Content uploaded by Lara Caeiro
Author content
All content in this area was uploaded by Lara Caeiro on Jul 08, 2016
Content may be subject to copyright.
Stroke is a major cause of death and disability world-
wide1. In developed countries, the acute treatment of
stroke has improved substantially in the past two dec-
ades with the implementation of stroke units and the
use of thrombo lysis and/or thrombectomy. As a conse-
quence, the mortality associated with acute stroke has
decreased and the proportion of survivors with mild
to moderate disability has increased2. Traditionally,
research into the functional impairments following
stroke and care of stroke sequelae has focused on motor
and sensory deficits, language disorders, visuospatial
neglect, and impairment of daily living. However, long
term follow-up of stroke survivors by multi disciplinary
teams shows that a substantial proportion of these
indivi duals are also affected by psycho logical distress
and numerous psychiatric disorders3. These disabling
psychiatric outcomes markedly reduce the quality of life
after stroke; they are a major source of burden, stress
and exhaustion for the caregiver, and often precipitate
institutionalization of the patient.
The psychiatric complications of stroke are under-
recognized and undertreated, despite growing evidence
for the beneficial effects of available pharmacological and
behavioural interventions. Health-care professionals are
becoming more aware of the prevalence and relevance
of neuropsychiatric disorders in patients with stroke.
Unfortunately, physicians, nurses and physio therapists
rarely receive formal training in the screening and
management of emotional and behaviouraldisorders.
This Review provides medical practitioners,
including neurologists, psychiatrists, neurosurgeons,
emergency and internal medicine physicians, family
physicians, nurses and rehabilitation specialists, with
an update on the acute and long-term psychi atric con-
sequences of stroke, with an emphasis on the clinical
aspects, bio logical and psychosocial determinants, and
management of stroke-related psychi atric symptoms.
We focus on disorders that are the most common,
that are preventable and treatable (such as mood and
anxiety disorders), and/or for which scientific advances
have accumulated in recent years (for example, post-
traumatic stress dis order and personality changes)
(TABLE1). Stroke-associated acute psychiatric disorders
(delirium, acute stress disorders, acute psychosis, hallu-
cinations and delusions) and chronic neurocognitive
disorders (vascular cognitive impairment and dementia)
will not be covered here. Disorders with predominantly
somatic manifestations (disorders of sleep, eating and
sexual function) are also not included because of the
confounding effect of other comorbidities with similar
symptoms that are common in elderly stroke survi-
vors. Finally, fatigue, pain and disorders that affect the
1Department of
Neurosciences, Centro
Hospitalar Lisboa Norte,
University of Lisbon,
Professor Egas Moniz Avenue,
1649–035 Lisbon, Portugal.
2Instituto de Medicina
Molecular, University of
Lisbon, Professor Egas Moniz
Avenue, 1649–028 Lisbon,
Portugal.
Correspondence to J.M.F.
jmferro@medicina.ulisboa.pt
doi:10.1038/nrneurol.2016.46
Published online 11 Apr 2016
Neuropsychiatric sequelae of stroke
José M.Ferro1, Lara Caeiro2 and Maria Luísa Figueira1
Abstract | Stroke survivors are often affected by psychological distress and neuropsychiatric
disturbances. About one-third of stroke survivors experience depression, anxiety or apathy,
whichare the most common neuropsychiatric sequelae of stroke. Neuropsychiatric sequelae
aredisabling, and can have a negative influence on recovery, reduce quality of life and lead to
exhaustion of the caregiver. Despite the availability of screening instruments and effective
treatments, neuropsychiatric disturbances attributed to stroke are currently underdiagnosed and
undertreated. Stroke severity, stroke-related disabilities, cerebral small vessel disease, previous
psychiatric disease, poor coping strategies and unfavourable psychosocial environment influence
the presence and severity of the psychiatric sequelae of stroke. Although consistent associations
between psychiatric disturbances and specific stroke locations have yet to be confirmed, functional
MRI studies are beginning to unveil the anatomical networks that are disrupted in stroke-associated
psychiatric disorders. Evidence regarding biochemical and genetic biomarkers for
stroke-associated psychiatric disorders is still limited, and better understanding of the biological
determinants and pathophysiology of these disorders is needed. Investigation into the
management of these conditions must be continued, and should include pilot studies to assess the
benefits of innovative behavioural interventions and large-scale cooperative randomized controlled
pharmacological trials of drugs that are safe to use in patients with stroke.
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
1
REVIEWS
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
control of expression of emotions will not be covered
in this Review, because they are not included as psy-
chiatric disorders in the fifth edition of the Diagnostic
and Statistical Manual of Mental Disorders (DSM-5)4.
The reader is referred to other excellent reviews3,5
and books6 on the neuro psychiatric manifestations of
cerebrovascular diseases that are not coveredhere.
Depressive disorders
Clinical and diagnostic features
After an unexpected and dramatic stressor event, such
as stroke, transient feelings of sadness are a nonpatho-
logical reaction. However, some patients with stroke
develop a prominent and persistent depressed mood
and/or diminished interest or pleasure in activities that
used to be enjoyable (anhedonia)4,7 (TABLE1). These two
criteria—depressed mood and anhedonia—define
depressive disorder due to stroke4. Other symptoms,
such as loss of energy, decreased concentration, psycho-
motor retardation and decreased appetite and insom-
nia, are also frequent. Suicidal thoughts and guilt can
also occur after stroke, but are less common8,9 (TABLE1).
Of note, some of the symptoms, in particular somatic
complaints, might be directly caused by stroke-induced
lesions or stroke complications, and can confound the
diagnosis of depression.
Depressive disorders after stroke are subdivided into
three categories. First, major depressive-like episodes are
defined as presentation with five or more of the follow-
ing symptoms for more than 2weeks: depressed mood,
anhedonia, weight loss or decrease in appetite, insom-
nia or hypersomnia, psychomotor agitation or retar-
dation, loss of energy, worthlessness or guilt, difficulty
concentrating, and suicidal ideation. Second, if patients
present with some of the above symptoms but do not
meet the criteria of a major depressive episode, they are
considered to have depressive features. Last, mixed fea-
tures are described as symptoms of mania in addition to
symptoms of depression4,10.
The diagnosis of depression (and of psychiatric dis-
turbances in general) requires the clinical judgement and
expertise of a qualified physician, and the use of validated
questionnaires11 and accepted diagnosticcriteria4,12.
Depression scales are often used to screen patients and
to quantify the severity of depressive symptoms. In most
of the recent studies, stroke-related depression was eval-
uated with several scales, including the Montgomery
and Åsberg Depression RatingScale (MADRS)13,
the Hamilton Depression Rating Scale(HDRS)14, the
Hospital Anxiety and DepressionScale (HADS)15,
the Beck Depression Inventory (BDI)16, and the Mini
International Neuropsychiatric Interview (M.I.N.I.)11
—a structured interview instrument that is widely used
for psychiatric diagnosis.
Clinician-administered structured clinical interviews
and screening scales for depression showed acceptable
toexcellent results for all validation measures when
used to screen for psychiatric disorders in patients
with stroke. No significant differences in performance
were observed between the screening scales, with the
exception of the Distress Thermometer, which was less
accurate than theother scales17.
Prevalence
Depressive disorders, which are considered to be distinct
from bipolar and bipolar-related disorders in the DSM-5
classification4, are much more common than bipolar
disorders in patients who have experienced stroke18,19.
In a systematic review of 61 observational studies, the
prevalence of depression at any time between 1year
and 5years after stroke was estimated at 31%, although
the figures at 1year and 5years were lower (25% and
23%, respectively)20. Another meta-analysis of 50 stud-
ies reported a similar figure (29% early after stroke)21;
however, in this study, the prevalence of depression
remained stable up to 10years after stroke, a finding
also supported by the analysis of the South London
Stroke Registry, which followed patients up for several
years21,22. Of note, patients who have experienced a tran-
sient ischaemic attack (TIA) show a comparable risk of
depression to stroke survivors23–26.
The variation in the prevalence of depression
between studies stems from several factors, includ-
ing the setting of the study (depression is least com-
mon in community- dwelling patients, intermediate
in patients undergoing rehabilitation, and highest in
outpatients)10,the type ofstroke (ischaemic versus
haemorrhagic), the amount of time elapsed since the
stroke, and the types of stroke-related deficits (for exam-
ple, severe aphasia or anosognosia)10. The different diag-
nostic criteria and the various methods used to diagnose
depression are another major source of variation27,28. In
most studies, the diagnosis of depression depends on the
score on a given scale. As stressed above, these scales
are more appropriate for screening for depression and
to grade theseverityof depressive symptoms than for
validatingthe diagnosis of depression.
Predictors
The major predictors of stroke-induced depressive
disorder are prestroke depression, anxiety and cogni-
tive impairment associated with stroke, and the sever-
ity of the neurological deficit and physical disability
followingstroke21,29.
Key points
• Neuropsychiatric sequelae of stroke are often disabling, have a negative effect on
stroke recovery, and decrease quality of life
• Neuropsychiatric disorders after stroke are relatively common: one-third of stroke
survivors experience depression, anxiety or apathy; recovery from these disorders is
only moderate, and the risk of recurrence is high
• Some of these disorders are treatable; for example, antidepressants reduce the
number and severity of depressive symptoms and episodes and decrease anxiety
scores in patients with stroke
• Research into the pathophysiology of stroke-associated neuropsychiatric
disturbances would greatly benefit from improved study design, including
incorporation of control groups in functional imaging studies and specification of
working hypotheses
• Pilot studies on the effects of behavioural interventions and large-scale randomized
trials of drugs that are safe to use in patients with stroke would improve the
management of neuropsychiatric sequelae of stroke
REVIEWS
2
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
Traditionally, stroke-induced depressive disorder,
especially in the first months after stroke, has been
associ ated with lesions in the left hemisphere, particu-
larly in the cortical or subcortical left anterior pole30.
