolitis obliterans organizing pneu-
monia and acute respiratory distress syndrome,” e Journal of
Immunology,vol.,no.,pp.–,.
[] G. Matute-Bello, W. C. Liles, K. P. Steinberg et al., “Soluble Fas
ligand induces epithelial cell apoptosis in humans with acute
lung injury (ARDS),” Journal of Immunology,vol.,no.,pp.
–, .
[] M. Tanaka, T. Suda, T. Takahashi, and S. Nagata, “Expression
of the functional soluble form of human Fas ligand in activated
Disease Markers
lymphocytes,” e EMBO Journal,vol.,no.,pp.–,
.
[] K.H.Albertine,M.F.Soulier,Z.Wangetal.,“Fasandfasligand
are up-regulated in pulmonary edema uid and lung tissue of
patients with acute lung injury and the acute respiratory distress
syndrome,” e American Journal of Pathology,vol.,no.,pp.
–, .
[] J. Farjanel, D. J. Hartmann, B. Guidet, L. Luquel, and G.
Oenstadt, “Four markers of collagen metabolism as possible
indicators of disease in the adult respiratory distress syndrome,”
American Review of Respiratory Disease,vol.,no.,pp.–
, .
[] A.N.Chesnutt,M.A.Matthay,F.A.Tibayan,andJ.G.Clark,
“Early detection of type iii procollagen peptide in acute lung
injury: pathogenetic and prognostic signicance,” American
JournalofRespiratoryandCriticalCareMedicine,vol.,no.
, pp. –, .
[]J.G.Clark,J.A.Milberg,K.P.Steinberg,andL.D.Hudson,
“Type III procollagen peptide in the adult respiratory distress
syndrome: association of increased peptide levels in bron-
choalveolar lavage uid with increased risk for death,” Annals
of Internal Medicine,vol.,no.,pp.–,.
[] J.-M. Forel, C. Guervilly, S. Hraiech et al., “Type III procollagen
is a reliable marker of ARDS-associated lung broproliferation,”
Intensive Care Medicine, vol. , no. , pp. –, .
[] C.M.Hendrickson,B.Crestani,andM.A.Matthay,“Biology
and pathology of broproliferation following the acute respira-
tory distress syndrome,” Intensive Care Medicine,vol.,no.,
pp.–,.
[] L. B. Ware, T. Koyama, D. D. Billheimer et al., “Prognostic
and pathogenetic value of combining clinical and biochemical
indices in patients with acute lung injury,” Chest,vol.,no.,
pp. –, .
[] J.-M. Constantin and E. Futier, “Lung imaging in patients with
acute respiratory distress syndrome: from an understanding of
pathophysiology to bedside monitoring,” Minerva Anestesiolog-
ica,vol.,no.,pp.–,.
[] L.Puybasset,P.Gusman,J.-C.Muller,P.Cluzel,P.Coriat,and
J.-J. Rouby, “Regional distribution of gas and tissue in acute
respiratory distress syndrome. III. Consequences for the eects
of positive end-expiratory pressure,” IntensiveCareMedicine,
vol. , no. , pp. –, .
[] J.-M. Constantin, S. Jaber, E. Futier et al., “Respiratory eects
of dierent recruitment maneuvers in acute respiratory distress
syndrome,” Critical Care,vol.,articleR,.
[] J.-M. Constantin, S. Cayot-Constantin, L. Roszyk et al.,
“Response to recruitment maneuver inuences net alveolar
uid clearance in acute respiratory distress syndrome,” Anesthe-
siology,vol.,no.,pp.–,.
[] R. Mayeux, “Biomarkers: potential uses and limitations,” Neu-
roRx,vol.,no.,pp.–,.
[] V.G.DeGruttola,P.Clax,D.L.DeMetsetal.,“Considerations
in the evaluation of surrogate endpoints in clinical trials: sum-
mary of a National Institutes of Health Workshop,” Controlled
Clinical Trials, vol. , no. , pp. –, .
[]P.Ray,Y.L.Manach,B.Riou,andT.T.Houle,“Statistical
evaluation of a biomarker,” Anesthesiology,vol.,no.,pp.
–, .
[]H.GerlachandS.Toussaint,“Sensitive,specic,predictive...
statisticalbasics:howtousebiomarkers,”Critical Care Clinics,
vol. , no. , pp. –, .
[] M. Bhargava and C. H. Wendt, “Biomarkers in acute lung
injury,” Tra nsla tio n al Res e arc h,vol.,no.,pp.–,.
Content uploaded by Matthieu Jabaudon
Author content
All content in this area was uploaded by Matthieu Jabaudon on Feb 15, 2016
Content may be subject to copyright.