Content uploaded by Jeong Kyung Kim
Author content
All content in this area was uploaded by Jeong Kyung Kim on Jan 09, 2016
Content may be subject to copyright.
Content uploaded by Jeong Kyung Kim
Author content
All content in this area was uploaded by Jeong Kyung Kim on Jan 09, 2016
Content may be subject to copyright.
1
Received March 15, 2011
1
P511 RR/ Dyslipidemia 2
Final
3
Cover title : Morning vs. Evening Administration of Ezetimibe/Simvastatin 4
5
Comparison of Effects of Morning Versus Evening Administration of Ezetimibe/Simvastatin on 6
Serum Cholesterol in Patients with Primary Hypercholesterolemia 7
8
Hyung Sik Yoon, Sung Ho Kim, Jeong Kyung Kim, Sang Hun Ko, Jae Ee Ko, Soo Jin Park, 9
Moon Gi Park, Jae Hwan Lee, and Min Soo Hyon
10
11
12
Background: Ezetimibe, a first-in-its-class inhibitor of cholesterol absorption, is an effective
13
agent for combined use with statins to achieve low-density lipoprotein cholesterol (LDL-C) goals. 14
Ezetimibe in combination with simvastatin as a single-tablet formulation has proven to be highly 15
effective in reducing serum LDL-C through the dual inhibition of cholesterol absorption and 16
biosynthesis. The effect of time of administration on efficacy of this combination therapy has not 17
been evaluated.
18
Objective: To compare the effects of morning versus evening administration of 19
ezetimibe/simvastatin on serum cholesterol levels of patients with primary hypercholesterolemia. 20
Methods: In this multicenter, open-label, randomized, 2-sequence, 2-period crossover study, 21
patients with primary hypercholesterolemia randomly received ezetimibe/simvastatin 22
10 mg/20 mg once daily, either in the morning (within 1 hour of breakfast) or in the evening,
23
Deleted: Specifically, e
Deleted: (ezetimibe/simvastatin),
Deleted:
,
Deleted: d
Deleted: However, t
Deleted: ed
Deleted: We aimed t
Deleted: the
2
(within 1 hour of dinner) for 6 weeks.
1
Results: Data on 171 patients (87 in the morning administration group and 84 in the evening 2
administration group) were analyzed. A significant reduction (p ≤ 0.001) in the total cholesterol,
3
triglyceride, high-density lipoprotein cholesterol, LDL-C, apo-lipoprotein B, and high-sensitivity 4
C-reactive protein (hs-CRP) from baseline was achieved after each treatment. Noninferiority of 5
morning administration versus evening administration was shown in the percentage reduction of 6
the LDL-C level from baseline (difference, -1.62%; 90% CI -4.94 to 1.70). No significant 7
difference was found between groups with respect to the percentage of changes in other lipid
8
parameters from baseline. Furthermore, there was no significant difference in the percentage of 9
change in hs-CRP as an antiinflammatory marker between the morning and evening
10
administration groups. The frequency of adverse events was similar between groups. 11
Conclusions: Morning administration of ezetimibe/simvastatin 10 mg/20 mg is noninferior to 12
evening administration with respect to LDL-C-lowering ability.
13
Key Words: chronobiologic variation, ezetimibe, high-sensitivity C-reactive protein, 14
hypercholesterolemia, simvastatin. 15
16
Clinical trials have demonstrated that a reduction in serum low-density lipoprotein cholesterol 17
(LDL-C) to the target level has favorable effects on cardiovascular disease (CVD) by reducing
18
the frequency of progressing coronary lesions, causing regression of atherosclerotic lesions, 19
decreasing cardiovascular morbidity and mortality, and decreasing the total mortality.
1-3
20
21
Ezetimibe, a novel agent that can inhibit the intestinal absorption of cholesterol from dietary and 22
biliary sources, blocking the Niemann-Pick C1-like 1 protein for cholesterol transport across the 23
Deleted: One hundred seventy-one
Deleted: administration
Deleted: the
3
intestinal wall.
4,5
The drug is rapidly metabolized in the intestine to aphenolic glucuronide; once 1
glucuronidated, it is excreted in the bile, thereby returning to the site of action. Cholesterol 2
absorption studies have indicated that the glucuronide form may be more potent than ezetimibe 3
itself, because glucuronidated ezetimibe localizes to a greater extent in the intestine. Ezetimibe 4
and its glucuronide have a half-life of approximately 22 hours in humans.
6
5
Ezetimibe 10 mg/day decreases the serum LDL-C level by about 16-22%.
7
Owing to the dual 6
point of inhibition (ie, cholesterol absorption and biosynthesis), combined therapy of ezetimibe
7
with a statin results in a greater decrease in LDL-C.
8,9
Furthermore, such therapy leads to a 8
significant reduction in C-reactive protein (CRP) compared with statin monotherapy.
10
9
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III 10
recommends hydroxymethylglutaryl coenzyme A reductase inhibitors as the first-line therapy for 11
reducing the LDL-C.
11
On the basis of the short half-lives of simvastatin,
12
lovastatin,
13
and
12
fluvastatin,
14
ranging from 1 hour to 5 hours, and peak cholesterol biosynthesis, occurring 13
between midnight and 0300 hours, the manufacturers have recommended evening administration
14
of these drugs.
15
Alternatively, atorvastatin,
16
atorvastatin-active ortho-hydroxylated and para-15
hydroxylated metabolites, pravastatin,
17
and rosuvastatin
18
have long half-lives (14, 20, 30, 22, 16
and 19 hours, respectively) and can be administered at any time of the day. The recent change in 17
the NCEP ATP III guidelines recommends a target LDL-C of 70 mg/dL or less for very high-risk 18
individuals; therefore, the appropriate time of drug administration to achieve the maximal
19
reduction is important.
19
However, with the long half-life of ezetimibe (22 hours), the effect of 20
the time of administration on the efficacy of combined therapy of ezetimibe with simvastatin has
21
not been established.
22
The aim of this study was to compare effects of morning versus evening administration of 23
Deleted: in
Deleted:
, namely the inhibition of
Deleted: (HMG-CoA)
Co mment: 3 A AUTHOR: Is this also
from ref 16? If not, provide reference
Deleted: ≤
4
ezetimibe and simvastatin as a combined therapy (ezetimibe/simvastatin) on serum cholesterol of
1
patients with primary hypercholesterolemia. 2
3
Methods 4
5
Design 6
This open-label, randomized, 2-period, 2-sequence, crossover study was conducted according to 7
Good Clinical Practice and in accordance with the Declaration of Helsinki (Helsinki, Finland,
8
1964) and all subsequent amendments at 2 centers from January 2008 to June 2009. The ethics 9
committee or institutional review board of each participating center approved the protocol, and
10
all study participants provided written informed consent at the time of study enrollment. 11
Random sequence generation was used to assign subjects in a crossover fashion (to prevent bias 12
from the open-label design) to 2 treatment sessions of equal duration (6 weeks).
13
Ezetimibe/simvastatin 10 mg/20 mg was administered as a single tablet daily within 1 hour of 14
breakfast (morning administration) or dinner (evening administration) in period 1 and the 15
regimen was interchanged in period 2. The study also included a 4-week lead-in period. 16
17
Main inclusion criteria
18
Patients 18 years or older with an LDL-C at or above the drug treatment threshold established by 19
the NCEP ATP III guidelines were eligible for enrollment if they met the following criteria: (1) 20
established CHD or risk equivalent, 2 or more risk factors conferring a 10-year risk larger than 21
20% for CHD (Framingham score), and an LDL-C level of 130 mg/dL or more; (2) no CHD or 22
risk equivalent, 2 or more risk factors conferring a 10-year risk less than 20% for CHD, and an
23
Deleted:
,
Deleted: subjects
Deleted: ≥
Deleted: or ≥
Deleted: >
Deleted: ≥
Deleted:
≥
Deleted: <
5
LDL-C level of 160 mg/dL or more; or (3) no CHD or risk equivalent, less than 2 risk factors
1
conferring a 10-year risk lower than 20% for CHD, and an LDL-C level of 190 mg/dL or more. 2
3
Main exclusion criteria 4
5
Exclusion criteria included allergy, hypersensitivity, or intolerance to ezetimibe or statin; 6
consumption of more than 2 alcoholic drinks per day or a history of substance abuse or 7
dependency within 12 months before screening; and active hepatitis or other liver disease.
