Content uploaded by Dominique Luneau
Author content
All content in this area was uploaded by Dominique Luneau on Oct 24, 2018
Content may be subject to copyright.
Current Opinion in Solid State and Materials Science 5 (2001) 123–129
Molecular magnets
*
Dominique Luneau
Laboratoire de Chimie Inorganique et Biologique
(
UMR
5046),
DRFMC
,
CEA-Grenoble
, 38054
Grenoble cedex
,
France
Abstract
This paper presents recent developments in the fields of molecular-based magnets and of single-molecule magnets. It concerns
compounds made with open shell molecules which may be organic, inorganic or both. 2001 Elsevier Science Ltd. All rights reserved.
1. Introduction that in these compounds the molecular magnetic centres
interact in a three-dimensional (3D) array and there is a net
In the 1970s the easiest access to crystal structure resulting magnetic moment at the crystal level.
determinations allowed numerous studies of the magnetic We also include in this review a fifth part dedicated to
properties versus the structural parameters. Within this the emerging field of single-molecule magnets. In contrast
framework the studies of organic or inorganic molecules to molecular-based magnets they are made of isolated
with few interacting magnetic centres were very helpful for clusters which have individual behaviours reminiscent of
the conception of qualitative models to predict the strength magnets. The challenge here for the chemist is to connect a
and the nature, ferro or antiferromagnetic, of the interac- finite number of magnetic centres to have a large resulting
tions [1–5]. They pioneered the engineering of the first magnetic moment in one discrete molecule.
molecular magnets in the 1980s.
As part of supramolecular science, molecular magnets
are at the interface of chemistry and physics. For the 2. Organic compounds
molecular chemist there is a real challenge to imagine the
molecules and to connect them in the solid state, and to get In this class of compounds the molecular bricks are
properties which are generally devoted to minerals. For the made of organic free radicals. A recent overview of their
theoreticians and the physicists, these compounds are use in magnetism may be found in the book Magnetic
useful to refine their models. Although this research field Properties of Organic Materials [8].
may look mainly basic it aims at future technologies such The first organic magnet was discovered during the last
as those devoted to information storage. decade and was based on the p-nitrophenyl nitronyl
In this paper, as is the purpose of this review, we point nitroxide radical (Fig. 1a) [9]. It exhibited spontaneous
only to the major results and prospects of the field in
recent years. For complementary reading there are two
recent reviews which give an overview of the field [6,7].
The developments in molecular-based magnets are
presented in four parts corresponding to the different types
of the constitutive magnetic centres which may be organic,
inorganic or organic–inorganic. Molecular-based magnets
are compounds which exhibit spontaneous magnetisation
below a critical (Curie) temperature, abbreviated T. This
C
transitions are associated with specific heat anomaly and
are a property of the crystal (bulk). This is due to the fact
*Tel.: 133-4-7688-4462; fax: 133-4-7688-5090. Fig. 1. Example of free radical used in organic molecular-based magnets:
E-mail address
:
luneau@drfmc.ceng.cea.fr (D. Luneau). (a) T50.6 K; (b) T51.48 K; (c) T535 K.
CC C
1359-0286/01 / $ – see front matter 2001 Elsevier Science Ltd. All rights reserved.
PII: S1359-0286(00)00043-7
124 D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
magnetisation below the Curie temperature T50.6 K but 3. Inorganic compounds
C
only the bcrystal phase among four (a,b,g,d) has such a
behaviour. Indeed, the spontaneous magnetisation results This part concerns compounds in which the spin carriers
from the long range ordering of the magnetic centres in the are metal ions (Fig. 2b). This is the field of polymetallic
solid state (3D ordering) and as a matter of fact relies on coordination complexes. In order to have a magnet the
the crystal packing. To have an organic-based magnet the metal ions have to interact throughout a three-dimensional
crystal packing has to favour ferromagnetic interactions network. This is done by using bridging ligands which
between the free radicals. In this regard, the predictions of both connect and magnetically couple the metal ions.