However, systematic reviews have shown that neither
the anterior–posterior nor the right–left hemispherical
localization of the lesions is relevant to the occurrence
of stroke-induced depression31,32. According to current
understanding, factors associated with increased risk
of depression after stroke include large lesion volume33,
silent infarcts34, and subcortical small vessel disease
(characterized by lacunes, white matter lesions35,36
and microbleeds37–40), presumably because they inter-
fere with frontal subcortical circuits and ascending
mono aminergic and serotonergic pathways, which are
essential components of the motivational circuit.
Besides the severity of the neurological impairment
and of stroke-associated disability, the personality of
thepatient, the subjective experience of the stroke by the
patient, and the patient’s coping strategies41,42, lifestyle
and lack of social support and networks also contribute
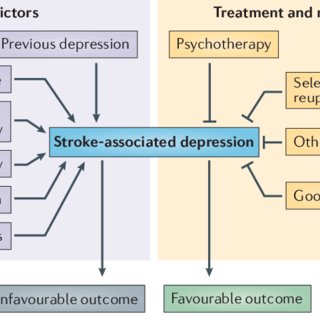
to the risk of depression43,44 (FIG.1).
Clinical course and outcome
According to the South of London Stroke Registry, most
episodes of stroke-associated depression begin within
1year after stroke21. In patients who are depressed a
few months after stroke, the depression recovery rate
at 1year after stroke is moderate (15–57%)21. Among
patients with stroke-associated depression, the fre-
quency of recurrent episodes of depression increases
gradually from 38% at 2years after stroke to 100% at
years 14–15 (REF.22). These figures illustrate the dynamic
pattern of depression after stroke: depression affects a
large subset of stroke survivors, and the risk of recurrent
depressive episodes ishigh.
Stroke-associated depressive disorder increases
mortality (HR = 1.27–1.41) up to 5years after stroke45;
the excess mortality is particularly notable in patients
younger than 65years and is not better explained by
other medical factors, or by comorbidities, smok-
ing, alcohol use, social support or compliance with
medication45. Stroke-induced depression also has a
negative effect on functional outcomes29 and quality of
life (mostly in the emotional and social domains), and
Table 1 | Neuropsychiatric disturbances after stroke
Disorder Prevalence in stroke
survivors (%)
Main clinical characteristics Screening tools Treatment options
Depression 31 • Depressed mood
• Anhedonia
• Loss of energy
• Decreased concentration
• Psychomotor retardation
• Decreased appetite
• Insomnia
• Suicidal thoughts
• Guilt
• Montgomery and Åsberg
Depression Rating Scale
(MADRS)
• Hamilton Depression Rating
Scale (HDRS)
• HADS
• Beck Depression Inventory (BDI)
• Antidepressants (SSRIs)
• Psychotherapy
Anxiety 18 • Anxiety or worry
• Restlessness
• Decreased energy
• Poor concentration
• Irritation
• Nervous tension
• Insomnia
HADS • Antidepressants (SSRIs)
• Benzodiazepines
• Buspirone
• Pregabalin
• Psychotherapy
• Lifestyle modifications
PTSD 10–25 • Unpleasant and uncontrollable
re-experiences of stroke
• Intrusion symptoms (memories,
dreams or flashbacks about stroke)
• Persistent avoidance of stimuli
associated with the stroke
• Stroke-related negative alterations
in cognition, mood, arousal and
reactivity
• Impact of Events Scale—Revised
• Interview
• Exposure psychotherapy
• Learning coping skills
Aggressive
personality
change
15–57 • Feelings of anger
• Aggressive reactions and
behaviour
• Hostility
• Personality scales and
questionnaires
• Neuropsychiatric Inventory
• SSRIs (fluoxetine)
• Neuroleptics (haloperidol or
atypical neuroleptics)
• Antiepileptic drugs or beta
blockers
Apathetic
personality
change
36 • Low motivation
• Reduced initiative
• Loss of self-activation
• Emotional indifference
• Personality scales and
questionnaires
• Apathy Evaluation Scale
• Apathy Scale
• Neuropsychiatric Inventory
• Dopaminergic agents
• Buproprion
• Noradrenergic
antidepressants
• Nefiracetam
• Cholinergic agents
• Stimulants
HADS, Hospital Anxiety and Depression Scale; PTSD, post-traumatic stress disorder; SSRI, selective serotonin reuptake inhibitor.
REVIEWS
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
3
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
Nature Reviews | Neurology
Treatment and management
Stroke-associated depression
Predictors
Psychotherapy
Selective serotonin
reuptake inhibitors
Other antidepressants
Good coping skills
Favourable outcome
Unfavourable outcome
Previous depression
Social isolation
Stroke severity and
associated disability
Small vessel disease
Anxiety
Poor coping skills
increases the risk of institutionalization, and family
andcaregiver depression22. Depression in stroke survi-
vors at 3months after stroke does not predict cognitive
impairment within 5years after stroke, but is associated
with an increased risk of anxiety22.
Pathophysiology and biomarkers
Two magnetic resonance spectroscopy studies showed
that patients with stroke-associated depressive disorder
had increased glutamate levels in the frontal lobe46,47.
Moreover, in stroke survivors with small vessel disease,
white matter damage (measured by fractional aniso tropy)
in the anterior limb of the internal capsule has been
shown to increase the risk of depression, possibly because
it impairs the frontal–subcortical circuits48. This biolog-
ical vulnerability, together with environmental stressors,
could precipitate stroke-associated depressive disorder35.
In one functional MRI (fMRI) study, disrupted default
mode network connectivity in the middle temporal cor-
tex and precuneus correlated with the severity of depres-
sive symptoms49. In another study, poststroke depression
was associated with dysfunction of the links between the
different areas of the affective network (prefrontal cor-
tex, amygdala, insula, ventral striatum, hippocampus and
anterior cingulate gyrus), with a correlation between the
intensity of depressive symptoms and altered functional
connectivity of the left orbital frontal gyrus50.
In addition to the alteration of imaging markers after
stroke, blood-based biomarker levels can be modified
in patients with poststroke depression. Patients with
stroke-induced depression show high homocysteine51
and bilirubin levels52, proteomic evidence of perturbed
lipid metabolism and altered immunoregulation53, and
increased levels of serum leptin54 and plasma glutamate55.
Some of these factors—in particular, bilirubin—are
potential markers of depression; however, these results
need replication, and the causal relationship between
these potential markers and depression remains to
beestablished.
In a meta-analysis of association studies of sero-
tonin transporter gene (SLC6A4) polymorphisms,
stroke patients who carried two ‘short’ variations of the
5-HTTLPR polymorphic region had a twofold increase
in the risk of stroke-induced depressive disorder56. The
expression of SLC6A4 is influenced by DNA methy-
lation: increased methylation in the SLC6A4 promoter
region can suppress SLC6A4 expression, and has been
linked to poststroke depression57. Brain-derived neuro-
trophic factor (BDNF) gene poly morphisms and the
methylation status of BDNF also affect the suscepti-
bility to depressive disorder after stroke57. SLC6A4 and
BDNF polymorphisms are potential genetic markers of
depressive disorders associated withstroke.
Management and treatment
In general, management of depression includes psycho-
therapy, antidepressants and neurostimulation58,59 (FIG.1).
In patients with stroke, the use of neurostimulation is
strictly limited because these individuals are at increased
risk of stimulation-triggered seizures. Psychotherapy has
a small preventive effect on stroke-associated depressive
disorder60, but there is no robust evidence to support
the use of psychotherapy to treat stroke-induced depres-
sive disorder61. Sending a monthly postcard to patients
—a practice that has been shown to reduce suicidal
behaviour in psychiatric inpatients and self-poisoning
patients—was not effective in patients with stroke62.
According to the European Stroke Organization,
pharmacotherapy is recommended for depressive dis-
order attributed to stroke63, because antidepressants
have been shown to reduce the number and severity of
depressive symptoms and episodes, despite treatment-
associated adverse events61. A Cochrane Review
including 56 randomized controlled trials (RCTs) and
4,059patients provided strong support for the use
of selective serotonin reuptake inhibitors (SSRIs) in
patients with stroke, most notably those with depres-
sion. SSRIs reduced dependence, disability, neuro logical
impairment, anxiety and depression, but had no effect
on mortality, cognitive function or motor deficits64. No
increase in severe adverse effects (seizures or gastro-
intestinal bleeding) was reported. Time elapsed since
stroke or stroke severity did not influence the beneficial
effect of SSRIs. As no SSRI has consistently demonstrated
superiority over the others64, selection of the most appro-
priate antidepressant for the individual patient must take
into account not only efficacy, but also the comorbidities
and adverse effects65,66 (TABLE2).
Folic acid and vitaminB1, B6 and B12 supplements have
some beneficial effects on depression associated with
stroke67. The efficacy of alternative therapies in stroke-
associated depressive disorder has also been assessed68.
In Asia, the use of acupuncture69, electro acupuncture70
and music therapy71 had encouraging beneficial effects,
but these results need confirmation.
Despite the high prevalence of stroke-associated
depressive disorder and the availability of affordable
and efficacious treatments, depression remains under-
diagnosed and undertreated in patients with stroke24,72,
and two-thirds of stroke survivors with depression do not
Figure 1 | Major psychosocial and cerebrovascular predictors of stroke-associated
depression. Anxiety and poor coping-strategies, among other factors, have a negative
influence on the course of depression. In stroke patients, depression is associated with
unfavorable outcomes, including death. Treatment and management strategies such as
the administration of antidepressants and good coping skills have beneficial effects on
stroke-associated depression.
REVIEWS
4
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
receive antidepressants. Such figures could be improved
by eradicating the misconception that low mood is
a transient normal reaction to stroke, and that the
associ ated deficits are not relevant to health outcomes.