8
Patients with the following conditions were also excluded: persistent uncontrolled hypertension; 9
unstable angina, non-ST-segment elevation myocardial infarction, or ST-segment elevation
10
myocardial infarction within the previous 12 months; angioplasty, stroke, transient ischemic 11
attack, or deep vein thrombosis within the previous 3 months; coronary artery bypass graft 12
surgery within the previous 6 months; uncontrolled cardiac arrhythmias; acute or subacute
13
peripheral artery disease occlusion; congestive heart failure (New York Heart Association class 14
III or IV); fasting serum triglyceride greater than 350 mg/dL, alanine aminotransferase or 15
aspartate aminotransferase 1.5 times or more the upper limit of normal, serum creatinine greater 16
than 1.5 mg/dL, creatine kinase 1.5 times or more the upper limit of normal, and hemoglobin 17
A1C level 9% or more in patients with diabetes; poorly controlled type 1 or type 2 diabetes;
18
active cancer or a diagnosis of cancer within the last 5 years; fibromyalgia, myopathy, 19
rhabdomyolysis, unexplained muscle pain, and/or discontinuation of a statin due to myalgia; or 20
life expectancy less than 2 years. 21
Patients taking non-statin lipid-lowering drugs, immunosuppressants, corticosteroids, or potent 22
CYP3A4 inhibitors were also excluded from participation. All lipid-altering drugs were
23
Deleted:
≥
Deleted:
Deleted: <
Deleted: <
Deleted: ≥
Co mment: 5 A AUTHOR: Allergy to any
statin?
Deleted:
Deleted: Other criteria included a
Deleted: >
Deleted:
≥
Deleted: normal
Deleted:
Deleted: level >
Deleted: .
Deleted: a
Deleted: level ≥
Deleted: normal
Deleted: an
Deleted:
≥
Deleted: <
Deleted:
cytochrome P-450
6
discontinued for 6 weeks (for statins) or 8 weeks (for fibrates) before randomization.
1
2
Screening, randomization, and treatment
3
4
At screening, patients underwent a complete physical examination, recording of medical history 5
(eg, medical conditions, smoking status, medications), and laboratory assessments. They then 6
entered a 4-week dietary lead-in in which they were encouraged to follow the Step II diet (<7% 7
saturated fat and <200 mg/day cholesterol) described in the NCEP ATP II guidelines
19
and all
8
lipid-lowering treatments were discontinued. After this lead-in, eligible patients randomly 9
received ezetimibe/simvastatin 10 mg/20 mg in the morning or evening for 6 weeks. During the
10
study, patients were not permitted to take alternative lipid-modifying medications or any of the 11
following agents: amiodarone, isotretinoin, cyclosporine, danazol, nefazodone, rifampin, oral 12
macrolide antibiotics, telithromycin, oral azole antifungals, preparations containing St. John’s
13
wort or Ginkgo biloba, verapamil, systemic androgens or anabolic steroids, and chronic systemic 14
corticosteroids. They were instructed to take the medicine orally, once daily, within 1 hour of 15
breakfast (morning administration) or dinner (evening administration) and to follow the NCEP 16
Adult Treatment Panel (ATP) III Therapeutic Lifestyle Change diet for the duration of the 17
study.
12
18
19
20
Laboratory tests 21
22
23
24
Efficacy variables were assessed using 12- to 14-hour fasting blood samples after dinner. 25
Samples were collected at the beginning of the dietary lead-in period (week −4), 2 weeks after
26
Formatted: Font:Italic
Co mment: 6 A AUTHOR: Do you mean
the ATP III guidelines? (see ref. 19).
Deleted: 3-
Deleted: e
Deleted: (TLC)
Co mment: 6B AUTHOR: Should this be
ref. 11?
7
the beginning of this period (week −2), and during randomization (week 0). The baseline fasting
1
lipid levels were the mean of these 3 measurements. Samples were collected at weeks 6 and 12 2
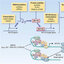
for lipid and other laboratory analyses (Figure 1). Laboratories at each clinical center performed
3
the lipid and other laboratory measurements using standardized procedures. The laboratories are 4
certified by the Korean Society of Clinical Pathologists and the Korean Society for Laboratory 5
Medicine. Plasma glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol 6
(HDL-C), aspartate aminotransferase, alanine aminotransferase, creatine kinase, and lactate 7
dehydrogenase levels were measured by using enzymatic kits (Sigma Diagnostics, Taufkirchen,
8
Germany) in a BM-Hitachi 747-200 autoanalyzer (Hitachi-Roche, Tokyo, Japan). LDL-C was 9
calculated by the Friedewald equation.
20
Serum apo-lipoprotein B (apo-B) and high-sensitivity
10
C-reactive protein (hs-CRP) levels were determined using an immunoturbidometric method 11
(Cobas Integra 800 automatic analyzer; Roche Diagnostics, Basel, Switzerland). The intraassay 12
coefficient of variation for each laboratory was in the range 3.2-4.7%.
13
14
Outcomes
15
16
17
18
Efficacy
19
The primary endpoint was percentage change in the LDL-C level from baseline to the end of 20
treatment. Secondary endpoints included percentage changes in total cholesterol, triglyceride,
21
HDL-C, apo-B, and hs-CRP levels from baseline to the end of treatment. 22
23
24
25
Safety
26
27
Safety analysis was based on data collected for adverse events, routine chemistry and 28
Deleted: Further, s
Deleted: by
Deleted: by
8
hematology analyses, urinalysis, vital sign measurements, and physical examinations. Safety data
1
were collected at each visit, including the last visit. 2
3
Adherence 4
5
Standard pill-count was used to assess adherence. At each visit, the number of remaining pills 6
in the containers was counted. Adherence was defined by the number of pills taken (dispensed 7
pills – returned pills) in relation to the theoretical number of prescribed doses. Patients with an
8
adherence rate lower than 75% were excluded from the per-protocol (PP) analysis. 9
10
11
Statistical analysis 12
13
14
Randomization was conducted by using a number generator in blocks of 6 patients with nQuery 15
Advisor 7.0 (Statistical Solutions Ltd., Cork, Ireland). We hypothesized that both strategies 16
would result in similar reductions of approximately 40% in serum LDL-C levels. The
17
noninferiority boundary was determined from Lund et al.,
21
who compared morning blood 18
samples and included morning versus evening administration, primarily of simvastatin 20 mg,
19
with a crossover design. In their study, the difference in mean percentage of change in LDL-C 20
from the baseline was 13.4% higher in the evening group, with a 95% CI of 7.59% to 18.67%. 21
We choose restrictive and smaller margin of 95% CI of the Lund study for noninferiority 22
referenced from Kaul and Diamond.
22
Therefore, the noninferiority boundary in our study was 23
set at 7%.
24
25
Deleted: Compliance
Deleted: compliance
Deleted: <
Deleted: made
Deleted:
a
Deleted: ~
Deleted:
Deleted: mostly
Deleted: simvastatin
Deleted: confidence interval (
Deleted: )
Deleted: et al..
9
The intent-to-treat (ITT) population comprised all randomized patients who received at least 1
1
dose of study medication and had at least 1 efficacy data point. The PP population was analyzed 2
for all efficacy endpoints because a PP population is associated with less variability than is an
3
ITT population (which includes nonconforming patients) and protocol violations are more likely 4
to reduce the treatment efficacy. Accordingly, analysis of a PP population is considered the more 5
conservative and statistically correct approach for demonstrating noninferiority.
23
All efficacy 6
analyses performed on the PP population were confirmed by ITT analysis. 7
8
The safety analysis population comprised all randomized patients who received at least 1 dose 9
of study medication and had at least 1 follow-up data point. A total sample size of 142 achieves
10
90% power to detect noninferiority of differences in a 2 × 2 crossover design by using a 1-sided 11
t-test. Morning administration of ezetimibe/simvastatin was considered noninferior to evening 12
administration if the upper limit of the 2-sided 90% CI for the difference in mean percent change
13
in LDL-C from baseline was less than 7%. The sample was divided equally between the 14
administration groups. The significance level was set at 0.05, and the square root of the mean 15
square error was 20.0. 16
17
Treatment effects were compared with respect to the changes from the baseline to the 2 efficacy
18
end points by using an analysis of covariance that included treatment, center, and baseline 19
measures as the covariate in the model. Power Analysis and Sample Size for Windows version 20
2008 (NCSS, Kaysville, Utah) was used to estimate the required sample size for noninferiority in 21
the 2 × 2 crossover design. Statistical analyses for comparison of least-squares means with 90% 22
CI for the treatment difference (morning administration – evening administration) in the 2 × 2
23
Deleted:
one
Deleted: one
Deleted: ,
Deleted: <
Deleted: among
10
crossover design were conducted in a model that included treatment, period, and carryover effect
1
by using NCSS for Windows version 2007, according to the methods described by Chow and 2
Liu
24
and Chow et al.
25
3
Results 4
One hundred seventy-one patients were enrolled and randomized (Figure 2); 145 patients 5
completed the study and 26 withdrew. Among adverse events, 3 patients developed myalgia, 2 6
patients experienced diarrhea, and 3 patients had indigestion. There were no significant 7
differences between the treatment groups with regard to the demographic and baseline disease
8
characteristics or maintenance medication (Table 1). 9
10
The ITT population consisted of 151 and 150 patients were included in the morning and evening 11
administration groups, respectively. In the PP population, there were 155 and 154 patients in the 12
morning and evening administration groups, respectively. In the PP population, marked
13
reductions in the LDL-C level from baseline were observed in morning (46.33%, p < 0.001) and 14
evening administration (47.95%, p < 0.001) groups, with no significant difference between the 15
groups. 16
17
18
Efficacy 19
20
Primary endpoint 21
22
In the PP population, noninferiority of morning administration compared with evening
23
Deleted: participants discontinued the trial.