the magnetic interactions between free radicals relies Compared with the organic-based magnets, the use of
mainly on the McConnell-I proposal [2] that intermolecu- metallic complexes as molecular bricks offer numerous
lar interactions are ferromagnetic when atoms with oppo- advantages. Metal ions have coordination number and
site sign of the spin population are in close contact. This geometries (tetrahedral, octahedral, dodecahedral . . . )
proposal is only qualitative and trials to rationalize it have which facilitate the building of supramolecular compounds
been recently made [4,10,11]. The crystal engineering in which the metal ions are 3D connected. Moreover there
strategies which are used to have the free radicals interact- is a wide palette of magnetic centres available, with spin
ing are inspired from supramolecular chemistry [12] and ranging from S51/2 to S55/2 for the transition metal
are based mostly on hydrogen bond networks to direct the ions, and up to S57/2 for the rare earth metal ions. The
crystal packing. This has been recently demonstrated to ferromagnetic nature of the exchange interaction depends
work quite efficiently in a few cases, so that ferromagnetic on the filling and the symmetry of the magnetic orbitals of
interactions and magnets were rationally achieved [13–15]. the metal ions which interact. The interactions are more
Due to their high stability, most of the free radicals generally antiferromagnetic. It is however possible in that
which are used as molecular bricks are nitroxide radicals. case to have a non zero resulting magnetic moment if
However, despite some success in the engineering of metal ions alternate with different spin values (ferrimag-
organic ferromagnets [13–15], these compounds have low netism). It must be stressed however that the magnetic
T. The highest Curie temperature obtained with nitroxide- properties, in particular T, depend greatly on the choice
C C
based magnets is T51.48 K (Fig. 1b) [16]. Because Tof the bridging ligand and this is one of the main
CC
increases with the strength of the magnetic interaction, the limitations. It is not sufficient to connect the metal centres
low Tmay be ascribed to the weakness of the inter- in a 3D network, they have also to interact strongly.
C
molecular magnetic interactions mediated by hydrogen Indeed, Tis proportional to the strength of the magnetic
C
bond networks or van der Walls contacts (Fig. 2a). This interaction between the magnetic centres. Generally the
has prompted the search for new types of radicals [17,18] shorter is the bridging ligand between the metal ions, the
able to interact more strongly but without dimerisation stronger is the magnetic interaction.
which would annihilate the magnetism. In this frame a The efficiency of the coordination chemistry approach
benzo-fused dithiazolyl radical (Fig. 1) was discovered to has been nicely demonstrated with compounds of the
be a weak ferromagnet below T535 K (Fig. 1c) [18]. Prussian-blue family (Fig. 3) [19] based on cyanide
C
This is the highest Tever observed for an organic chemistry [20]. For compounds of this family Trange
C C
compound. from a few Kelvin to values largely above room tempera-
Fig. 2. Schematic representation of the supramolecular building: (a) in organic and (b) inorganic magnets.
D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
125
conditions the way the molecules interact in the solid state
and the strength of the magnetic interactions. With the
organometallic–radical compounds, as for the organic-
based magnets, the main difficulty is to control the crystal
packing of the molecules (metallocene and radicals), with
non-covalent intermolecular interactions to have a 3D
magnetic order with strong magnetic interactions (Fig. 2a).
5. Metal–radical complexes
The second way to combine inorganic and organic spin
carriers for the engineering of molecular magnets is to use
free radicals as bridging ligands in polymetallic com-
pounds (Fig. 2b). Indeed, as we have seen above, coordina-
tion chemistry is really efficient for the building of 3D
extended compounds. Secondly, the use of free radicals as
Fig. 3. Schematic representation of a Prussian blue type crystal structure. bridging ligands is favourable to strong magnetic interac-
Cyanide (CN) groups are used to connect the metal ions sand d.tions because of the direct metal–radical bonding [8].
The record for this family of compounds is held by
V[TCNE] .yCH Cl which order at T5400 K [31] but
x22 C
ture [7,19,21] depending on the metal ions. Due to their decomposes in air. The structure of this compound is not
?2
cubic symmetry they are generally soft magnets with small yet known but it is proposed to involve the [TCNE]
II
coercive magnetic field. To overcome this, anisotropy has radical anion bound to V ions through the four CN
been recently introduced with the molecular brick groups. Similar compounds which are stable in air but
III 42
[Mo (CN) ] , 3D compounds have been synthesized exhibit lower Taround 100 K have been synthesized with
7 C
with T551 K [22] but there is little effect on the coercive other metal ions [7].