Implementation of routine screening for depression in
stroke and rehabilitation units could also be beneficial.
Suicidality after stroke
As stated above, recurrent thoughts of death—that
is, suicidal ideation—or suicide plans or attempts are
among the diagnostic symptoms of a major depressive
episode4. However, although the suicide rate is higher in
patients with stroke than in the general population73,74,
suicide is still uncommon after stroke75. Suicidal
thoughts, which can develop shortly after stroke but
are more often observed after a delay76, are particularly
common in patients with low education, previous mood
disorder, and/or stroke-associated depressive disorder75.
Suicidality after stroke is also associated with younger
age, functional limitations73, insomnia, pain, apathy and
lobar cerebral microbleeds77–80.
In patients with acute depression after stroke, com-
pleted suicide has sometimes been linked with argyro-
philic grain disease or early progressive supranuclear
palsy, suggesting that an underlying tauopathy can
aggravate poststroke depression, potentially leading to
suicide. Such an effect could, plausibly, occur through
frontal disinhibition81.
Bipolar and related disorders
Manic episodes and bipolar disorder are rare psychi-
atric complications of stroke (reported in 1–2%
ofstroke survivors)18,19. Attribution of these disorders
to strokeshould only be made when the onset of mania
coincideswith or follows stroke: mania can occur con-
comitantly withstroke or follow it by days, months or
years. The most common clinical manifestations of
poststroke mania are elevated mood, hyperactivity,
increased rate or amount of speech, and insomnia or
decreased need for sleep19 (TABLE1). Other symptoms
include irritability, flights of ideas, grandiosity, lack of
insight, and social disinhibition. Mania is more common
after infarcts of the right hemisphere than of the left
hemi sphere19. Patients with stroke-associated mania can
experience recurrent episodes of mania or, as described
in a few reports, alternate manic and depressive bouts
(bipolar disorder)19.
Evidence to support management strategies for
manic episodes and bipolar disorder after stroke is
limited to case reports and small case series19. Current
treatment guidelines for bipolar disorder recommend
mood stabilizers such as lithium, valproate or lamo-
trigine, neuroleptics during severe maniac episodes, and
antidepressants in depressive periods82.
Anxiety disorders
Clinical and diagnostic features
Anxiety disorders are common after stroke, but they
are less well studied than depressive symptoms. Panic
attacks and phobias attributed to stroke have been
described in isolated reports, but the most common
stroke- associated anxiety disorder is generalized anxiety
dis order83. Generalized anxiety disorder is defined
as almost permanent anxiety or worry about a vari-
ety of topics that is difficult to control, to the extent of
having a negative impact on well-being and everyday
functioning4. In addition to permanent anxiety, the
DSM-5 criteria require that the patient presents with
three or more other symptoms (restlessness, decreased
energy, poor concentration, irritation, nervous ten-
sion and/or insomnia)4. The Hospital Anxiety and
Depression Scale (HADS) is commonly used to screen
and rate the intensity of the symptoms in patients with
anxiety after stroke15.
Table 2 | Selection of an antidepressant for the individual patient with depression after stroke
Class Adverse effects Drug Co-morbidities leading to
preferential use
Selective serotonin
reuptake inhibitor (SSRI)
Nausea, vomiting, gastric pain,
anxiety, tremor, decreased
threshold for seizures, and
withdrawal syndrome
Escitalopram Anxiety
Paroxetine Anxiety, weight loss
Fluoxetine Hypersexuality, weight gain
Sertraline Weight gain
TeCA, TriCA Dry mouth, blurred vision,
increased ocular tension,
drowsiness, increased heart
rate, cardiac arrhythmias,
constipation, urine retention,
postural hypotension, tremor and
decreased threshold for seizures
Mirtazapine (TeCA) Sleep disturbances,
weightloss
Amitriptyline (TriCA) Sleep disturbances,
weightloss, pain
Serotonin–noradrenaline
reuptake inhibitor (SNRI) Nausea, headache, somnolence,
ejaculation disorder, yawning,
decreased threshold for seizures
Duloxetine Pain
Venlafaxine Pain, weight gain
Serotonin antagonist and
reuptake inhibitor (SARI)
Dry mouth, constipation, blurred
vision, drowsiness
Trazodone Sleep disturbances
Dopaminergic Dry mouth, headache, nausea,
weight loss, insomnia, agitation Bupropion Apathy, weight gain
TeCA, tetracyclic antidepressant; TriCA, tricyclic antidepressant.
REVIEWS
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
5
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
Prevalence
In a systematic review and meta-analysis of 42 obser-
vational studies comprising 5,760 patients with stroke,
the prevalence of anxiety was 18% when assessed by
clinical interview and 25% when assessed by a rating
scale84. The prevalence of anxiety is stable over time: 20%
of patients experience anxiety within the first month
after stroke, compared with 23% 1–5months and 24%
>6months after stroke84. Two community-based studies
published after the meta-analysis confirmed most of
thesefindings27,85.
Predictors
Previous depression, previous anxiety and alcohol
abuse are the most consistent psychiatric predictors
of stroke-induced anxiety. Demographic predictors of
anxiety include young age and female sex. Aphasia, his-
tory of insomnia and cognitive impairment also predict
anxiety associated with stroke. Functional and social
predictors of anxiety after stroke include impairment in
activities of daily living, impairment in social function-
ing, inability to work, being single, and living alone or
having no social contacts outside the family85,86.
Clinical course and outcome
25–50% of patients with acute anxiety after stroke
develop permanent chronic anxiety. The co-occurrence
of depression with poststroke anxiety increases the
likelihood that the anxiety will be permanent or long-
standing86. Anxiety without depression does not influ-
ence functional recovery from stroke but is associated
with worse social functioning and quality of life85,87.
Advances in pathophysiology
Stroke-triggered anxiety could be especially common
after strokes affecting the anterior circulation: one study
suggested an association between anxiety and right fron-
tal infarcts88. A small-sample genetic association study
performed in China suggested that polymorphisms in
the tryptophan hydroxylase 2 (TPH2) gene are involved
in the development of stroke-associated anxiety89.
Management and treatment
Management of generalized anxiety disorder includes
patient education and lifestyle modifications, psycho-
therapy, and pharmacotherapy with antidepressants
(SSRIs or serotonin–noradrenaline reuptake inhib-
itors), benzodiazepines, buspirone or pregabalin90.
Pharmacological treatments are also efficacious in the
treatment of anxiety attributed to stroke. In a system-
atic review that included two trials involving a total of
175stroke patients with anxiety and comorbid depres-
sion, paroxetine and buspirone were found to substan-
tially reduce anxiety scores91. A systematic review of
SSRIs for stroke recovery assessed eight trials with a total
of 413 participants, and reported that SSRIs decrease
anxiety scores64. A few small pilot trials of relaxation
therapies to reduce stroke- associated anxiety have pro-
duced encouraging results92,93. The use of benzodiaze-
pines or pregabalin in patients with poststroke anxiety
has not yet been evaluated byRCT.
Post-traumatic stress disorder
Clinical and diagnostic features
Stroke and TIA are unexpected events that have the poten-
tial to be life-threatening and cause serious dis ability;
moreover, they can be re-experienced in an unpleasant
and uncontrollable way after the actual event94. Stroke-
associated post-traumatic stress disorder (PTSD) is char-
acterized by intrusive symptoms (memories, dreams, and
flashbacks of the stroke or TIA), persistent avoidance of
stimuli associated with the stroke, negative alterations
in cognition and mood, marked alterations in arousal,
and increased reactivity (irritability, angry outbursts,
exaggerated startle response)4.
The PTSD Impact of Events Scale—Revised and
Interview95 can be used to screen patients for PTSD. PTSD
can be diagnosed with questionnaires, scales or formal
psychiatric interview, with varying results: when assessed
with a scale, PTSD was diagnosed in 25% of patients with
stroke, whereas only 10% of patients were diagnosed with
PTSD when assessed with a formal psychiatric interview96.
Prevalence
In stroke survivors, the estimated prevalence of PTSD
ranges from 10% to 31%94,96,97. These percentages are
probably overestimates because, in most studies, PTSD
was diagnosed with questionnaires and scales96,98, rather
than with formal psychiatric interviews.
Predictors
PTSD after stroke is more common in women, younger
patients, and patients with low educational level, recur-
rent strokes, more-severe disability, comorbidities
(including depression and anxiety), prestroke neuroti-
cism, or prior psychiatric morbidity. Moreover, PTSD is
more common in patients with a subjectively rated high
stroke risk or a negative appraisal of the stroke or TIA
experience (peri traumatic distress)94,96,97. Peritraumatic
distress predicts acute PTSD symptoms after a first
stroke99 and correlates with the intensity of PTSD symp-
toms, although this correlation tends to decline over time
after the stroke99.
Course and outcome
PTSD has a negative effect on mental health and qual-
ity of life. It is also associated with an increased risk of
nonadherence to medication100.
Management and treatments
The use of behavioural therapeutic strategies, such as
exposure therapy, has been shown to reduce PTSD in
combat veterans101 and could be tested in patients with
stroke. Approaches in which patients are taught more-
effective coping skills and are cautiously briefed about
the realistic risk of stroke recurrence should be tested for
effectiveness in PTSD afterstroke.
Personality changes
Personality has a complex two-way relationship with
stroke. Some personality traits4,102, including anger,
typeA behaviour and pessimism, increase stroke
risk103, and affect stroke outcome104 and response to
REVIEWS
6
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
Nature Reviews | Neurology
Negative affectivity
Detachment
Antagonism
Disinhibition
Psychoticism
Emotional stability
Extraversion
Agreeableness
Conscientiousness
Lucidity
Healthy individual
Patient with stroke
Negative pole Positive polePersonality traits domains
therapeuticinterventions. In a pooled analysis of three
cohort studies, high extraversion was linked to an
increased risk of stroke; high neuroticism was associated
to increased stroke-related mortality, whereas high con-
sciousness was associated with decreased mortality105.