Deleted: suffered from
Deleted: had
11
administration was shown in percentage reduction in LDL-C from baseline because the 90% CI
1
did not deviate from the prespecified difference of 7% to satisfy the noninferiority criterion. The 2
corresponding analyses performed on the ITT population supported the findings of the PP
3
population, with no period effects (p = 0.09) or carryover effects (p = 0.87) (Table 2). 4
5
Secondary endpoints 6
7
There were no significant differences between groups in terms of percentage changes in total
8
cholesterol, triglyceride, HDL-C, and apo-B (Table 3). The baseline and follow-up hs-CRP 9
concentrations showed significant differences (p < 0.001) for both groups. At week 6, the mean
10
hs-CRP decreased from 2.64 mg/dL to 2.01 mg/dL in the morning administration group and from 11
2.48 mg/dL to 1.92 mg/dL in the evening administration group. The mean percentage changes 12
were 21.04% and 23.75% in the morning and the evening administration groups, respectively,
13
with no significant between-administration period difference. 14
15
Safety 16
Study treatments were well tolerated. The adverse events profile was generally similar between 17
groups; most adverse events were mild to moderate and were considered unrelated to the
18
treatment. In addition, there were no deaths, and no treatment-related serious adverse events 19
were reported. At least 1 clinical adverse event was observed in 26 patients in the morning 20
administration group and 27 patients in the evening administration group (Table 4). There were 21
no reports of a clinically significant increase in serum creatine kinase (>10 times the upper limit 22
of normal) or cases of rhabdomyolysis.
23
Deleted: . Analyses demonstrated that there
was
Deleted: 8
Deleted: s
Deleted: T
Deleted: normal
12
1
Discussion 2
3
To our knowledge, this study is the first to assess the effect of time of ezetimibe/simvastatin 4
administration on the serum cholesterol level. The results show that morning administration of 5
ezetimibe/simvastatin is noninferior to evening administration. 6
7
C-Reactive protein measured by high-sensitivity assays is widely accepted as an independent
8
marker of cardiovascular risk in otherwise healthy populations, on the basis of multiple 9
observational studies and randomized clinical trials.
26-28
The use of hs-CRP for risk stratification
10
of patients with established coronary artery disease has not been recommended in the American 11
Heart Association/Centers for Disease Control and Prevention guidelines, but is supported by the 12
results of clinical trials, including a large trial conducted in patients with stable symptoms.
29
13
Statins reduce hs-CRP levels in a manner largely independent of LDL-C,
30
and statin plus 14
ezetimibe therapy reduces hs-CRP more effectively than statin monotherapy.
31
15
16
Although ezetimibe alone reduces cholesterol by 15-20%, when used in conjunction with statins 17
(ie, dual therapy), it can enhance the cholesterol-lowering of statins an additional 20%.
32
Dose-
18
scheduling studies of ezetimibe monotherapy in Phase 2 trials evaluated 2 doses, 5 mg/day and 19
10 mg/day, and morning versus evening dosing with and without food in approximately 40 20
patients per group. This series of studies found no significant difference between morning and 21
evening dosing, with 10 mg/day resulting in a 17.5% reduction in LDL-C versus an 18.2% 22
reduction with evening dosing.
33
23
Deleted: The present
Deleted: and
Deleted: s
Deleted: (AHA/CDC)
Deleted: so-called
13
1
Simvastatin and its active metabolites have short half-lives (<5 hours), which may explain the 2
differences detected in clinical trials favoring evening administration.
34
With its short half-life (2
3
hours) and an active metabolite with an unknown duration of action, lovastatin also revealed a 4
trend toward a greater decrease in LDL-C on evening administration.
35
However, pravastatin and 5
rosuvastatin, which have long half-lives (22 hours and 19 hours, respectively), do not have active 6
metabolites, and their lipid-lowering effects may wane at the end of a 24-hour interval, possibly 7
explaining a slight trend toward more reduction in LDL-C with evening administration in clinical
8
trials.
36,37
Atorvastatin has a long half-life (14 hours) and also has active metabolites with half-9
lives in the range 20-30 hours, which may explain why evening versus morning administration of
10
atorvastatin demonstrated no significant difference between study groups.
38
To our knowledge, 11
there has been no study conducted to evaluate the chronobiologic effects of the administration of 12
fluvastatin, which has a short half-life (<3 hours) and no active metabolites, but its extended-
13
release formulation (longer pharmacodynamic effect of 7.3-10.5 hours) showed equivalent lipid-14
lowering efficacy between morning and evening administration.
39
15
16
Therefore, ezetimibe, with a long half-life and active metabolites, in combination with 17
simvastatin was expected to have equivalent lipid-lowering efficacy between morning and
18
evening administration. However, this is not a full explanation of the 19
pharmacologic/pharmacokinetic plausibility for the fact that no significant differences were 20
found in LDL-C reduction between the 2 regimens. We agree that this result cannot be 21
generalized in Asian or white patients, because there may be pharmacogenomic differences 22
between these populations.
23
Deleted: But
Deleted: Caucasian
Deleted:
an Asian patient population and a
Caucasian patient
14
1
Although the results are very encouraging, the study has several limitations. First, its open-label, 2
unblinded design limits generalizability beyond the study groups; however, the randomized
3
assignment of patients and the crossover design could reduce the bias. Second, no washout 4
period was used. However, patients with angina, diabetes, and hypertension were included in this 5
study; treatment with antilipid agents for primary or secondary prevention of CVD should not be 6
interrupted in these patients. We believed that avoiding the unethical action of stopping the 7
antilipid agent during a washout period was more important than inclusion of a washout period.
8
The results of testing possible period effects and carryover effects according to Chow and Liu
24
9
and Chow et al.
25
can lessen this limitation.
10
11
Recently, results of the SHARP (Study of Heart and Renal Protection) trial,
40
which evaluated 12
the effects of lowering LDL-C levels with ezetimibe 10 mg and simvastatin 20 mg daily versus
13
placebo in 9438 patients with chronic kidney disease (CKD), were published. The findings 14
suggest that the addition of ezetimibe to statin therapy is effective and safe in treating 15
dyslipidemia in patients with CKD and those who have undergone kidney transplant. CVD is 16
the main cause of morbidity and mortality in kidney transplant recipients. The SHARP trial 17
provides evidence about the efficacy and safety of lowering LDL-C with the combination of
18
ezetimibe and simvastatin among wide range of patients with CKD. 19
20
In conclusion, daily morning administration of ezetimibe/simvastatin 10 mg/20 mg is noninferior 21
to daily evening administration in reduction of LDL-C levels.
22
23
24
Deleted: its
Deleted: of this study
Deleted: presented in this study
Deleted: This study included
Deleted: ve patients
Deleted: ,
Deleted: these patients should continue to
maintain
Deleted: cardiovascular disease
Deleted: have considered
Deleted: problem induced by
Deleted: is more serious than the degree of
completion of the study. We expect t
Deleted: can weaken the
Deleted: (SHARP
Deleted: s
Deleted:
the
Deleted: the
Deleted: patients. Cardiovascular disease
Deleted: This
Deleted: results
Deleted: ity with respect
Deleted: as daily evening administration.
15
Hyung Sik Yoon BSc MD, Resident, Department of Internal Medicine, Sun General Hospital,
1
Cardiovascular Center, Daejeon, Korea 2
Sung Ho Kim, MD BSc
,
Resident, Department of Internal Medicine, Sun General Hospital,
3
Cardiovascular Center, Daejeon 4
Jeong Kyung Kim MD MSc,
,
Director, of Department of Internal Medicine, Sun General 5
Hospital, Cardiovascular Center, Daejeon 6
Sang Hun Ko BSc MD
,
Resident, Division of Endocrinology, Department of Internal Medicine, 7
Sun General Hospital
8
Jae Ee Ko BSc MD
,
Resident, Department of Internal Medicine, Division of Endocrinology, Sun 9
General Hospital
10
Soo Jin Park MSc MD
,
Staff, Department of Internal Medicine, Sun General Hospital; 11
Cardiology physician, Cardiovascular Center, Daejeon 12
Moon Gi Park BSc MD
,
Chief of Vascular Surgery, Department of General Surgery, Sun General
13
Hospital 14
Jae Hwan Lee MD PhD
,
Associate Professor, Department of Internal Medicine, 15
Chungnam National University; Cardiology physician, Department of Internal Medicine, 16
Cardiovascular Center, Daejeon 17
Min Soo Hyon MD PhD
,
Professor, Department of Internal Medicine, College of Medicine,
18
Soonchunhyang University; Cardiology physician, Cardiovascular Institution, Seoul, Korea 19
Correspondence: Dr. Jeong Kyung Kim, dr_doom@naver.com 20
Reprints/Online Access: www.theannals.com/cgi/reprint/aph1.P511 21
Conflict of interest: Authors reported none 22
References
23
Co mment: 15A AUTHOR: Please
provide city; Asan ?