C
magnetic field. Apart from these examples, most of the free radicals
Other recent strategies consist in using cyanide and used in metal–radical coordination complexes are nitrox-
other bridging ligands in an effort to tune the Ttempera- ides because of their high stability per se and when
C
ture [23,24]. Pseudohalide ligands such as dicyanamide or coordinated to metal ions. This has allowed tremendous
tricyanamide have also been used with success to build a work on their coordination chemistry [8]. The first magnets
3D framework structure but with rather low T,10 K of this kind were synthesized at the end of the 1980s as
C
[25]. Otherwise, the study of layered compounds with chain compounds and exhibited spontaneous magnetisation
T,30 K and in which inorganic layers are separated by below T,10 K. The low Twere ascribed to the low
CCC
organic molecules have brought new insight in the com- dimensionality of the compounds. To increase Tone way
C
prehension of super-exchange and dipolar magnetic inter- has been the synthesis of polyradicals with several nitrox-
actions [26]. ide groups able to anchor metal ions in a 3D network [27].
This works well and magnets with T543 K have been
C
obtained.
4. Organometallic–radical compounds In Grenoble we follow another approach which is to
synthesise chelating radicals such as the nitronyl nitronyl
Instead of synthesising pure organic or inorganic-based nitroxide radicals substituted by imidazolyl or ben-
magnets the idea in the following two parts is to combine zimidazolyl groups (Fig. 4). With these radicals we
both types of spin carriers in the same compound. One way obtained complexes in which the metal ion is surrounded
is to use an approach developed for the charge transfer by three or four radicals, respectively, for transition and
salts. The inorganic spin carriers are organometallic cat- lanthanide ions [28,29]. These complexes are molecular
III ?1
ions, generally metallocenes [M Cp*2] . The organic bricks for the building of extended networks. With man-
?2
counterparts are the radical cations [TCNQ] or ganese(II) we synthesised chain compounds [30] which are
?2
[TCNE] (TCNQ, 7,7,8,8-tetracyanoquinodimethane; magnets with T,5 K. The main advance however is the
C
TCNE, tetracyanoethylene). These compounds have been synthesis of layered compounds in which the sheets have a
II
among the first molecular magnets reported. However in honeycomb structure made of Mn ions alternating with
this family of compounds, magnets have low T,30 K, radicals (Fig. 4). These compounds are magnets with T
C C
and there is no recent improvement [7]. As we have up to 55 K [31] depending mainly on the counter anions
already pointed out, Trely on the crystal packing which used for neutrality which are between the layers.
C
126 D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
Fig. 4. Layered structure of the compounds obtained with manganese(II) and nitronyl nitroxide substituted with imidazolyl or benzimidazolyl substituents
[31].
6. Single-molecule magnets with oxo, hydroxo and carboxylato bridging ligands re-
ceive special attention [34–36], mainly because the early
This is the most recent domain in the field of molecular studies of bioinorganic models have allowed a good
magnetism but it has its root in the studies of polymetallic knowledge in their chemistry and magnetic properties.
biological systems such as the iron metabolism or the Clusters based on cyanide chemistry are also widely
manganese core in photosynthetic water oxidation. synthesised. The method is to use ligands in the coordina-
The first single-molecule magnet was a Mn cluster tion sphere of the external metal ions (Fig. 5) to hinder the
12
II,IV
[Mn O (MeCO )(H O) ].2MeCO H.4H O [32] which expansion of the compound toward a 3D structure of the
12 12 2 2 4 2 2
has a ground spin state S510. The relaxation time of the Prussian-blue type (Fig. 4) [19,37–40]. In that way two
magnetisation is so slow at low temperature (1 K) that the clusters with large ground spin state values have been
individual clusters behave as a magnet such as the evi- recently synthesised but one (S539/2) [39] has a low
dence of hysteresis loops of the magnetisation. The blocking temperature (,2 K) while the other (S551/ 2)
experiments showed clearly that it is not a behaviour due (Fig. 6) [40] has no single-molecule magnet behaviour.
to 3D long range magnetic order but is of molecular origin Along with cyanide chemistry another approach is the use
and is associated with thermally assisted quantum tunnel-
ling. The temperature at which it becomes observable is
called the blocking temperature. The behaviour was con-
firmed by the same team for an Fe iron(III) oxo cluster
8
[Fe (tacn) O (OH) ]Br .9H O which also has an S510
8621282
ground spin state [33].
This discovery has generated tremendous activity to find
new high spin molecules. From the basic point of view
these compounds open a way to understand the limits in
size for the transition from molecular to bulk properties, or
to have both coexisting. From the point of view of applied
technologies they are in the framework of the search for
nanoscale memories. In this regard, this is the bottom-up
approach in contrast to the up-bottom one which is mainly
used to produce nanosize objects.