Neuroticism was also found to predispose to poststroke
depression and poor quality of life105. Negative affectivity
hampers improvement in response to speech therapy106.
Conversely, stroke can affect personality; such person-
ality changes are more marked and intense than those
reported in association with other chronic diseases.
In a pooled analysis of four cohort studies including
17,493patients with chronic diseases, stroke induced a
long-term change in personality traits, even after adjust-
ment for age. Stroke was associated with a decrease in the
‘positive pole’ of personality traits domains (FIG.2), includ-
ing extraversion, emotional stability, consciousness and
openness to experience. Furthermore, a trend towards
a ‘dose–response’ relationship between the severity and
chronicity of stroke and the intensity of personality trait
changes was observed107.
Clinical and diagnostic features
Personality disorders are grouped in three main clusters,
A, B and C, according to the DSM-5 classification, and
are defined as a repeated deviation of behaviour from
what can be expected from the individual’s culture4.
Personality changes are classified into five types (labile,
disinhibited, aggressive, apathetic, and paranoid)4, and
represent a change from the individual’s previous per-
sonality pattern. Diagnosis of personality disorders and
changes requires multiple examinations by a medical
expert, usually a psychiatrist. Personality scales can help
establish the correct diagnosis104,108, and simple instru-
ments, such as the Neuropsychiatric Inventory109, are
often used for screening purposes. In the sections that
follow, we will focus on the apathetic and aggressive
types of personality change, as the information on other
personality changes after stroke is limited.
Apathetic personality change. Apathy is a disorder of
motivation characterized by decreased spontaneous men-
tal and physical activity and emotional indiffer ence110,111.
Apathetic patients have markedly decreased motor, ver-
bal and behavioural initiative: they do not start a new
activity by themselves, but they can adequately perform
the same activity following the commands or actions of
others. Characteristic symptoms in apathetic patients are
lack of interest in their previous activities and hobbies,
and preference for passive activities. As apathetic patients
are emotionally indifferent, even to their symptoms, they
can seem depressed, but if questioned whether they are
sad, they deny low mood, in contrast with depressed
patients who express sadness and are affected by not
being able to start and maintain actions and to experience
pleasure in activities.
Some patients with apathy after stroke are also
depressed112–115, and depression is particularly common
and severe in apathetic patients114,116. However, the co-
occurrence of apathy and depression could be lower than
reported, because apathy can be erroneously labelled as
anhedonia or inhibition, thereby leading to an incorrect
diagnosis of depression110. Indeed, apathy and depression
are typically dissociated: apathy without depression was
reported in 21% and depression without apathy in 12%
of stroke survivors113. These disorders also have different
evolutions during follow-up and different responses to
treatment112,117–120.
Apathetic personality change should be diagnosed by
a skilled physician, using accepted diagnostic criteria4.
Validated scales, such as the Apathy Scale, which was
adapted from the original Apathy Evaluation Scale121, can
be used for screening and to grade the severity of apathy
symptoms, but cannot replace the expert diagnosis of
apathetic personalitychange.
Aggressive personality change. In patients with
stroke, the three components of anger (emotional,
cognitive andbehavioural122,123), the subjective experi-
ence of anger,and anger-associated behaviour can be
dissociated124,125. Patients with stroke and aggressive
personality change can behave aggressively without feel-
ing angry or, conversely, experience only hostility without
showing aggressive behaviour.
In stroke survivors, anger or aggressive behaviour can
be symptomatic of several neuropsychiatric disorders,
including delirium, mania, psychosis, vascular cognitive
impairment and catastrophic reaction126. A tendency to
react and behave aggressively can also be the prominent
feature of another type of long-standing personality
change after stroke. Patients with dorsolateral pre frontal
or basofrontal infarcts (for example, in the context of
anterior communicating artery aneurysmal rupture) can
display such personality change as part of a dysexecutive
syndrome that impairs inhibition of aggressive responses
and decreases mental flexibility. Similarly, patients with
severe Wernicke aphasia are almost deprived of language
comprehension and can develop intense suspicion, anger
and aggressive behaviour126.
Prevalence
Stroke-related personality disorders of the three clusters
(A, B and C) are rare, and are estimated to occur in less
than 1% of stroke survivors127. However, stroke- associated
Figure 2 | Shift towards the negative pole of personality trait domains after
stroke. Personality changes attributed to stroke can be conceptualized as changes
towards the ‘negative pole’ of personality traits. Examples include detachment observed
in apathetic personality, negative affectivity seen in labile personality, disinhibition in
disinhibited personality, psychoticism in paranoid personality and negative affectivity,
detachment, antagonism, disinhibition and/or psychoticism in aggressive personality.
REVIEWS
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
7
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
personality changes seem to be quite common, although
their frequency is not well defined, except in the case
of apathy, as detailed below. Reasons for these hetero-
geneous results include different definitions of person-
ality changes, and the multiplicity of instruments that
have been used to detect and grade the severity ofsuch
changes of personality. The reported frequencyof per-
sonality changes after subarachnoid haemorrhage is
59%128. Depending on the study, 8–32% of stroke survi-
vors experience a labile personality change and 6–76%
of patients with stroke show disinhibited personality
change3. Similarly, a 2015 systematic review reported a
high prevalence of anger, with high variability between
studies (15–57%)129.
Although stroke-associated apathy has received less
attention than depression, potentially owing to the fact
that apathy can be misdiagnosed as depression, their
prevalence is similar. In a systematic review of 19studies,
we found a prevalence of stroke-associated apathy of
36.3%116. The prevalence was similar in acute (up to
15days after stroke onset) and post-acute phases. Two
studies, published in 2013 and 2015, confirmed the high
prevalence of stroke-related apathy117,130.
Predictors
In our systematic review, apathy was more common
in older patients, and in patients with recurrent stroke,
cognitive impairment or depression116. The preva-
lence of apathy was similar in both sexes, after ischae-
mic and haemorrhagic strokes, and after right and left
hemispheric strokes112–116.
Case reports and small case series have described
apathy to be a prominent clinical feature in patients
with strokes in locations that are essential for motiva-
tion, such as the anterior cingulate–pallidum–thalamic
circuit. For example, apathetic personality changes were
induced by unilateral or bilateral anterior cerebral artery
infarcts131, anterior and paramedian ischaemic thalamic
strokes132, striatocapsular strokes118, and anterior com-
municating artery aneurysmal rupture with basofrontal
or mesial infarcts128,133. However, in most systematically
evaluated series of patients with apathy after stroke, the
anatomical location of the stroke was not significantly
associated with apathy, with the exception of one patient
series131, in which apathy was more common after
bilateral lesions (67%), followed by left-hemisphere and
right-hemisphere strokes (51% and 25%, respectively).
In this study, apathy was more common when the stroke
damaged the frontal pole, gyrus rectus, corpus callosum,
cingulate gyrus or superior frontal lobe. In another
study, pontine infarcts were linked to increased risk of
apathy after stroke134.
In patients with stroke, the association of anger with
demographic, clinical, neuroradiological, psychological
and social variables is not consistent between studies129.
Some studies reported anger to be more frequent in
patients with haemorrhagic strokes, with lesions close
to the frontal pole, and in strokes involving the frontal,
lenticulocapsular and basal pontine areas, whereas other
studies found no association with a specific stroke type
or localization129.
Course and outcome
The course of apathy after stroke has been evaluated
in only a few longitudinal studies112,115,130,135. In general,
apathetic patients with stroke show little improvement of
apathy over time. Apathy in the acute phase of stroke pre-
dicts longer-term poststroke apathy112. Persistent apathy
after stroke is associated with cognitive impairment,
more-severe functional deficits, less functional improve-
ment, depression, recurrent strokes, and suicide80. Apath y
interferes with rehabilitation, and impairs health-related
quality of life114,116,119,120,130,131,135,136. Nevertheless, no differ-
ence in functional outcomes has been observed between
patients with and without apathy114.
Advances in pathophysiology
A few small functional neuroimaging studies have
provided important hints concerning the cerebral net-
work dysfunction that underlies apathy. Matsuokaetal.
found delayed atrophy in the posterior cingulum
inpatients with poststroke apathy137. The fMRI study of
a patientwith poststroke apathy demonstrated aberrant
functional connectivity in the default mode network
and in the cingulo-opercular network, and identified
these two networks as an apathy-related functional net-
work138. A voxel-based analysis of fractional anisotropy
in 54patients with stroke demonstrated that apathy is
related to damage of the genu and splenium of the corpus
callosum, left anterior corona radiata and white matter
of the right inferior frontal lobe139. Poor reward sensi-
tivity, which is linked to damage to the ventral putamen
and pallidum, dorsal thalamus, left insula and prefrontal
cortex, was also associated with apathy in an fMRI study
of 55 patients with stroke and 15 controls140. Another
fMRI study showed that the pathways associated with
affective (serotonergic) and apathetic (dopaminergic)
depression after stroke were different141.
In patients with acute stroke, failure of inhibitory
control of behaviour is probably the primary cause of
aggressive behaviour126. The hospital environment can
be perceived as hostile or humiliating, thereby contrib-
uting to the development of angry behaviour. Premorbid
anger can also increase the intensity of the manifestations
of anger after stroke. fMRI studies in healthy indivi-
duals have implicated the ventromedial, prefrontal and
orbitofrontal cortices in anger142. Stroke rarely involves
these frontal areas, with the notable exception of sub-
arachnoid haemorrhage caused by rupture of an aneu-
rism in the anterior communicating artery. This type and
location of stroke often results in aggressive behaviour143.