16
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering
1
in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 2
Lancet 1994;344:1383-9.
3
2. Shepherd J, Cobbe SM, Ford I, et al.; The West of Scotland Coronary Prevention Study Group. 4
Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl 5
J Med 1995;333:1301-7. 6
3. Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol 7
2003;91(suppl):4-8.
8
4. van Heek M, Farley C, Compton DS, Hoos LM, Smith-Torhan A, Davis HR. Ezetimibe 9
potently inhibits cholesterol absorption but does not affect acute hepatic or intestinal cholesterol
10
synthesis in rats. Br J Pharmacol 2003;138:1459-64. 11
5. Altmann SW, Davis HR Jr, Zhu LJ et al. Niemann-Pick C1 like 1 protein is critical for 12
intestinal cholesterol absorption. Science 2004;303:1201-4.
13
6. Darkes MJ, Poole RM, Goa KL. Ezetimibe. Am J Cardiovasc Drugs 2003;3:67-76. 14
7. Kosoglou T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction between the new 15
selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol 16
2002;54:309-19. 17
8. Ose L, Shah A, Davis MJ, et al. Consistency of lipid-altering effects of ezetimibe/simvastatin
18
across gender, race, age, baseline low density lipoprotein cholesterol levels, and coronary heart 19
disease status: results of a pooled retrospective analysis. Curr Med Res Opin 2006;22:823-35. 20
9. Kalogirou M, Tsimihodimos V, Saougos V, Lagos K, Tselepis AD, Elisa M. Effect of 21
ezetimibe on lipoprotein subfraction concentrations: the role of atorvastatin pretreatment. Arch 22
Med Sci 2007;3:344-50.
23
17
10. Pearson T, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Comparison of effects of
1
ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and 2
low-density lipoprotein cholesterol levels. Am J Cardiol 2007;99:1706-13.
3
11. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 4
Executive Summary of The Third Report of the National Cholesterol Education Program 5
(NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in 6
Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97. 7
12. Package insert. Zocor (simvastatin) tablets. Whitehouse Station, NJ: Merck Pharmaceuticals,
8
November 2009. 9
13. Package insert. Mevacor (lovastatin) tablets. Whitehouse Station, NJ: Merck Pharmaceuticals,
10
November 2009. 11
14. Package insert. Lescol (fluvastatin) tablets. East Hanover, NJ: Novartis Pharmaceuticals, 12
May 2009.
13
15. Parker TS, McNamara DJ, Brown C, et al. Mevalonic acid in human plasma: relationship of 14
concentration and circadian rhythm to cholesterol synthesis rates in man. Proc Natl Acad Sci U S 15
A 1982;79:3037-4. 16
16. Package insert. Lipitor (atorvastatin calcium) tablets. Morris Plains, NJ: Pfizer 17
Pharmaceuticals, July 2009.
18
17. Package insert. Pravachol (pravastatin) tablets. Princeton, NJ: Bristol-Myers Squibb, 19
November 2009. 20
18. Package insert. Crestor (rosuvastatin) tablets. Wilmington, DE: AstraZeneca, March 2009. 21
19. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the 22
National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation
23
18
2004;110:227-39.
1
20. Bairaktari E, Hatzidimou K, Tzallas C, et al. Estimation of LDL cholesterol based on the 2
Friedewald formula and on apo B levels. Clin Biochem 2000;33:549-555. DOI 10.1016/S0009-
3
9120(00)00162-4 4
21. Lund TM, Torsvik H, Falch D, Christophersen B, Skårdal R, Gullestad L. Effect of morning 5
versus evening intake of simvastatin on the serum cholesterol level in patients with coronary 6
artery disease. Am J Cardiol 2002;90:784-6. 7
22. Kaul S, Diamond GA. Making sense of noninferiority: a clinical and statistical perspective
8
on its application to cardiovascular clinical trials. Prog Cardiovasc Dis 2007;49:284-99. 9
23. Lewis JA. Statistical principles for clinical trials (ICH E9): an introductory note on an
10
international guideline. Statist Med 1999;18:1903-42. 11
24. Chow S-C, Liu J-P. Design and analysis of bioavailability and bioequivalence studies. 2nd ed. 12
New York, NY: Marcel Dekker, Inc., 1999:73-7.
13
25. Chow S-C, Shao J, Wang H. Sample size calculations in clinical research. 1st ed. New York, 14
NY: Marcel Dekker, Inc., 2003:63-8. 15
26. Koenig W, Sund M, Frölich M, et al. C-Reactive protein, a sensitive marker of inflammation, 16
predicts future risk of coronary heart disease in initially healthy middle-aged men: results from 17
the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg
18
Cohort Study, 1984 to 1992. Circulation 1999;99:237-42. 19
27. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular 20
disease: application to clinical and public health practice. A statement for healthcare 21
professionals from the Centers for Disease Control and Prevention and the American Heart 22
Association. Circulation 2003;107:499-511.
23
19
28. Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein associated phospholipase A2,
1
high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged 2
men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation
3
2004;109:837-42. 4
29. Sabatine MS, Morrow DA, Jablonski KA, et al. Prognostic significance of the Centers for 5
Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for 6
cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 7
2007;115:1528-36.
8
30. Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting 9
of statin therapy in the primary prevention of acute coronary events. N Engl J Med
10
2001;344:1959- 65. 11
31. Gagné C, Bays HE, Weiss SR, et al.; Ezetimibe Study Group. Efficacy and safety of 12
ezetimibe added to ongoing statin therapy for treatment of patients with primary
13
hypercholesterolemia. Am J Cardiol 2002;90:1084-91. 14
32. Gagné C, Gaudet D, Bruckert E, for the Ezetimibe Study Group. Efficacy and safety of 15
ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial 16
hypercholesterolemia. Circulation 2002;105:2469-75. 17
33. Stein EA. An investigative look: selective cholesterol absorption inhibitors—embarking on a
18
new standard care. Am J Manag Care 2002;8(2 suppl):S36-9;discussion S45-7. 19
34. Wallace A, Chinn D, Rubin G. Taking simvastatin in the morning compared with in the 20
evening: randomised controlled trial. BMJ 2003;327:788. 21
35. Illingworth RD. Comparative efficacy of once versus twice daily mevinolin in the therapy of 22
familial hypercholesterolemia. Clin Pharmacol Ther 1986;40:338-43.
23
20
36. Hunninghake DB, Mellies MJ, Goldberg AC, et al. Efficacy and safety of pravastatin in
1
patients with primary hypercholesterolemia. II. Once-daily versus twice-daily dosing. 2
Atherosclerosis 1990;85:219-27.
3
37. Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and pharmacokinetics of a 4
new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in 5
healthy volunteers. Br J Clin Pharmacol 2002;54:472-7. 6
38. Cilla DD Jr, Gibson DM, Whitfield LR, Sedman AJ. Pharmacodynamic effects and 7
pharmacokinetics of atorvastatin after administration to normo-cholesterolemic subjects in the
8
morning and evening. J Clin Pharmacol 1996;36:604-9. 9
39. Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T, März W. Efficacy and
10
safety of fluvastatin-extended release in hypercholesterolemic patients: morning administration 11
is equivalent to evening administration. Cardiology 2006;106:241-8. 12
40. Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial
13
to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with 14
chronic kidney disease. Am Heart J 2010;160:785-794.e10. 15
Table 1, CHD/CHD risk equivalents, evening group 16
Table 1, ARBs, morning and evening groups17
Co mment: 20A AUTHOR: 33/84 =
39.28 (ie, 39.3%). OK to change?
Co mment: 20B AUTHOR; 38/ 87=
43.67 (43.7%) and 36/84 = 42.85
(42.9%) OK to change?
C
linical trials have demonstrated that
a reduction in serum low-density
lipoprotein cholesterol (LDL-C) to the
target level has favorable effects on car-
diovascular disease (CVD) by reducing
the frequency of progressing coronary
lesions, causing regression of atheroscle-
rotic lesions, decreasing cardiovascular
morbidity and mortality, and decreasing
the total mortality
.
1-3
Ezetimibe, a novel agent that can in-
hibit the intestinal absorption of choles-
terol from dietary and biliary sources,
blocking the Niemann-Pick C1
-like 1
protein for cholesterol transport across
the intestinal wall.
4,5
The drug is rapidly
metabolized in the intestine to aphenolic
glucuronide; once glucuronidated, it is
excreted in the bile, thereby returning to
the site of action. Cholesterol absorption
studies have indicated that the glu-
curonide form may be more potent than
ezetimibe itself, because glucuronidated
ezetimibe localizes to a greater extent in
the intestine. Ezetimibe and its glu-
curonide have a half-life of approximate-
ly 22 hours in humans.