The challenge here for the chemist is to assemble in a
controlled manner a great number of magnetic centres in
one molecule (cluster), and isolated from the neighbours.
Indeed, to have a single-molecule magnet with a high
blocking temperature, a large value of the ground spin state
and no interactions between the clusters are required. Fig. 5. Schematic representation of a polymetallic cluster based on
The synthesis of large clusters relies mostly on poly- cyanide chemistry. The external ligands (L) are used to block the
metallic coordination complexes. In this frame, compounds extension of the compound.
D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
127
II V
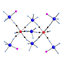
Fig. 6. Molecular structure of the cyano-bridged cluster Mn Mo molecular cluster with an S551/2 reproduced by permission from Wiley-VCH [40].
96
of free radicals in the coordination sphere of the external interesting alternatives to develop. The metal–radical
metal ions to build a cluster of finite size and ground spin approach has already proved to work and opens the way
with successive shells of spin carriers like an onion. This for versatile magnets. The organic-based magnets up to
has been successfully demonstrated to work in the case of now have low Tbut recent results show that it could be
C
a cluster with a ground spin S57 but no single-molecule greatly improved.
magnet behaviour [41]. Other approaches are based on the Molecular-based magnets are also interesting because
design of new ligands to have polymetallic clusters [42]. their molecular nature is promising in respect to multi-
functional materials. In that frame, new trends in the field
are the studies of combined magnetic with conducting or
7. Summary and prospects optical properties [43–46]. The studies of combined prop-
erties are also interesting for the progress of each field
The first molecular-based magnets were produced less because it brings in close contact different but complemen-
than 20 years ago. Since then the Curie temperature has tary expertise.
increased from a few Kelvin and is now 100 K above room
temperature. Different approaches have been developed
using either organic or inorganic magnetic centres. The References
inorganic approach based on polymetallic coordination
complexes has proved to be the most efficient for the [1] McConnell HM. Ferromagnetism in solid free radicals. J Chem Phys
synthesis of molecular-based magnets. In particular, the 1963;39:1910.
results obtained with cyanide chemistry demonstrate the [2] Hay PJ, Thibeault JC, Hoffmann R. Orbital interaction in metal
dimer complexes. J Am Chem Soc 1975;97:4884–99.
feasibility to synthesise such magnets with high T.
C
[3] Kahn O, Briat B. J Chem Soc Faraday II 1976;72:268.
However, they must not close the way for new routes using [4] Kollmar C, Kahn O. Ferromagnetic spin alignment in molecular
different bridging ligands. Indeed, molecular magnets will systems: an orbital approach. Acc Chem Res 1993;26:259–65.
be interesting if it is possible to play with their chemistry [5] Kahn O, editor, Molecular magnetism, New York: VCH, 1993.
to make them adjustable to many applications. In this [6] Ovcharenko VI, Sagdeev RZ. Molecular ferromagnets. Russ Chem
regard, organic and metal–radical-based compounds are Rev 1999;68:345.
128 D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
[7] Miller JS. Organometallic and organic-based magnets: new chemis- layered compounds prepared by anion exchange reaction: correlation
try and new materials for the new millennium. Inorg Chem between structure and magnetic properties. J Mater Chem
2000;39:4392–408. 1999;9:169–74.
[8] Lahti PM, editor, Magnetic properties of organic materials, New [27] Inoue K, Hayamizu T, Iwamura H et al. Assemblage and alignment
York: Marcel Dekker, 1999. of the spins of the organic trinitroxide radical with a quartet ground
[9] Kinoshita M, Turek P, Tamura M, Nozawa K, Shiomi D, Nakazawa state by means of complexation with magnetic metal ions. A
Y et al. An organic radical ferromagnet. Chem Lett 1991:1225. molecule-based magnet with three-dimensional structures and high
[10] Deumal M, Novoa JJ, Bearpark MJ, Celani P, Olivucci M, Robb Tof 46 K. J Am Chem Soc 1996;118:1803–4.
C
MA. On the validity of the McConnell-I model of ferromagnetic [28] Fegy K, Sanz N, Luneau D, Belorizky E, Rey P. Proximate nitroxide
interactions: the [2.2] paracyclophane example. J Phys Chem A ligands in the coordination sphere of manganese(II) and nickel(II)
1998;102:8404–12. ions. Precursors for high-dimensional molecular magnetic materials.