Aggressiveness attributed to stroke can also be secondary
to the loss of empathy. Recent MRI studies in patients
with right hemispheric stroke point towards a crucial role
for the right uncinate fasciculus in emotional empathy144,
and a function of the temporal pole and anterior insula
in affectiveempathy145.
Management and treatment
No large high-quality RCTs have yet been conducted
to guide the treatment of apathetic personality change
attributed to stroke. Evidence regarding potential treat-
ments for apathy is limited to case reports and small
REVIEWS
8
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
case series. Therefore, the treatment of apathy after
stroke currently follows indirect low-level evidence
collected in the context of apathy treatment in other
neurologicalconditions.
Behavioural interventions for apathy prevention
have been assessed in two small RCTs. Coping-strategy
training146 and problem-solving therapy147 both show
promise for the prevention ofapathy.
Given the role of dopamine in motivation, dopamin-
ergic agents could represent a first-line pharmacological
treatment to ameliorate apathy66,148. If the patient is also
depressed, antidepressants with dopaminergic activity
(for example, buproprion) or noradrenergic activity (for
example, reboxetine) could be used. Asmall randomized
trial showed improvement of poststroke apathy with the
nootropic nefiracetam, which enhances GABAergic,
choli nergic and monoaminergic signalling149. Other
choli nergic agents, such as donepezil150, and stimu-
lants, such as modafinil151 or methylphenidrate152, have
also been reported to alleviate apathy, although their
cardio vascular adverse effects limit their use in elderly
patients with stroke and comorbid hypertension or car-
diac diseases. Surprisingly, a case report claimed that
the sedative zolpidem was effective in the treatment of
poststrokeapathy153.
No studies have specifically evaluated interventions to
manage severe aggressive personality change in patients
with stroke. Recommendations have been made for
dealing with aggressive behaviour after other neuro-
logical conditions, such as traumatic brain injury154, but
post-traumatic and poststroke aggressiveness might have
different pathophysiologies. We advocate psychological
counselling to establish realistic goals for recovery, coping
strategies to deal with the stroke-associated deficits, and
explaining to the caregiver how to deal with the aggressive
patients. Anger after stroke can be treated with SSRIs such
as fluoxetine155. In patients with severe aggressive behav-
iour, neuroleptics (either haloperidol or atypical neuro-
leptics) could be used to prevent harm to the patient and
to others. The starting dose should be low and titrated
according to the control of aggression gained with treat-
ment and to the intensity of the adverse effects (sedation,
confusion or cognitive impairment, rigidity, walking
difficulty and falls). Cardiovascular adverse effects and
lowering of the seizure threshold, especially if the drugs
are prescribed concomitantly with SSRIs, should also
be monitored. If aggressive behaviour is under control
or decreases to acceptable levels, the dose should be
reduced, and the drug should eventually be discontinued.
In patients who do not respond to SSRIs and neuroleptics,
antiepileptic drugs or beta blockers can be used154.
Conclusions and future directions
Over the past decade, researchers have successfully
described the high prevalence of the neuropsychiatric
sequelae of stroke and their main clinical and psycho-
social correlates. One-third to one-half of stroke survi-
vors are affected by a neuropsychiatric disorder despite
evidence that pharmacological treatment of neuro-
psychiatric disorders—in particular, depression—is eff i-
cacious in patients recovering from stroke. Moreover,the
neuro psychiatric disturbances that occur after stroke
are currently underdetected25,72. This underestimation
is observed even in developed countries where access
to health care is easy. Antidepressants can have the
additional benefit of improving physical and cognitive
recovery after stroke. These results could justify anti-
depressant prescription to almost all stroke survivors, but
larger trials are needed before such a treatment policy is
implemented64,156.
Most of the studies published on the psychiatric com-
plications of stroke have several recurrent methodological
limitations. Almost all studies analysed patients from hos-
pitals, clinics or rehabilitation centres, and very few were
population-based. Patients with aphasia and cognitive
deficits were often excluded. Stroke type and location were
not always specified. The coexistence of imaging mark-
ers of cerebral small vessel disease or Alzheimer disease,
which are confounding factors, was only rarely assessed.
In general, the diagnosis of the psychiatric condition was
made after a single examination, and by following cut-off
scores on a scale. The diagnosis of a psychiatric condition
requires the expertise of an experienced psychiatrist, using
validated diagnostic criteria and multiple observations
of the patient. Moreover, different studies used differ-
ent scales, making interstudy comparisons and system-
atic reviews challenging. The use of scales also leads to
theinclusion of mild cases and minor disturbances in the
same group as psychiatric disorders, which can obscure
or dilute the results of studies that investigate risk factors
and prognostic variables. Another limitation of the studies
on the neuropsychiatric sequelae of stroke is that psychi-
atric models, such as personality models, are only rarely
integrated when testing hypotheses on the development
of neuropsychiatric disorders afterstroke.
The majority of the studies on the neuropsychi atric
consequences of stroke failed to confirm consistent associ-
ations between psychiatric disturbances and anatomical
locations of stroke lesions. Some studies indicate that
lesions in particular locations trigger certain psychiatric
conditions; however, such claims can only be validated
by comparing patients with psychiatric disorders pre-
sumably caused by stroke lesions with a control group of
patients with stroke-associated lesions in other locations.
In addition, most fMRI studies that evaluated the influ-
ence of lesion location on psychiatric symptoms—for
example, the study on right hemispheric stroke and
apathy144—selected patients with a specific stroke loca-
tion, and did not include a control group. The results of
fMRI and network analysis studies are often difficult to
interpret, owing to the multiple roles of functional nodes
that are deemed important for a specific disturbance.
There is still a paucity of studies that analysed serum or
cerebrospinal fluid biomarkers or examined genetic poly-
morphisms that could predispose individuals to psychiat-
ric disturbances after stroke. Many of the available studies
tested hypotheses that were too general, such as the cat-
echolamine hypothesis, which premise is that depression
is associated with a decrease in central catecholamine lev-
els, or asked questions that were too broad (for example,
“is inflammation involved in stroke-associated depressive
disorder?”), and the results are yet to be replicated.
REVIEWS
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
9
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
Improved study designs and expansion of the
research on the biological determinants and pathophys-
iology of stroke-associated psychiatric disorders are
clearly needed. Management of poststroke psychiatric
symptoms also needs further investigation, which should
include pilot studies of innovative behavioural interven-
tions and large-scale RCTs of drugs that are safe to use
in patients withstroke.
1. Feigin,V.L. etal. Global and regional burden of stroke
during 1990–2010: findings from the Global Burden
of Disease Study 2010. Lancet 383, 245–254
(2014).
2. Bejot,Y., Daubail,B. &Giroud,M. Epidemiology of
stroke and transient ischemic attacks: current
knowledge and perspectives. Rev. Neurol. (Paris) 172,
59–68 (2016).
3. Hackett,M.L., Kohler,S., O’Brien,J.T. &Mead,G.E.
Neuropsychiatric outcomes of stroke. Lancet Neurol.
13, 525–534 (2014).
4. American Psychiatric Association. Diagnostic and
Statistical Manual of Mental Disorders 5th edn
(American Psychiatric Association, 2013).
5. Piechowski-Jozwiak,B. &Bogousslavsky,J.
Neurobehavioral syndromes. Front. Neurol. Neurosci.
30, 57–60 (2012).
6. Ferro,J. NeuropsychiatricSymptoms of
Cerebrovascular Diseases (Springer, 2013).
7. American Psychiatric Association. Diagnostic and
StatisticalManual of Mental Disorders‑TR 4th edn
(American Psychiatric Association, 2002).
8. Caeiro,L., Ferro,J., Santos,C. &Figueira,M.
Depression in acute stroke. J.Psychiatry Neurosci.
31, 377–383 (2006).
9. Spalletta,G., Ripa,A. &Caltagirone,C. Symptom
profile of DSM-IV major and minor depressive
disorders in first-ever stroke patients. Am. J.Geriatr.
Psychiatry 13, 108–115 (2005).
10. Robinson,R.G. &Jorge,R.E. Post-stroke depression:
a review. Am. J.Psychiatry 173, 221–231 (2016).
11. Sheehan,D.V. etal. The Mini-International
Neuropsychiatric Interview (M.I.N.I.): the development
and validation of a structured diagnostic psychiatric
interview for DSM-IV and ICD-10. J.Clin. Psychiatry
59 (Suppl. 20), 22–33 (1998).
12. Robinson,R.G. &Spalletta,G. Poststroke depression:
a review. Can. J.Psychiatry 55, 341–349 (2010).
13. Montgomery,S.A. &Asberg,M. A new depression
scale designed to be sensitive to change.
Br.J.Psychiatry 134, 382–389 (1979).
14. Hamilton,M. A rating scale for depression.
J.Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
15. Zigmond,A.S. &Snaith,R.P. The hospital anxiety
and depression scale. Acta Psychiatr. Scand. 67,
361–370 (1983).
16. Beck,A.T., Ward,C.H., Mendelson,M., Mock,J.
&Erbaugh,J. An inventory for measuring depression.
Arch. Gen. Psychiatry 4, 561–571 (1961).
17. Turner,A. etal. Depression screening in stroke:
acomparison of alternative measures with the
structured diagnostic interview for the diagnostic and
statistical manual of mental disorders, fourth edition
(major depressive episode) as criterion standard.
Stroke 43, 1000–1005 (2012).
18. Starkstein,S.E. &Robinson,R.G. Affective disorders
and cerebral vascular disease. Br. J.Psychiatry 154,
170–182 (1989).
19. Santos,C., Caeiro,L., Ferro,J. &Figueira,M.
Mania and stroke: a systematic review.
Cerebrovasc. Dis. 32, 11–21 (2011).