6
Ezetimibe 10 mg/day decreases the
serum LDL-C level by about 16
-22%.
7
Owing to the dual point of inhibition (ie,
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
n
1
Comparison of Effects of Morning Versus Evening Administration
of Ezetimibe/Simvastatin on Serum Cholesterol in Patients with
Primary Hypercholesterolemia
Hyung Sik Yoon, Sung Ho Kim, Jeong Kyung Kim, Sang Hun Ko, Jae Ee Ko, Soo Jin Park, Moon Gi Park,
Jae Hwan Lee, and Min Soo Hyon
theannals.com
Dyslipidemia
job no. RR P511
date
Author information provided at end of text.
RESEARCH REPORTS
BACKGROUND: Ezetimibe, a first-in-its-class inhibitor of cholesterol absorption, is
an effective agent for combined use with statins to achieve low-density lipoprotein
cholesterol (LDL-C) goals. Ezetimibe in combination with simvastatin as a single-
tablet formulation has proven to be highly effective in reducing serum LDL-C
through the dual inhibition of cholesterol absorption and biosynthesis. The effect
of time of administration on efficacy of this combination therapy has not been
evaluated.
OBJECTIVE: To compare the effects of morning versus evening administration of
ezetimibe/simvastatin on serum cholesterol levels of patients with primary
hypercholesterolemia.
METHODS: In this multicenter, open-label, randomized, 2-sequence, 2-period
crossover study, patients with primary hypercholesterolemia randomly received
ezetimibe/simvastatin 10 mg/20 mg once daily, either in the morning (within 1
hour of breakfast) or in the evening (within 1 hour of dinner) for 6 weeks.
RESULTS: Data on 171 patients (87 in the morning administration group and 84 in
the evening administration group) were analyzed. A significant reduction (p
≤
0.001) in the total cholesterol, triglyceride, high-density lipoprotein cholesterol,
LDL-C, apo-lipoprotein B, and high-sensitivity C-reactive protein (hs-CRP) from
baseline was achieved after each treatment. Noninferiority of morning
administration versus evening administration was shown in the percentage
reduction of the LDL-C level from baseline (dif
ference, –1.62%; 90% CI –4.94 to
1.70). No significant difference was found between groups with respect to the
percentage of changes in other lipid parameters from baseline. Furthermore,
there was no significant difference in the percentage of change in hs-CRP as an
antiinflammatory marker between the morning and evening administration
groups. The frequency of adverse events was similar between groups.
CONCLUSIONS: Morning administration of ezetimibe/simvastatin 10 mg/20 mg is
noninferior to evening administration with respect to LDL-C–lowering ability
.
KEY WORDS: chronobiologic variation, ezetimibe, high-sensitivity C-reactive
protein, hypercholesterolemia, simvastatin.
Ann Pharmacother 2011;45:xxxx.
Published Online, xx XXX 201
1,
theannals.com, DOI 10.1345/aph.1P51
1
Abbreviated title for Cover: Morning vs. Evening Administration of Ezetimibe/Simvastatin
cholesterol absorption and biosynthesis), combined thera-
py of ezetimibe with a statin results in a greater decrease in
LDL-C.
8,9
Furthermore, such therapy leads to a significant
r
eduction in C-reactive protein (CRP) compared with statin
monotherapy.
10
The National Cholesterol Education Program (NCEP)
Adult Treatment Panel III (ATP III) recommends hydroxy-
methylglutaryl coenzyme A reductase inhibitors as the first-
line therapy for reducing the LDL-C.
11
On the basis of the
short half-lives of simvastatin,
12
lovastatin,
13
and fluvastatin,
14
ranging from 1 hour to 5 hours, and peak cholesterol biosyn-
thesis, occurring between midnight and 0300 hours, the man-
ufacturers have recommended evening administration of
these drugs.
15
Alternatively, atorvastatin,
16
atorvastatin-active
ortho-hydroxylated and para-hydroxylated metabolites,
pravastatin,
17
and rosuvastatin
18
have long half-lives (14, 20,
30, 22, and 19 hours, respectively) and can be administered at
any time of the day. The recent change in the NCEP ATP III
guidelines recommends a target LDL-C of 70 mg/dL or less
for very high-risk individuals; therefore, the appropriate time
of drug administration to achieve the maximal reduction is
important.
19
However, with the long half-life of ezetimibe (22
hours), the effect of the time of administration on the efficacy
of combined therapy of ezetimibe with simvastatin has not
been established.
The aim of this study was to compare effects of morning
versus evening administration of ezetimibe and simvastatin
as a combined therapy (ezetimibe/simvastatin) on serum
cholesterol of patients with primary hypercholesterolemia.
Methods
DESIGN
This o
pen-label, randomized, 2-period, 2-sequence, cross-
over study was conducted according to Good Clinical Prac-
tice and in accordance with the Declaration of Helsinki
(Helsinki, Finland, 1964) and all subsequent amendments at
2 centers from January 2008 to June 2009. The ethics com-
mittee or institutional review board of each participating cen
-
ter approved the protocol, and all study participants provided
written informed consent at the time of study enrollment.
Random sequence generation was used to assign sub-
jects in a crossover fashion (to prevent bias from the open-
label design) to 2 treatment sessions of equal duration (6
weeks). Ezetimibe/simvastatin 10 mg/20 mg was adminis
-
tered as a single tablet daily within 1 hour of breakfast
(morning administration) or dinner (evening administra-
tion) in period 1 and the regimen was interchanged in peri
-
od 2. The study also included a 4-week lead-in period.
MAIN INCLUSION CRITERIA
Patients 18 years or older with an LDL-C at or above
the drug treatment threshold established by the NCEP A
TP
III guidelines were eligible for enrollment if they met the
following criteria: (1) established CHD or risk equivalent,
2 or more risk factors conferring a 10-year risk larger than
2
0% for CHD (Framingham score), and an LDL-C level of
130 mg/dL or more; (2) no CHD or risk equivalent, 2 or
more risk factors conferring a 10-year risk less than 20%
for CHD, and an LDL-C level of 160 mg/dL or more; or
(3) no CHD or risk equivalent, less than 2 risk factors con-
ferring a 10-year risk lower than 20% for CHD, and an
LDL-C level of 190 mg/dL or more.
MAIN EXCLUSION CRITERIA
Exclusion criteria included allergy, hypersensitivity, or
intolerance to ezetimibe or statin; consumption of more than
2 alcoholic drinks per day or a history of substance abuse or
dependency within 12 months before screening; and active
hepatitis or other liver disease. Patients with the following
conditions were also excluded: persistent uncontrolled hyper-
tension; unstable angina, non-ST-segment elevation myocar-
dial infarction, or ST-segment elevation myocardial infarction
within the previous 12 months; angioplasty, stroke, transient
ischemic attack, or deep vein thrombosis within the previous
3 months; coronary artery bypass graft surgery within the
previous 6 months; uncontrolled cardiac arrhythmias; acute
or subacute peripheral artery disease occlusion; congestive
heart failure (New York Heart Association class III or IV);
fasting serum triglyceride greater than 350 mg/dL, alanine
aminotransferase or aspartate aminotransferase 1.5 times or
more the upper limit of normal, serum creatinine greater than
1.5 mg/dL, creatine kinase 1.5 times or more the upper limit
of normal, and hemoglobin A
1C
level 9% or more in patients
with diabetes; poorly controlled type 1 or type 2 diabetes; ac-
tive cancer or a diagnosis of cancer within the last 5 years; fi-
bromyalgia, myopathy
, rhabdomyolysis, unexplained muscle
pain, and/or discontinuation of a statin due to myalgia; or life
expectancy less than 2 years.
Patients taking non-statin lipid-lowering drugs, im-
munosuppressants, corticosteroids, or potent CYP3A4 in-
hibitors were also excluded from participation. All lipid-al-
tering drugs were discontinued for 6 weeks (for statins) or
8 weeks (for fibrates) before randomization.
SCREENING, RANDOMIZA
TION, AND TREATMENT
At screening, patients underwent a complete physical
examination, recording of medical history (eg, medical
conditions, smoking status, medications), and laboratory
assessments. They then entered a 4-week dietary lead-in in
which they were encouraged to follow the Step II diet
(<7% saturated fat and <200 mg/day cholesterol) described
in the NCEP ATP II guidelines
19
and all lipid-lowering
treatments were discontinued. After this lead-in, eligible
patients randomly received ezetimibe/simvastatin 10
2
n
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
theannals.com
HS Yoon et al.
mg/20 mg in the morning or evening for 6 weeks. During
the study, patients were not permitted to take alternative
lipid-modifying medications or any of the following agents:
a
miodarone, isotretinoin, cyclosporine, danazol, nefazodone,
rifampin, oral macrolide antibiotics, telithromycin, oral azole
antifungals, preparations containing St. John’s wort or
Gink-
go biloba
, verapamil, systemic androgens or anabolic
steroids, and chronic systemic corticosteroids. They were in-
structed to take the medicine orally, once daily, within 1 hour
of breakfast (morning administration) or dinner (evening ad-
ministration) and to follow the NCEP ATP III Therapeutic
Lifestyle Change diet for the duration of the study.