[11] Novoa JJ, Veciana J, Deumal M. Crystal engineering of purely Inorg Chem 1998;37:4518–23.
organic molecular magnets: what can ab initio computations tell us. [29] Lescop C, Luneau D, Belorizky E, Fries P, Guillot M, Rey P.
NATO ASI Ser Ser C 1999;518:105–25. Unprecedented antiferromagnetic metal–ligand interactions in
[12] Desiraju GR. The crystal as a supramolecular entity, Chichester: gadolinium-nitroxide derivatives. Inorg Chem 1999;38:5472–3.
John Wiley, 1996. [30] Fegy K, Luneau D, Belorizky E, Novac M, Tholence J-L, Paulsen C
[13] Togashi K, Imachi R, Tomioka K, Tsuboi H, Ishida T, Nogami T et et al. 1D-Manganese(II) derivatives of an imidazole substituted
al. Organic radicals exhibiting intermolecular ferromagnetic interac- nitronyl nitroxide. An approach toward molecular magnetic materi-
tions with high probability. 4-Arylmethyleneamino-2,3,6,6-tetra- als of high dimensionality. Inorg Chem 1998;37:4524–32.
methylpiperidin-1-yloxyls and related compounds. Bull Chem Soc [31] Fegy K, Luneau D, Ohm T, Paulsen C, Rey P. Two-dimensional
Jpn 1996;69:2821–30. nitroxide-based molecular magnetic materials. Angew Chem Int Ed
[14] Matsushita MM, Izuoka A, Sugawara T, Kobayashi T, Wadu N, 1998;37:1270–3.
Takeda N et al. Hydrogen-bonded organic ferromagnets. J Am [32] Sessoli R, Gatteschi D, Caneschi A, Novak MA. Magnetic bi-
Chem Soc 1997;119:4369–79. stability in a metal-ion cluster. Nature 1993;365:141–3.
[15] Romero FM, Ziessel R, Bonnet M, Pontillon Y, Ressouche E, [33] Wernsdorfer W, Sessoli R. Quantum phase interference and parity
Schweizer J et al. Evidence for transmission of ferromagnetic effects in molecular clusters. Science 1999;284:133–5.
interactions through hydrogen bonds in alkyne-substituted nitroxide [34] Gatteschi D, Sessoli R, Cornia A. Single-molecule magnets based on
radicals: magnetostructural correlations and polarized neutron dif- iron(III) oxo clusters. Chem Commun 2000:725–32.
fraction studies. J Am Chem Soc 2000;122:1298–309. [35] Yoo J, Brechin EK,Yamaguchi A, Nakano M, Huffman JC, Maniero
[16] Chiarelli R, Novak MA, Rassat A, Tholence JL. A ferromagnetic AL et al. Single-molecule magnets: a new class of tetranuclear
transition at 1.48 K in organic nitroxide. Nature 1993;363:147–9. manganese magnets. Inorg Chem 2000;39:3615–23.
[17] Barr CL, Chase PA, Hicks RG, Lemaire MT, Stevens CL. Synthesis [36] Benelli C, Parsons S, Solan A, Winpenny REP. Ferric wheels and
and characterization of verdazyl radicals bearing pyridine or py- cages: decanuclear iron complexes with carboxylato and pyridonato.
rimidine substituents: a new family of chelating spin-bearing Angew Chem Int Ed Engl 1996;35:1825–8.
ligands. J Org Chem 1999;64:8893–7. ``
[37] Rogez G, Marvilliers A, Riviere E, Audiere J-P, Lloret F,Varret F et
[18] Rawson JM, McManus GD. Benzo-fused dithiazolyl radicals: from al. A mixed-valence mixed-spin Prussian-blue-like heptanuclear
chemical curiosities to materials chemistry. Coord Chem Rev complex. Angew Chem Int Ed Engl 2000;39:2885–7.
1999;189:135–68. [38] Oshio H, Tamada O, Onodera H, Ito T, Ikoma T, Tero-Kubota S.