20. Hackett,M.L. &Pickles,K. Part I: frequency of
depression after stroke: an updated systematic
review and meta-analysis of observational studies.
Int.J.Stroke 9, 1017–1025 (2014).
21. Ayerbe,L., Ayis,S., Wolfe,C.D. &Rudd,A.G.
Natural history, predictors and outcomes of
depression after stroke: systematic review and meta-
analysis. Br.J.Psychiatry 202, 14–21 (2013).
22. Ayerbe,L., Ayis,S., Crichton,S., Wolfe,C.D.
&Rudd,A.G. The natural history of depression up
to15years after stroke: the South London Stroke
Register. Stroke 44, 1105–1110 (2013).
23. Wu,K.Y., Liu,C.Y., Chau,Y.L. &Chang,C.M.
Transient ischemic attack and incidence of depression
in old age: evidence from a population-based analysis
in Taiwan. Am. J.Geriatr. Psychiatry 18, 382–387
(2010).
24. Luijendijk,H.J. etal. Transient ischemic attack and
incident depression. Stroke 42, 1857–1861 (2011).
25. El Husseini,N. etal. Depression and antidepressant
use after stroke and transient ischemic attack. Stroke
43, 1609–1616 (2012).
26. Broomfield,N.M., Quinn,T.J., Abdul-Rahim,A.H.,
Walters,M.R. &Evans,J.J. Depression and anxiety
symptoms post-stroke/TIA: prevalence and
associations in cross-sectional data from a regional
stroke registry. BMC Neurol. 14, 198 (2014).
27. Schramke,C.J., Stowe,R.M., Ratcliff,G., Goldstein,G.
&Condray,R. Poststroke depression andanxiety:
different assessment methods result in variations in
incidence and severity estimates.
J.Clin. Exp. Neuropsychol. 20, 723–737 (1998).
28. Berg,A., Lonnqvist,J., Palomaki,H. &Kaste,M.
Assessment of depression after stroke: a comparison
of different screening instruments. Stroke 40,
523–529 (2009).
29. Kutlubaev,M.A. &Hackett,M.L. Part II: predictors of
depression after stroke and impact of depression on
stroke outcome: an updated systematic review of
observational studies. Int. J.Stroke 9, 1026–1036
(2014).
30. Starkstein,S.E., Robinson,R.G. &Price,T.R.
Comparison of cortical and subcortical lesions in the
production of poststroke mood disorders. Brain 110,
1045–1059 (1987).
31. Carson,A.J. etal. Depression after stroke and lesion
location: a systematic review. Lancet 356, 122–126
(2000).
32. Wei,N. etal. Post-stroke depression and lesion
location: a systematic review. J.Neurol. 262, 81–90
(2015).
33. Zhang,T. etal. A prospective cohort study of lesion
location and its relation to post-stroke depression
among Chinese patients. J.Affect. Disord. 136,
e83–87 (2012).
34. Wu,R.H., Li,Q., Tan,Y., Liu,X.Y. &Huang,J.
Depression in silent lacunar infarction: a cross-
sectional study of its association with location of
silentlacunar infarction and vascular risk factors.
Neurol. Sci. 35, 1553–1559 (2014).
35. Yasuno,F. etal. Microstructural abnormality in white
matter, regulatory T lymphocytes, and depressive
symptoms after stroke. Psychogeriatrics 14, 213–221
(2014).
36. Pavlovic,A.M. etal. Baseline characteristic of patients
presenting with lacunar stroke and cerebral small
vessel disease may predict future development of
depression. Int. J.Geriatr. Psychiatry 31 , 58–65
(2016).
37. Tang,W.K. etal. Cerebral microbleeds and depression
in lacunar stroke. Stroke 42, 2443–2446 (2011).
38. Tang,W.K. etal. Cerebral microbleeds and symptom
severity of post-stroke depression: a magnetic
resonance imaging study. J.Affect. Disord. 129,
354–358 (2011).
39. Tang,W.K. etal. Cerebral microbleeds as a predictor
of 1-year outcome of poststroke depression. Stroke
45, 77–81 (2014).
40. Tang,W.K. etal. Pontine microbleeds and depression
in stroke. J.Geriatr. Psychiatry Neurol. 27, 159–164
(2014).
41. van Mierlo,M.L., van Heugten,C.M., Post,M.W.,
deKort,P.L. &Visser-Meily,J.M. Psychological factors
determine depressive symptomatology after stroke.
Arch. Phys. Med. Rehabil. 96, 1064–1070 (2015).
42. Visser,M.M. etal. Coping, problem solving,
depression, and health-related quality of life in
patients receiving outpatient stroke rehabilitation.
Arch. Phys. Med. Rehabil. 96, 1492–1498 (2015).
43. Ouimet,M.A., Primeau,F. &Cole,M.G. Psychosocial
risk factors in poststroke depression: a systematic
review. Can. J.Psychiatry 46, 819–828 (2001).
44. Hinojosa,R., Haun,J., Hinojosa,M.S. &Rittman,M.
Social isolation poststroke: relationship between
race/ethnicity, depression, and functional
independence. Top. Stroke Rehabil. 18, 79–86 (2011).
45. Ayerbe,L., Ayis,S., Crichton,S.L., Rudd,A.G.
&Wolfe,C.D. Explanatory factors for the increased
mortality of stroke patients with depression.
Neurology 83, 2007–2012 (2014).
46. Glodzik-Sobanska,L. etal. Single voxel proton
magnetic resonance spectroscopy in post-stroke
depression. Psychiatry Res. 148, 111–120 (2006).
47. Wang,X. etal. Glutamate level detection by magnetic
resonance spectroscopy in patients with post-stroke
depression. Eur. Arch. Psychiatry Clin. Neurosci. 262,
33–38 (2012).
48. Brookes,R.L., Herbert,V., Lawrence,A.J.,
Morris,R.G. &Markus,H.S. Depression in small-
vessel disease relates to white matter ultrastructural
damage, not disability. Neurology 83, 1417–1423
(2014).
49. Lassalle-Lagadec,S. etal. Linking MRI to daily life
experience: the example of poststroke depression.
Neurology 78, 322–325 (2012).
50. Zhang,P. etal. Dysfunction of affective network in
post ischemic stroke depression: a resting-state
functional magnetic resonance imaging study.
Biomed. Res. Int. 2014, 846830 (2014).
51. Pascoe,M.C. etal. Homocysteine as a potential
biochemical marker for depression in elderly stroke
survivors. Food Nutr. Res. http://dx.doi.org/10.3402/
fnr.v56i0.14973 (2012).
52. Tang,W.K. etal. Association between high
serumtotal bilirubin and post-stroke depression.
Psychiatry Clin. Neurosci. 67, 259–264 (2013).
53. Zhan,Y. etal. Plasma-based proteomics reveals lipid
metabolic and immunoregulatory dysregulation in
post-stroke depression. Eur. Psychiatry 29, 307–315
(2014).
54. Li,Y.T., Zhao,Y., Zhang,H.J. &Zhao,W.L. The
association between serum leptin and post stroke
depression: results from a cohort study. PLoS ONE 9,
e103137 (2014).
55. Cheng,S.Y. etal. Plasma levels of glutamate during
stroke is associated with development of post-stroke
depression. Psychoneuroendocrinology 47,
126–135 (2014).
56. Mak,K.K., Kong,W.Y., Mak,A., Sharma,V.K.
&Ho,R.C. Polymorphisms of the serotonin
transporter gene and post-stroke depression:
a meta-analysis. J.Neurol. Neurosurg. Psychiatry 84,
322–328 (2013).
57. Kim,J.M. etal. A longitudinal study of SLC6A4 DNA
promoter methylation and poststroke depression.
J.Psychiatr. Res. 47, 1222–1227 (2013).
58. Harmandayan,M., Romanowicz,M. &Sola,C.
Successful use of ECT in post-stroke depression.
Gen. Hosp. Psychiatry 34, 102.e5–102.e6 (2012).
59. Bueno,V.F., Brunoni,A.R., Boggio,P.S.,
Bensenor,I.M. &Fregni,F. Mood and cognitive
effects of transcranial direct current stimulation in
post-stroke depression. Neurocase 17, 318–322
(2011).
60. Anderson,C.S., Hackett,M.L. &House,A.O.
Interventions for preventing depression after stroke.
Cochrane Database Syst. Rev. 3, CD003689
(2004).
61. Hackett,M.L., Anderson,C.S., House,A. &Xia,J.
Interventions for treating depression after stroke.
Cochrane Database Syst. Rev. 4, CD003437 (2008).
62. Hackett,M.L. etal. ImProving Outcomes after STroke
(POST): results from the randomized clinical pilot
trial. Int. J.Stroke 8, 707–710 (2013).
63. Committee,E.S.O.E.E. &Committee,E.W.
Guidelines for management of ischaemic stroke and
transient ischaemic attack 2008. Cerebrovasc. Dis.
25, 457–507 (2008).
64. Mead,G.E. etal. Selective serotonin reuptake
inhibitors (SSRIs) for stroke recovery.
CochraneDatabase Syst. Rev. 11 , CD009286
(2012).
65. Cleare,A. etal. Evidence-based guidelines for treating
depressive disorders with antidepressants: a revision
of the 2008 British Association for
Psychopharmacology guidelines. J.Psychopharmacol.
29, 459–525 (2015).
66. Sami,M.B. &Faruqui,R. The effectiveness of
dopamine agonists for treatment of neuropsychiatric
symptoms post brain injury and stroke.
Acta Neuropsychiatr. 27, 317–326 (2015).
67. Almeida,O.P. etal. B-vitamins reduce the long-term
risk of depression after stroke: the VITATOPS-DEP
trial. Ann. Neurol. 68, 503–510 (2010).
68. Peng,L., Zhang,X., Kang,D.Y., Liu,X.T. &Hong,Q.
Effectiveness and safety of Wuling capsule for post
stroke depression: a systematic review. Complement.