12
LABORATORY TESTS
Efficacy variables were assessed using 12- to 14-hour
fasting blood samples after dinner. Samples were collected
at the beginning of the dietary lead-in period (week – 4), 2
weeks after the beginning of this period (week –2), and
during randomization (week 0). The baseline fasting lipid
levels were the mean of these 3 measurements. Samples
were collected at weeks 6 and 12 for lipid and other labora-
tory analyses (Figure 1). Laboratories at each clinical cen-
ter performed the lipid and other laboratory measurements
using standardized procedures. The laboratories are certi-
fied by the Korean Society of Clinical Pathologists and the
Korean Society for Laboratory Medicine. Plasma glucose,
total cholesterol, triglyceride, high-density lipoprotein
cholesterol (HDL-C), aspartate aminotransferase, alanine
aminotransferase, creatine kinase, and lactate dehydroge
-
nase levels were measured by using enzymatic kits (Sigma
Diagnostics, Taufkirchen, Germany) in a BM-Hitachi 747-
200 autoanalyzer (Hitachi-Roche, Tokyo, Japan). LDL-C
was calculated by the Friedewald equation.
20
Serum apo-
lipoprotein B (apo-B) and high-sensitivity C-reactive pro-
tein (hs-CRP) levels were deter
mined using an immuno
-
turbidometric method (Cobas Integra 800 automatic ana-
lyzer; Roche Diagnostics, Basel, Switzerland). The
intraassay coefficient of variation for each laboratory was
in the range 3.2- 4.7%.
OUTCOMES
E
fficacy
The primary endpoint was percentage change in the
LDL-C level from baseline to the end of treatment. Sec-
ondary endpoints included percentage changes in total
cholesterol, triglyceride, HDL-C, apo-B, and hs-CRP lev-
els from baseline to the end of treatment.
Safety
Safety analysis was based on data collected for adverse
events, routine chemistry and hematology analyses, urinal-
ysis, vital sign measurements, and physical examinations.
Safety data were collected at each visit, including the last
visit.
ADHERENCE
Standard pill count was used to assess adherence. At
each visit, the number of remaining pills in the containers
was counted. Adherence was defined by the number of
pills taken (dispensed pills returned pills) in relation to the
theoretical number of prescribed doses. Patients with an
adherence rate lower than 75% were excluded from the
per-protocol (PP) analysis.
STATISTICAL ANALYSIS
Randomization was conducted by using a number gen-
erator in blocks of 6 patients with nQuery Advisor 7.0
(Statistical Solutions Ltd., Cork, Ireland). We hypothesized
that both strategies would result in similar reductions of ap
-
proximately 40% in serum LDL-C levels. The noninferior
-
ity boundary was determined from Lund et al.,
21
who com-
pared mor
ning blood samples and included morning ver-
sus evening administration, primarily of
simvastatin 20 mg, with a crossover design. In
their study
, the dif
ference in mean percentage
of change in LDL-C from the baseline was
13.4% higher in the evening group, with a
95% CI of 7.59% to 18.67%. We chose a re-
strictive and smaller mar
gin of 95% CI of the
Lund study for noninferiority referenced from
Kaul and Diamond.
22
Therefore, the noninferi-
ority boundary in our study was set at 7%.
The intent-to-treat (ITT) population com-
prised all randomized patients who received at
least 1 dose of study medication and had at least
1 efficacy data point. The PP population was an-
alyzed for all ef
ficacy endpoints because a PP
population is associated with less variability than
Morning vs. Evening Administration of Ezetimibe/Simvastatin
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
n
3
theannals.com
.Figure 1. T
imeline of the lipid sampling.
is an ITT population (which includes nonconforming pa-
tients) and protocol violations are more likely to reduce the
treatment efficacy. Accordingly, analysis of a PP population
i
s considered the more conservative and statistically correct
approach for demonstrating noninferiority.
23
All efficacy
analyses performed on the PP population were confirmed by
ITT analysis.
The safety analysis population comprised all random-
ized patients who received at least 1 dose of study medica-
tion and had at least 1 follow-up data point. A total sample
size of 142 achieves 90% power to detect noninferiority of
differences in a 2
× 2 crossover design by using a 1-sided t-
test. Morning administration of ezetimibe/simvastatin was
considered noninferior to evening administration if the up-
per limit of the 2-sided 90% CI for the difference in mean
percent change in LDL-C from baseline was less than 7%.
The sample was divided equally between the administra-
tion groups. The significance level was set at 0.05, and the
square root of the mean square error was 20.0.
Treatment effects were compared with respect to the
changes from the baseline to the 2 efficacy endpoints by
using an analysis of covariance that included treatment,
center, and baseline measures as the covariate in the mod
-
el. Power Analysis and Sample Size for Windows version
2008 (NCSS, Kaysville, Utah) was used to estimate the re-
quired sample size for noninferiority in the 2
× 2 crossover
design. Statistical analyses for comparison of least-squares
means with 90% CI for the treatment difference (morning
administration – evening administration) in the 2 × 2 cross-
over design were conducted in a model that included treat-
m
ent, period, and carryover effect by using NCSS for Win-
dows version 2007, according to the methods described by
Chow and Liu
24
and Chow et al.
25
Results
One hundred seventy-one patients were enrolled and
randomized (Figure 2); 145 patients completed the study
and 26 withdrew. Among adverse events, 3 patients devel-
oped myalgia, 2 patients experienced diarrhea, and 3 pa-
tients had indigestion. There were no significant differ-
ences between the treatment groups with regard to the de-
mographic and baseline disease characteristics or
maintenance medication (Table 1).
The ITT population consisted of 151 and 150 patients
who were included in the morning and evening administra-
tion groups, respectively. In the PP population, there were
155 and 154 patients in the morning and evening adminis-
tration groups, respectively. In the PP population, marked
reductions in the LDL-C level from baseline were ob-
served in morning (46.33%, p < 0.001) and evening ad-
ministration (47.95%, p < 0.001) groups, with no signifi-
cant difference between the groups.
4
n
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
theannals.com
HS Yoon et al.
Figure 2. Profile of patients in study. “Failure to return” includes the patients who did not cooperate or withdrew consent. “Others” includes violation of
selection criteria at entry and other protocol violation. PP = per-protocol.
EFFICACY
Primary Endpoint
In the PP population, noninferiority of morning admin-
istration compared with evening administration was
shown in percentage reduction in LDL-C from baseline
because the 90% CI did not deviate from the prespecified
difference of 7% to satisfy the noninferiority criterion.
The corresponding analyses performed on the ITT popu-
lation supported the findings of the PP population, with no
period effects (p = 0.09) or carryover effects (p = 0.87)
(Table 2).
Secondary Endpoints
There were no significant differences between groups in
terms of percentage changes in total cholesterol, triglyc-
eride, HDL-C, and apo-B (Table 3). The baseline and fol-
low-up hs-CRP concentrations showed significant differ-
ences (p < 0.001) for both groups. At week 6, the mean hs-
CRP decreased from 2.64 mg/dL to 2.01 mg/dL in the
morning administration group and from 2.48 mg/dL to
1.92 mg/dL in the evening administration group. The mean
percentage changes were 21.04% and 23.75% in the morn-
ing and the evening administration groups, respectively,
with no significant between-administration period differ-
ence.
Safety
Study treatments were well tolerated. The adverse events
profile was generally similar between groups; most adverse
events were mild to moderate and were considered unrelated
to the treatment. In addition, there were no deaths, and no
treatment-related serious adverse events were reported. At
least 1 clinical adverse event was observed in 26 patients in
Morning vs. Evening Administration of Ezetimibe/Simvastatin
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
n
5
theannals.com
Table 2. Least Squares Mean Percent Change of LDL-C at Week 6
Intent-to-Treat Per Protocol
Morning Evening Morning Evening
Set
(n = 151)
(n = 150)
(n = 155)
(n = 154)
Least-squares mean of % change 45.87 48.28 46.33 47.95
Difference in treatment (p value) –2.41% (0.22) –1.62% (0.42)
90% CI –5.94 to 0.61 –4.94 to 1.70
Carryover/period effect (p value) 0.74/0.29 0.87 /0.09
LDL-C = low-density lipoprotein cholesterol.
T
-test (one-sided) for analyzing noninferiority of morning vs evening treatment, with a predefined non-inferiority margin of 7% for the dif
ference of the
% changes between treatments.