[19] Verdaguer M, Bleuzen A, Marvaud V,Vaissermann J, Seuleiman M, Cyanide-bridged iron–copper molecular squares with doublet and
Desplanches C et al. Molecules to build solids: high Tmolecule-
C
quintet spin ground states. Inorg Chem 1999;38:5686–9.
based magnets by design and recent revival of cyano complexes
chemistry. Coord Chem Rev 1999;190–192:1023–47. [39] Zhong ZJ, Seino H, Mizobe Y, Hidai M, Fujishima A, Ohkoshi S et
[20] Dunbar KR, Heintz RA. Chemistry of transition metal cyanide al. A high-spin cyanide-bridged Mn W cluster (S539/2) with a
96
compounds: modern perspectives. In: Karlin KD, editor, Progress in full-capped cubane structure. J Am Chem Soc 2000;122:2952–3.
inorganic chemistry, vol. 45, John Wiley, 1997, pp. 283–391. [40] Larionova J, Gross M, Pilkington M, Andres H, Stoeckli-Evans H,
¨
[21] Holmes SD, Girolami GJ. Sol–gel synthesis of Gudel HU et al. High-spin molecules: a novel cyano-bridged
II III II V
KV [Cr (CN) ].2H O: a crystalline molecule-based magnet with a Mn Mo molecular cluster with a S551/2 ground state and
62 96
magnetic ordering temperature above 1008C. J Am Chem Soc ferromagnetic intercluster ordering at low temperatures. Angew
1999;121:5593–4. Chem Int Ed 2000;39:1605–9.
[22] Kahn O, Larionova J, Ouahab L. Magnetic anisotropy in cyano- [41] Vostrikova KE, Luneau D, Verdaguer M, Wernsdorfer W, Rey P. A
42
bridged bimetallic ferromagnets synthesized from the [Mo(CN) ] . polynuclear nitroxide metal complex with a S57 ground spin state.
7
Chem Commun 1999:945–52. J Am Chem Soc 2000;122:718–9.
´
[23] Smith JA, Galans-Mascaro JR, Clerac R, Dunbar KR. [42] Zhao L, Xu Z, Thompson LK, Heath SL, Miller DO, Ohba M.
Mn(OH) [Mn(bpym)(OH) ] [Fe(CN) ] j: a two dimensional fer- Synthesis, structure, and magnetism of a novel alkoxide bridged
22262
rimagnet with a partial cubane motif. Chem Commun 2000:1077–8. nonacopper(II) (Cu O ) [333] square grid generated by a strict
912
[24] Armentano D, De Munno G, Lloret F, Julve M. Novel three- self-assembly process. Angew Chem Int Ed 2000;39:3114–7.
`
dimensional cage assembly of a m4-carbonato-bridged cobalt(II) [43] Lescop C, Luneau D, Bussiere G, Triest M, Reber C. Ligand-
compound [Co (bpm)(H O) (CO )(OH)]NO .4H O. Inorg Chem centered near-infrared luminescence from lanthanide complexes
2223 32
1999;38:3744–7. with chelating nitronyl-nitroxide free radicals. Inorg Chem
[25] Jensen P, Price JD, Batten SR, Moubaraki B, Murray KS. Self- 2000;39:3740–1. `
penetration – a structural compromise between single networks and [44] Cador O, Mathoniere C, Kahn O. Single-crystal polarized optical
interpenetration: magnetic properties and crystal structures of absorption spectroscopy of the one-dimensional ferrimagnet
II II
[Mn(dca) (H O)] and [M(dca)(tcm)], M5Co, Ni, Cu, dca5Mn Cu (pba)(H O) .2H O (pba51,3-propylenebis(oxamato)).
22 23 2
22
dicyanamide, N(CN) , tcm5tricyanomethanide, C(CN) . Chem Inorg Chem 2000;39:3799–804.
23
Eur J 2000;6:3186–95. [45] Pejakovic DA, Manson JL, Miller JS, Epstein AJ. Cluster glass state
[26] Laget V, Hornick C, Rabu P, Drillon M. Hydrid organic–inorganic and photoinduced effects on the freezing dynamics in
D
.
Luneau /Current Opinion in Solid State and Materials Science
5 (2001) 123
–
129
129
22
K Co[Fe(CN) ] .zHO (x|0.16, y|0.72, z|4.4). J Appl Phys chromic nitroprusside complex [Fe(CN) NO] , and organic p-
x6y2 5
2000;87:6028–30. electron donors. Synthesis, structure and properties of the radical
[46] Clemente-Leon M, Coronado E, Galan-Mascaro JR, Gomez-Garcia salt (TTF) [Fe(CN) NO] (TTF5tetrathiafulvalene). Inorg Chem
752
CJ, Canadell E. Hybrid molecular materials based upon the photo- 2001; in press.