Ther. Med. 22, 549–566 (2014).
REVIEWS
10
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
69. Zhang,G.C. etal. Meta analysis of the curative
effectof acupuncture on post-stroke depression.
J.Tradit. Chin. Med. 32, 6–11 (2012).
70. Man,S.C. etal. A pilot controlled trial of a
combination of dense cranial electroacupuncture
stimulation and body acupuncture for post-stroke
depression. BMC Complement. Altern. Med. 14, 255
(2014).
71. Kim,D.S. etal. Effects of music therapy on mood in
stroke patients. Yonsei Med. J. 52, 977–981 (2011).
72. Herrmann,N. etal. Detection and treatment of post
stroke depression: results from the registry of the
Canadian stroke network. Int. J.Geriatr. Psychiatry 26,
1195–1200 (2011).
73. Fuller-Thomson,E., Tulipano,M.J. &Song,M. The
association between depression, suicidal ideation, and
stroke in a population-based sample. Int. J.Stroke 7,
188–194 (2012).
74. Yamauchi,T. etal. Death by suicide and other externally
caused injuries after stroke in Japan (1990–2010): the
Japan Public Health Center-based prospective study.
Psychosom. Med. 76, 452–459 (2014).
75. Santos,C., Caeiro,L., Ferro,J. &Figueira,M.A.
Study of suicidal thoughts in acute stroke patients.
J.Stroke Cerebrovasc. Dis. 21, 749–754 (2012).
76. Pompili,M. etal. Do stroke patients have an increased
risk of developing suicidal ideation or dying by suicide?
An overview of the current literature. CNSNeurosci.
Ther. 18, 711–721 (2012).
77. Tang,W.K. etal. Is insomnia associated with suicidality
in stroke? Arch. Phys. Med. Rehabil. 92, 2025–2027
(2011).
78. Tang,W.K. etal. Cerebral microbleeds and suicidality
in stroke. Psychosomatics 53, 439–445 (2012).
79. Tang,W.K., Liang,H., Mok,V., Ungvari,G.S.
&Wong,K.S. Is pain associated with suicidality in
stroke? Arch. Phys. Med. Rehabil. 94, 863–866
(2013).
80. Tang,W.K. etal. Apathy and suicide-related ideation
3months after stroke: a cross-sectional study.
BMCNeurol. 15, 60 (2015).
81. Nishida,N., Hata,Y., Yoshida,K. &Kinoshita,K.
Neuropathologic features of suicide victims who
presented with acute poststroke depression:
significance of association with neurodegenerative
disorders. J.Neuropathol. Exp. Neurol. 74, 401–410
(2015).
82. Podawiltz,A. A review of current bipolar disorder
treatment guidelines. J.Clin. Psychiatry 73, e12
(2012).
83. Vataja,R. &Kaste,M. in Neuropsychiatric Symptoms
of Cerebrovascular Disease Neuropsychiatric
Symptoms of Neurological Disease (ed. Ferro, J.M.)
81–108 (Springer, 2013).
84. Campbell Burton,C.A. etal. Frequency of anxiety after
stroke: a systematic review and meta-analysis of
observational studies. Int. J.Stroke 8, 545–559
(2013).
85. Ayerbe,L., Ayis,S.A., Crichton,S., Wolfe,C.D.
&Rudd,A.G. Natural history, predictors and
associated outcomes of anxiety up to 10years after
stroke: the South London Stroke Register. Age Ageing
43, 542–547 (2014).
86. Morrison,V., Pollard,B., Johnston,M. &MacWalter,R.
Anxiety and depression 3years following stroke:
demographic, clinical, and psychological predictors.
J.Psychosom. Res. 59, 209–213 (2005).
87. Tang,W.K., Lau,C.G., Mok,V., Ungvari,G.S.
&Wong,K.S. Impact of anxiety on health-related
quality of life after stroke: a cross-sectional study.
Arch. Phys. Med. Rehabil. 94, 2535–2541
(2013).
88. Tang,W.K. etal. Frontal infarcts and anxiety in stroke.
Stroke 43, 1426–1428 (2012).
89. Chi,S. etal. Tryptophan hydroxylase 2 gene
polymorphisms and poststroke anxiety disorders.
J.Affect. Disord. 144, 179–182 (2013).
90. Stein,M.B. &Sareen,J.Generalized anxiety disorder.
N.Engl. J.Med. 373, 2059–2068 (2015).
91. Campbell Burton,C.A. etal. Interventions for treating
anxiety after stroke. Cochrane Database Syst. Rev. 12,
CD008860 (2011).
92. Kneebone,I., Walker-Samuel,N., Swanston,J.
&Otto,E. Relaxation training after stroke: potential to
reduce anxiety. Disabil. Rehabil. 36, 771–774 (2014).
93. Golding,K., Kneebone,I. &Fife-Schaw,C. Self-help
relaxation for post-stroke anxiety: a randomised,
controlled pilot study. Clin. Rehabil. 30, 174–180
(2016).
94. Kiphuth,I.C., Utz,K.S., Noble,A.J., Kohrmann,M.
&Schenk,T. Increased prevalence of posttraumatic
stress disorder in patients after transient ischemic
attack. Stroke 45, 3360–3366 (2014).
95. Weiss,D. &Marmar,C. in Assessing Psychological
Trauma and PTSD (eds Wilson, J. &Keane, T. M.)
168–189 (The Guilford Press, 2004).
96. Favrole,P. etal. Frequency and predictors of post-
traumatic stress disorder after stroke: a pilot study.
J.Neurol. Sci. 327, 35–40 (2013).
97. Goldfinger,J.Z. etal. Correlates of post-traumatic
stress disorder in stroke survivors. J.Stroke
Cerebrovasc. Dis. 23, 1099–1105 (2014).
98. Bruggimann,L. etal. Chronic posttraumatic stress
symptoms after nonsevere stroke. Neurology 66,
513–516 (2006).
99. Letamendia,C. etal. Peritraumatic distress predicts
acute posttraumatic stress disorder symptoms after a
first stroke. Gen Hosp Psychiatry 35, e11–e13 (2012).
100. Kronish,I.M., Edmondson,D., Goldfinger,J.Z., Fei,K.
&Horowitz,C.R. Posttraumatic stress disorder and
adherence to medications in survivors of strokes and
transient ischemic attacks. Stroke 43, 2192–2197
(2012).
101. Roy,M.J., Costanzo,M.E., Blair,J.R. &Rizzo,A.A.
Compelling evidence that exposure therapy for PTSD
normalizes brain function. Stud. Health Technol. Inform.
199, 61–65 (2014).
102. Wright,A.G. etal. Stability of the DSM-5 Section III
pathological personality traits and their longitudinal
associations with psychosocial functioning in personality
disordered individuals. J.Abnorm. Psychol. 124,
199–207 (2015).
103. Guiraud,V. &Touzé,E. in Neuropsychiatric Symptoms
of Cerebrovascular Diseases Neuropsychiatric
Symptoms of Neurological Disease (ed. Ferro, J.M.)
255–298 (Springer, 2013).
104. Afanasiev,S., Aharon-Peretz,J. &Granot,M.
Personality type as a predictor for depressive symptoms
and reduction in quality of life among stroke survivals.
Am. J.Geriatr. Psychiatry 21, 832–839 (2013).
105. Jokela,M., Pulkki-Raback,L., Elovainio,M.
&Kivimaki,M. Personality traits as risk factors for
stroke and coronary heart disease mortality: pooled
analysis of three cohort studies. J.Behav. Med. 37,
881–889 (2014).
106. Votruba,K.L., Rapport,L.J., Whitman,R.D.,
Johnson,A. &Langenecker,S. Personality differences
among patients with chronic aphasia predict
improvement in speech–language therapy.
Top. Stroke Rehabil. 20, 421–431 (2013).
107. Jokela,M., Hakulinen,C., Singh-Manoux,A.
&Kivimaki,M. Personality change associated with
chronic diseases: pooled analysis of four prospective
cohort studies. Psychol. Med. 44, 2629–2640
(2014).
108. Marijnissen,R.M. etal. Depression in context of low
neuroticism is a risk factor for stroke: a 9-year cohort
study. Neurology 83, 1692–1698 (2014).
109. Cummings,J.L. etal. The Neuropsychiatric Inventory:
comprehensive assessment of psychopathology in
dementia. Neurology 44, 2308–2314 (1994).
110 . Starkstein,S.E. &Leentjens,A.F. The nosological
position of apathy in clinical practice. J.Neurol.
Neurosurg. Psychiatry 79, 1088–1092 (2008).
111. Habib,M. in Behavior and Mood Disorders in Focal
Brain Lesions (eds Bogousslavsky, J. &Cummings, J.L.)
261–284 (Cambridge Univ. Press, 2000).
112 . Caeiro,L., Ferro,J., e Melo,T., Canhao,P.
&Figueira,M. Post-stroke apathy: an exploratory
longitudinal study. Cerebrovasc. Dis. 35, 507–513
(2013).
113 . Caeiro,L., Ferro,J. &Figueira,M. Apathy in acute
stroke patients. Eur. J.Neurol. 19, 291–297 (2012).
114 . van Dalen,J.W., Moll van Charante,E.P.,
Nederkoorn,P.J., van Gool,W.A. &Richard,E.
Poststroke apathy. Stroke 44, 851–860 (2013).
115 . Withall,A., Brodaty,H., Altendorf,A. &Sachdev,P.S. A
longitudinal study examining the independence of
apathy and depression after stroke: the Sydney Stroke
Study. Int. Psychogeriatr. 23, 264–273 (2011).
116 . Caeiro,L., Ferro,J. &Costa,J. Apathy secondary to
stroke: a systematic review and meta-analysis.