Table 1. Demographic Characteristics
Morning
Evening
Administration Administration Total
Characteristic (n = 87) (n = 84) p Value (N = 171)
Age (y), mean (SD) 57.9 (11.3) 60.1 (10.8) 0.74 59.2 (11.6)
Female sex, n (%) 36 (41.3) 34 (40.4) 0.80 70 (40.9)
BMI (kg /m
2
), mean (SD) 23.7 (2.6) 22.9 (3.1) 0.79 22.7 (5.6)
Risk factors, n (%)
current smoking 14 (16.1) 12 (14.3) 0.44 26 (15.2)
hypertension 52 (59.8) 49 (58.3) 0.88 101 (59.1)
diabetes mellitus
39 (44.8)
35 (41.7) 0.31 74 (43.3)
CHD/CHD risk equivalents 31 (36.6) 33 (39.2) 0.37 64 (37.4)
Medication, n (%)
calcium channel blocker 47 (54.0) 46 (54.8) 0.84 93 (54.4)
β-Blocker 43 (49.4) 40 (47.6) 0.53 83 (48.5)
ACE inhibitor
44 (50.6)
41 (48.8)
0.54 85 (49.7)
ARB 38 (40.3) 36 (37.7) 0.49 74 (43.3)
nitrate
8 (9.2) 9 (10.7) 0.19 17 (9.9)
diuretic
17 (19.5)
13 (15.5)
0.09
30 (17.5)
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; CHD = coronary heart disease.
the morning administration group and 27 patients in the
evening administration group (Table 4). There were no re-
ports of a clinically significant increase in serum creatine
k
inase (>10 times the upper limit of normal) or cases of
rhabdomyolysis.
Discussion
To our knowledge, this study is the first to assess the ef-
fect of time of ezetimibe/simvastatin administration on the
serum cholesterol level. The results show that morning ad-
ministration of ezetimibe/simvastatin is noninferior to
evening administration.
CRP measured by high-sensitivity assays is widely ac-
cepted as an independent marker of cardiovascular risk in
otherwise healthy populations, on the basis of multiple ob-
servational studies and randomized clinical trials.
26-28
The
use of hs-CRP for risk stratification of patients with estab-
lished coronary artery disease has not been recommended
in the American Heart Association/Centers for Disease
Control and Prevention guidelines, but is supported by the
results of clinical trials, including a large trial conducted in
patients with stable symptoms.
29
Statins reduce hs-CRP
levels in a manner largely independent of LDL-C,
30
and
statin plus ezetimibe therapy reduces hs-CRP more effec
-
tively than statin monotherapy.
31
Although ezetimibe alone reduces cholesterol by 15-
20%, when used in conjunction with statins (ie, dual thera-
py), it can enhance the cholesterol-lowering of statins an
additional 20%.
32
Dose-scheduling studies of ezetimibe
monotherapy in Phase 2 trials evaluated 2 doses, 5 mg/day
and 10 mg/day, and morning versus evening dosing with
and without food in approximately 40 patients per group.
This series of studies found no significant difference be-
tween morning and evening dosing, with 10 mg/day result-
ing in a 17.5% reduction in LDL-C versus an 18.2% re-
duction with evening dosing.
33
Simvastatin and its active metabolites have short half-
lives (<5 hours), which may explain the differences detect-
ed in clinical trials favoring evening administration.
34
With its short half-life (2 hours) and an active metabolite
with an unknown duration of action, lovastatin also re-
vealed a trend toward a greater decrease in LDL-C on
evening administration.
35
However, pravastatin and rosu-
vastatin, which have long half-lives (22 hours and 19
hours, respectively), do not have active metabolites, and
their lipid-lowering ef
fects may wane at the end of a 24-
hour interval, possibly explaining a slight trend toward
more reduction in LDL-C with evening administration in
clinical trials.
36,37
Atorvastatin has a long half-life (14
hours) and also has active metabolites with half-lives in
the range 20-30 hours, which may explain why evening
versus morning administration of atorvastatin demonstrat-
ed no significant difference between study groups.
38
To
our knowledge, there has been no study conducted to
evaluate the chronobiologic effects of the administration
of fluvastatin, which has a short half-life (<3 hours) and
no active metabolites, but its extended-release formulation
(longer pharmacodynamic effect of 7.3-10.5 hours)
showed equivalent lipid-lowering efficacy between morn-
ing and evening administration.
39
6
n
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
theannals.com
HS Yoon et al.
T
able 3.
Results of Laboratory T
ests
a
Treatment
Morning Evening Difference
Variable (n = 155) (n = 154) (90% CI) p Value
T
otal cholesterol
baseline 219.17 (39.36) 215.24 (38.90) 3.93 (–5.68 to 13.55) 0.500
% change 31.90 (1.54) 32.60 (1.30) –0.70 (–3.21 to 1.91) 0.699
Triglycerides
baseline 199.18 (39.36) 189.38 (65.34) 9.79 (–3.5 to 23.17) 0.228
% change
35.89 (1.50)
34.76 (1.34)
1.12 (–2.01 to 4.45)
0.583
HDL-C
baseline 46.69 (10.86) 45.46 (11.00) 1.23 (–1.46 to 3.91) 0.452
% change –20.27 ± (5.92) –19.06 (5.29) –1.21 (–6.21 to 3.99) 0.735
Apo-B
baseline
146.73 (17.81) 147.97 (17.42) –1.24 (–5.57 to 3.09) 0.635
% change 42.53 ( 0.78) 42.49 (0.79) 0.04 (–1.09 to 1.54) 0.964
hs-CRP
baseline 2.64 (1.37) 2.48 (1.49) 0.16 (–1.05 to –0.38) 0.457
% change
21.04 (1.96)
23.75 (1.40)
–2.71 (–6.92 to 2.10) 0.314
APO-B = apo-lipoprotein B; HDL-C = high-density lipoprotein cholesterol; hs-CRP = high-sensitivity C-reactive protein.
a
Baseline and overall mean percent changes of lipid and hs-CRP levels in per-protocol analysis measured at 6 weeks. The baseline values (mg/dL)
represent the mean ± SD and % change values represent the mean ± SE.
Therefore, ezetimibe, with a long half-life and active
metabolites, in combination with simvastatin was expected
to have equivalent lipid-lowering efficacy between morn-
i
ng and evening administration. However, this is not a full
explanation of the pharmacologic/pharmacokinetic plausi-
bility for the fact that no significant differences were found
in LDL-C reduction between the 2 regimens. We agree that
this result cannot be generalized in Asian or white patients,
because there may be pharmacogenomic differences be-
tween these populations.
Although the results are very encouraging, the study has
several limitations. First, its open-label, unblinded design
limits generalizability beyond the study groups; however,
the randomized assignment of patients and the crossover
design could reduce the bias. Second, no washout period
was used. However, patients with angina, diabetes, and hy-
pertension were included in this study; treatment with an-
tilipid agents for primary or secondary prevention of CVD
should not be interrupted in these patients. We believed
that avoiding the unethical action of stopping the antilipid
agent during a washout period was more important than in-
clusion of a washout period. The results of testing possible
period effects and carryover effects according to Chow and
Liu
24
and Chow et al.
25
can lessen this limitation.
Recently, results of the SHARP (Study of Heart and Re-
nal Protection) trial,
4
0
which evaluated the effects of lower-
ing LDL-C levels with ezetimibe 10 mg and simvastatin
20 mg daily versus placebo in 9438 patients with chronic
kidney disease (CKD), were published. The findings sug-
gest that the addition of ezetimibe to statin therapy is effec-
tive and safe in treating dyslipidemia in patients with CKD
and those who have undergone kidney transplant. CVD is
the main cause of morbidity and mortality in kidney trans-
plant recipients. The SHARP trial provides evidence about
the efficacy and safety of lowering LDL-C with the combi-
nation of ezetimibe and simvastatin among wide range of
patients with CKD.
In conclusion, daily mor
ning administration of ezetimibe/
simvastatin 10 mg/20 mg is noninferior to daily evening ad
-
ministration in reduction of LDL-C levels.
Hyung Sik Yoon BSc MD, Resident, Department of Internal
Medicine, Sun General Hospital, Cardiovascular Center
, Daejeon,
Korea
Sung Ho Kim M
D BSc, Resident, Department of Internal Medicine,
S
un General Hospital, Cardiovascular Center
J
eong Kyung Kim
MD MSc, Director, Department of Internal
Medicine, Sun General Hospital, Cardiovascular Center
Sang Hun Ko BSc MD, Resident, Division of Endocrinology, De-
partment of Internal Medicine, Sun General Hospital
Jae Ee Ko BSc MD, Resident, Department of Internal Medicine, Di-
vision of Endocrinology, Sun General Hospital
Soo Jin Park MSc MD, Staff, Department of Internal Medicine, Sun
General Hospital; Cardiology Physician, Cardiovascular Center
Moon Gi Park BSc MD, Chief of Vascular Surgery, Department of
General Surgery, Sun General Hospital
Jae Hwan Lee MD PhD, Associate Professor, Department of In-
ternal Medicine, Chungnam National University, Daejeon; Cardiology
Physician, Department of Internal Medicine, Cardiovascular Center
Min Soo Hyon MD PhD, Professor, Department of Internal
Medicine, College of Medicine, Soonchunhyang University; Cardi-
ology Physician, Cardiovascular Institution, Seoul, Korea
C
orrespondence:
Dr. Jeong Kyung Kim, dr_doom@naver.com
Reprints/Online Access: www.theannals.com/cgi/reprint/aph1.P511
Conflict of interest: Authors reported none
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of
cholesterol lowering in 4444 patients with coronary heart disease: the
Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-9.
2. Shepherd J, Cobbe SM, Ford I, et al.; The West of Scotland Coronary
Prevention Study Group. Prevention of coronary heart disease with
pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:
1301-7.
3. Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am
J Cardiol 2003;91(suppl):4-8.
4. van Heek M, Farley C, Compton DS, Hoos LM, Smith-Torhan A, Davis
HR. Ezetimibe potently inhibits cholesterol absorption but does not affect
acute hepatic or intestinal cholesterol synthesis in rats. Br J Pharmacol
2003;138:1459-64.
5. Altmann SW, Davis HR Jr, Zhu LJ, et al. Niemann-Pick C1 like 1 pro-
tein is critical for intestinal cholesterol absorption. Science
2004;303:1201-4.
6. Darkes MJ, Poole RM, Goa KL. Ezetimibe. Am J Cardiovasc Drugs
2003;3:67-76.
7. Kosoglou T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction be-
tween the new selective cholesterol absorption inhibitor ezetimibe and
simvastatin. Br J Clin Pharmacol 2002;54:309-19.
8.
Ose L, Shah A, Davis MJ, et al. Consistency of lipid-altering ef
fects of
ezetimibe/simvastatin across gender, race, age, baseline low density
lipoprotein cholesterol levels, and coronary heart disease status: results of
a pooled retrospective analysis. Curr Med Res Opin 2006;22:823-35.
9.
Kalogirou M, Tsimihodimos V, Saougos V, Lagos K, Tselepis AD, Elisaf
M. Effect of ezetimibe on lipoprotein subfraction concentrations: the role
of atorvastatin pretreatment. Arch Med Sci 2007;3:344-50.
10.
Pearson T
, Ballantyne C, Sisk C, Shah A, Veltri E, Maccubbin D. Com-
parison of ef
fects of ezetimibe/simvastatin versus simvastatin versus
atorvastatin in reducing C-reactive protein and low-density lipoprotein
cholesterol levels. Am J Cardiol 2007;99:1706-13.
11. Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults. Executive Summary of The Third Report of the
National Cholesterol Education Program (NCEP) Expert Panel on De
-
tection, Evaluation, and Treatment of High Blood Cholesterol in Adults
(Adult Treatment Panel III). JAMA 2001;285:2486-97.
12. Package insert. Zocor (simvastatin) tablets. Whitehouse Station, NJ:
Merck Phar
maceuticals, November 2009.
13.
Package insert. Mevacor (lovastatin) tablets. Whitehouse Station, NJ:
Merck Phar
maceuticals, November 2009.
14.
Package insert. Lescol (fluvastatin) tablets. East Hanover
, NJ: Novartis
Pharmaceuticals, May 2009.
Morning vs. Evening Administration of Ezetimibe/Simvastatin
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
n
7
theannals.com
T
able 4.
Incidence of Adverse Reactions
Morning Evening
Administration,
Administration,
n (%) n (%)
Variable (n = 87) (n = 84)
Any adverse event 26 (30) 27 (32)
Leading to study discontinuation 4 (4.6) 4 (4.8)
Serious adverse event 0 (0) 1 (1.2)
15. Parker TS, McNamara DJ, Brown C, et al. Mevalonic acid in human
plasma: relationship of concentration and circadian rhythm to cholesterol
synthesis rates in man. Proc Natl Acad Sci U S A 1982;79:3037- 4.
16. Package insert. Lipitor (atorvastatin calcium) tablets. Morris Plains, NJ:
Pfizer Pharmaceuticals, July 2009.
17. Package insert. Pravachol (pravastatin) tablets. Princeton, NJ: Bristol-
Myers Squibb, November 2009.
18. Package insert. Crestor (rosuvastatin) tablets. Wilmington, DE: Astra-
Zeneca, March 2009.
19. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical
trials for the National Cholesterol Education Program Adult Treatment
Panel III guidelines. Circulation 2004;110:227-39.
20. Bairaktari E, Hatzidimou K, Tzallas C, et al. Estimation of LDL choles-
terol based on the Friedewald formula and on apo B levels. Clin
Biochem 2000;33:549-555. DOI 10.1016/S0009-9120(00)00162- 4
21. Lund TM, Torsvik H, Falch D, Christophersen B, Skårdal R, Gullestad
L. Effect of morning versus evening intake of simvastatin on the serum
cholesterol level in patients with coronary artery disease. Am J Cardiol
2002;90:784-6.
22. Kaul S, Diamond GA. Making sense of noninferiority: a clinical and sta-
tistical perspective on its application to cardiovascular clinical trials. Prog
Cardiovasc Dis 2007;49:284-99.
23. Lewis JA. Statistical principles for clinical trials (ICH E9): an introductory
note on an international guideline. Statist Med 1999;18:1903- 42.
24. Chow S-C, Liu J-P. Design and analysis of bioavailability and bioequiva-
lence studies. 2nd ed. New York, NY: Marcel Dekker, Inc., 1999:73-7.
25. Chow S-C, Shao J, Wang H. Sample size calculations in clinical re-
search. 1st ed. New York, NY: Marcel Dekker, Inc., 2003:63-8.
26. Koenig W, Sund M, Frölich M, et al. C-Reactive protein, a sensitive
marker of inflammation, predicts future risk of coronary heart disease in
initially healthy middle-aged men: results from the MONICA (Monitor-
ing Trends and Determinants in Cardiovascular Disease) Augsburg Co-
hort Study, 1984 to 1992. Circulation 1999;99:237-42.
27. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation
and cardiovascular disease: application to clinical and public health prac-
tice. A statement for healthcare professionals from the Centers for Dis-
ease Control and Prevention and the American Heart Association. Circu-
lation 2003;107:499-511.
28. Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein associated
phospholipase A2, high-sensitivity C-reactive protein, and risk for inci-
dent coronary heart disease in middle-aged men and women in the
Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004;
109:837- 42.
29. Sabatine MS, Morrow DA, Jablonski KA, et al. Prognostic significance
of the Centers for Disease Control/American Heart Association high-
sensitivity C-reactive protein cut points for cardiovascular and other out
-
comes in patients with stable coronary artery disease. Circulation 2007;
115:1528-36.
30.
Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive pro
-
tein for the targeting of statin therapy in the primary prevention of acute
coronary events. N Engl J Med 2001;344:1959- 65.
31. Gagné C, Bays HE, Weiss SR, et al.; Ezetimibe Study Group. Efficacy
and safety of ezetimibe added to ongoing statin therapy for treatment of
patients with primary hypercholesterolemia. Am J Cardiol 2002;90:
1084-91.
32. Gagné C, Gaudet D, Bruckert E, for the Ezetimibe Study Group. Effica-
cy and safety of ezetimibe coadministered with atorvastatin or simvas
-
tatin in patients with homozygous familial hypercholesterolemia. Circu
-
lation 2002;105:2469-75.
33. Stein EA. An investigative look: selective cholesterol absorption in-
hibitors—embarking on a new standard care. Am J Manag Care 2002;
8(2 suppl):S36
-9;discussion S45
-7.
34. Wallace A, Chinn D, Rubin G. Taking simvastatin in the morning com-
pared with in the evening: randomised controlled trial. BMJ 2003;327:
788.
35. Illingworth RD. Comparative efficacy of once versus twice daily mevi-
nolin in the therapy of familial hypercholesterolemia. Clin Pharmacol
Ther 1986;40:338
-
43.
36. Hunninghake DB, Mellies MJ, Goldberg AC, et al. Efficacy and safety
of pravastatin in patients with primary hypercholesterolemia. II. Once-
daily versus twice-daily dosing. Atherosclerosis 1990;85:219-27.
37. Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and
pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin,
after morning or evening administration in healthy volunteers. Br J Clin
Pharmacol 2002;54:472-7.
38. Cilla DD Jr, Gibson DM, Whitfield LR, Sedman AJ. Pharmacodynamic
effects and pharmacokinetics of atorvastatin after administration to nor-
mo-cholesterolemic subjects in the morning and evening. J Clin Pharma-
col 1996;36:604-9.
39. Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T, März
W. Efficacy and safety of fluvastatin-extended release in hypercholes-
terolemic patients: morning administration is equivalent to evening ad-
ministration. Cardiology 2006;106:241-8.
40. Sharp Collaborative Group. Study of Heart and Renal Protection
(SHARP): randomized trial to assess the effects of lowering low-density
lipoprotein cholesterol among 9,438 patients with chronic kidney dis-
ease. Am Heart J 2010;160:785-794.e10.
8
n
The Annals of Pharmacotherapy
n
2011 Month, Volume 45
theannals.com
HS Yoon et al.