Cerebrovasc. Dis. 35, 23–39 (2013).
117 . van Almenkerk,S., Smalbrugge,M., Depla,M.F.,
Eefsting,J.A. &Hertogh,C.M. Apathy among
institutionalized stroke patients: prevalence and clinical
correlates. Am. J.Geriatr. Psychiatry 23, 180–188
(2015).
118 . Brodaty,H. etal. Frequency and clinical,
neuropsychological and neuroimaging correlates of
apathy following stroke—the Sydney Stroke Study.
Psychol. Med. 35, 1707–1716 (2005).
119 . Hama,S. etal. Depression or apathy and functional
recovery after stroke. Int. J.Geriatr. Psychiatry 22,
1046–1051 (2007).
120. Santa,N. etal. Apathy and functional recovery
following first-ever stroke. Int. J.Rehabil. Res. 31,
321–326 (2008).
121. Marin,R.S., Biedrzycki,R.C. &Firinciogullari,S.
Reliability and validity of the Apathy Evaluation Scale.
Psychiatry Res. 38, 143–162 (1991).
122. Martin,R., Watson,D. &Wan,C.K. A three-factor
model of trait anger: dimensions of affect, behavior,
and cognition. J.Pers 68, 869–897 (2000).
123. Ishikawa,S. &Raine,A. in Neuropsychiatry (eds
Schiffer, R.B. etal.) 660–678 (Lippincott Williams
&Wilkins, 2003).
124. Ghika-Schmid,F., van Melle,G., Guex,P.
&Bogousslavsky,J. Subjective experience and
behavior in acute stroke: the Lausanne Emotion
inAcute Stroke Study. Neurology 52, 22–28
(1999).
125. Santos,C., Caeiro,L., Ferro,J., Albuquerque,R.
&Figueira,M. Anger, hostility and aggression in the
first days of acute stroke. Eur. J.Neurol. 13, 351–358
(2006).
126. Carota,A., Bogousslavsky,J. &Calabrese,P.
inNeuropsychiatric Symptoms of Cerebrovascular
Diseases Neuropsychiatric Symptoms of Neurological
Disease (ed. Ferro, J.M.) 161–188 (Springer, 2013).
127. Moran,P. etal. Personality disorder and cardiovascular
disease: results from a national household survey.
J.Clin. Psychiatry 68, 69–74 (2007).
128. Wermer,M.J., Kool,H., Albrecht,K.W. &Rinkel,G.J.
Subarachnoid hemorrhage treated with clipping: long-
term effects on employment, relationships, personality,
and mood. Neurosurgery 60, 91–97; discussion
97–98 (2007).
129. Ramos-Perdigues,S., Mane-Santacana,A.
&Pintor-Perez,L. Prevalence and associated factors of
anger post stroke: a systematic review. Rev. Neurol.
60, 481–489 (in Spanish) (2015).
130. Brodaty,H., Liu,Z., Withall,A. &Sachdev,P.S. The
longitudinal course of post-stroke apathy over five
years. J.Neuropsychiatry Clin. Neurosci. 25,
283–291 (2013).
131. Kang,S.Y. &Kim,J.S. Anterior cerebral artery
infarction: stroke mechanism and clinical-imaging
study in 100 patients. Neurology 70, 2386–2393
(2008).
132. Bogousslavsky,J. etal. Loss of psychic self-activation
with bithalamic infarction. Acta Neurol. Scand. 83,
309–316 (1991).
133. Caeiro,L., Santos,C., Ferro,J. &Figueira,M.
Neuropsychiatric disturbances in acute subarachnoid
haemorrhage. Eur. J.Neurol. 18, 857–864 (2011).
134. Tang,W.K. etal. Location of infarcts and apathy in
ischemic stroke. Cerebrovasc. Dis. 35, 566–571
(2013).
135. Mayo,N.E., Fellows,L.K., Scott,S.C., Cameron,J.
&Wood-Dauphinee,S. A longitudinal view of apathy
and its impact after stroke. Stroke 40, 3299–3307
(2009).
136. Angelelli,P. etal. Development of neuropsychiatric
symptoms in poststroke patients: a cross-sectional
study. Acta Psychiatr. Scand. 110 , 55–63 (2004).
137. Matsuoka,K. etal. Delayed atrophy in posterior
cingulate cortex and apathy after stroke.
Int.J.Geriatr. Psychiatry 30, 566–572 (2015).
138. Siegel,J.S. etal. The circuitry of abulia: insights from
functional connectivity MRI. NeuroImage Clin. 6,
320–326 (2014).
139. Yang,S.R., Shang,X.Y., Tao,J., Liu,J.Y. &Hua,P.
Voxel-based analysis of fractional anisotropy in post-
stroke apathy. PLoS ONE 10, e116168 (2015).
140. Rochat,L. etal. Poor reward sensitivity and apathy
after stroke: implication of basal ganglia. Neurology
81, 1674–1680 (2013).
141. Murakami,T. etal. Neuroanatomic pathways
associated with poststroke affective and apathetic
depression. Am. J.Geriatr. Psychiatry 21 , 840–847
(2013).
142. Raine,A. &Yang,Y. Neural foundations to moral
reasoning and antisocial behavior. Soc. Cogn. Affect.
Neurosci. 1, 203–213 (2006).
143. Wong,G.K. etal. Neuropsychiatric disturbance
afteraneurysmal subarachnoid hemorrhage.
J.Clin. Neurosci. 21, 1695–1698 (2014).
144. Oishi,K. etal. Critical role of the right uncinate
fasciculus in emotional empathy. Ann. Neurol. 77,
68–74 (2015).
145. Leigh,R. etal. Acute lesions that impair affective
empathy. Brain 136, 2539–2549 (2013).
REVIEWS
NATURE REVIEWS
|
NEUROLOGY ADVANCE ONLINE PUBLICATION
|
11
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
146. Skidmore,E.R. Training to optimize learning after
traumatic brain injury. Curr. Phys. Med. Rehabil. Rep.
3, 99–105 (2015).
147. Mikami,K. etal. Prevention of poststroke apathy using
escitalopram or problem-solving therapy.
Am.J.Geriatr. Psychiatry 21, 855–862 (2013).
148. Kohno,N. etal. Successful treatment of post-stroke
apathy by the dopamine receptor agonist ropinirole.
J.Clin. Neurosci. 17, 804–806 (2010).
149. Robinson,R.G., Jorge,R.E., Clarence-Smith,K.
&Starkstein,S. Double-blind treatment of apathy in
patients with poststroke depression using nefiracetam.
J.Neuropsychiatry Clin. Neurosci. 21, 144–151
(2009).
150. Waldemar,G. etal. Effect of donepezil on emergence
ofapathy in mild to moderate Alzheimer’s disease.
Int.J.Geriatr. Psychiatry 26, 150–157 (2011).
151. Frakey,L.L., Salloway,S., Buelow,M. &Malloy,P. A
randomized, double-blind, placebo-controlled trial of
modafinil for the treatment of apathy in individuals
with mild-to-moderate Alzheimer’s disease. J.Clin.
Psychiatry 73, 796–801 (2012).
152. Rosenberg,P.B. etal. Safety and efficacy of
methylphenidate for apathy in Alzheimer’s disease:
arandomized, placebo-controlled trial. J.Clin.
Psychiatry 74, 810–816 (2013).
153. Autret,K., Arnould,A., Mathieu,S. &Azouvi,P.
Transient improvement of poststroke apathy
withzolpidem: a single-case, placebo-controlled
double-blind study. BMJ Case Rep. http://dx.doi.org/
10.1136/bcr-2012-007816 (2013).
154. Luaute,J., Plantier,D., Wiart,L. &Tell,L.
Caremanagement of the agitation or
aggressivenesscrisis in patients with TBI. Systematic
review of the literature and practice
recommendations. Ann. Phys. Rehabil. Med. 59,
58–67 (2016).
155. Choi-Kwon,S. etal. Fluoxetine treatment in
poststroke depression, emotional incontinence, and
anger proneness: a double-blind, placebo-controlled
study. Stroke 37, 156–161 (2006).
156. Mead,G. etal. The FOCUS, AFFINITY and EFFECTS
trials studying the effect(s) of fluoxetine in patients
with a recent stroke: a study protocol for three
multicentre randomised controlled trials. Trials 16,
369 (2015).
Acknowledgements
This review was partially supported by a post-doctoral grant
(“Rehabilitation of apathetic stroke patients”, SFRH/
BPD/100399/2014) from the Fundação para Ciência e
Tecnologia to Lara Caeiro.
Author contributions
All authors contributed substantially to the discussion of
the content and researched the data for the article. J.M.F.
and L.C. wrote and reviewed and/or edited the manuscript
before submission.
Competing interests statement
The authors declare no competing interests.
Review criteria
In this narrative review, we did not follow the Preferred
Reporting Items for Systematic Reviews and Meta-analysis
(PRISMA). We retrieved background information from pub-
lications and books and performed a MEDLINE/PubMed
search of relevant publications from 2010 to 2015 using
the following keywords: “depression”, “poststroke depres-
sion”, “suicide”, “bipolar”, “mania”, “psychosis”, “anxiety”,
“post-traumatic stress disorder”, “stress disorder”, “per-
sonality”, “personality disorders”, “lability”, “emotional
control”, “disinhibition”, “aggressiveness”, “aggression”,
“hostility”, “irritability”, “anger”, “poststroke apathy” and
“apathy AND stroke”. We also searched the Cochrane
Central Register of Controlled Trials, the Internet Stroke
Centre and ClinicalTrials.gov. References were selected on
hierarchy of evidence, study quality, clinical relevance
andinnovation.
REVIEWS
12
|
ADVANCE ONLINE PUBLICATION www.nature.com/nrneurol
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.
©
2016
Macmillan
Publishers
Limited.
All
rights
reserved.