Figure 2 - uploaded by Vincent Gauci
Content may be subject to copyright.
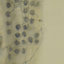
(top) Total monthly rainfall, (middle) peat temperature 10 cm below water table, and (bottom) mean water table position over the course of the experiment.
Source publication
The effect of acid rain SO42− deposition on peatland CH4 emissions was examined by manipulating SO42− inputs to a pristine raised peat bog in northern Scotland. Weekly pulses of dissolved Na2SO4 were applied to the bog over two years in doses of 25, 50, and 100 kg S ha−1 yr−1, reflecting the range of pollutant S deposition loads experienced in acid...
Contexts in source publication
Context 1
... 1997 and1998, distinct interannual differences in emission are evident, with total emis- sions in 1997 (7.7 g CH 4 m À2 ), 51% lower than over the same measurement period in 1998. These differences correspond to a lower water table in 1997 than in 1998 (Figure 2), which is due to lower than average rainfall in 1997 (670 mm, 26% lower than the 1916 -1950 mean) and slightly lower than average rainfall in 1998 (860 mm, 4% lower than the mean). Over the April to September growing season, when most CH 4 is emitted, there was 26% less rainfall in 1997 than 1998. ...
Context 2
... indicates that some other variable (or variables) was driving most of the variability in 1998. Changes in water table were of a smaller magnitude in 1998 than in 1997 (Figure 2), and methane emissions during the warm summer months of 1998 exhibited spikes that were unattributable to measured variables. ...
Context 3
... Overall, CH 4 fluxes exhibited typical seasonal changes which broadly followed changes in temperature (Figures 1 and 2b), i.e., higher in the warm summer months and early autumn and low during cool winter periods [Dise, 1993;Shannon and White, 1994;Saarnio et al., 1997]. There is a major difference in CH 4 flux between the two sampling years, which reflects large differences in the water table between the two years, with the field site receiving 26% less rainfall in 1997 than in 1998. ...
Context 4
... in late June and July, while temperatures remained high, fluxes from treatment plots were reduced to a level 45 -60% lower than fluxes from controls. Since this enhanced suppression accompanies a low- ering in water table (Figure 2), it is also described in the regression equation (effect of water table; Table 3 and Figure 6). While we have no pore water data from this period, we have already shown that later on in the year, CH 4 concentrations were significantly smaller in treated plots than in controls. ...
Similar publications
Bitumen fume condensate (BFC) is hazardous wastewater generated at asphalt reclamation and production sites. BFC contains a wide variety of potentially toxic organic pollutants that negatively affect anaerobic processes. In this study, we chemically characterized BFC produced at an industrial site and evaluated its degradation under anaerobic condi...
Citations
... This observation implied that these microorganisms sustained themselves due to the substantial reservoir of stored carbon. The existence of a sulfuric horizon within the wetland reduces the availability of easily degradable organic material at greater depths, as noted by (Gauci et al., 2002;Segers, 1998). ...
Climate change has emerged as a central concern with far-reaching consequences including a rise in global temperatures and sea levels which is attributed to an increase in greenhouse gas emissions. This phenomenon extends beyond environmental realms, impacting economies, human health, and social stability. Amidst this backdrop, acid sulfate soils present a unique challenge. These soils, found in waterlogged areas possess distinct characteristics due to sulfidic materials and extremely low pH values below 4. The objective of this study is to review in detail the role of acid sulfate soils in climate change adaptation and mitigation. Acid sulfate soils can undergo oxidation, causing acidification and the release of toxic elements, posing threats to ecosystems, agriculture, and infrastructure. The discharge of acidic water enriched with metals into water bodies further exacerbates the problem, especially under changing climate conditions. Acid sulfate soils can also emit sulfur-containing gases like hydrogen sulfide (H2S) and sulfur dioxide (SO2) alongside other gases like methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) impacting global concerns like acid rain and climate shifts. Coastal wetlands with acid sulfate soils can release carbon when drained, contributing to emissions, and affecting global warming. Research suggests that proper wetland management, water control, and carbon sequestration practices can mitigate these issues. However, gaps in research exist, such as understanding the carbon sequestration potential of acid sulfate soils, the factors influencing greenhouse gas emissions, and the impacts of climate change on acid sulfate soil properties. Continuous monitoring is essential for observing changes in pH levels, mineral composition, and the composition of microbial communities over a long period of time.
... Methanogenesis generally occurs only after complete sulfate reduction (Andersen et al., 2013;Gauci et al., 2002;Koebsch et al., 2019;Reddy and DeLaune, 2008). Consistently, the bog waters showed very low sulfate concentrations suggesting that S was fixed within the bog in reduced form (Eq. (6)), favoring the activity of methanogens. ...
Continental hydrosystems and in particular peatlands play an important role in the carbon cycle of the Critical Zone (CZ). Peatlands are important sinks for organic carbon and have therefore been extensively studied. However, peatlands are not only important for the fate of organic carbon, but they also affect the cycle of Dissolved Inorganic Carbon (DIC) of the peatland and the surrounding watershed. The fate of DIC is particularly complex in peatlands in limestone-dominated regions, because bicarbonate concentrations in surface and groundwater are high and the interaction between peatlands and surrounding hydrosystems are facilitated by the presence of highly permeable karst aquifers. In the present paper we study the origin and the fractionation of DIC in a peatland located on top of a karst aquifer. The study is based on hydrochemical and isotopic (δ¹³CDIC) data from samples recovered during 2 campaigns (low flow, high flow) at various depths within the Forbonnet peatland (Jura Mountains, eastern France), at the peatland outlet and at adjacent karst springs representing the underlying aquifer. In order to evaluate secondary fractionation processes, the measured δ¹³CDIC compositions were compared to modeled values considering the origin of DIC and potentially associated fractionation and speciation processes. The main results are: (1) DIC is lost at the bog surface by CO2 outgassing. (2) The δ¹³CDIC compositions of deep catotelm pore waters from the bog were much heavier than the modeled values. We relate this discrepancy to methanogenesis and show that this process is favored by reduced conditions at pH ~ 6 and a HCO3⁻ content of ~1 mmol/L, most probably due to punctual groundwater inflows at the base of the bog. Finally, contrasted δ¹³CDIC compositions between the bog and the fen of the peatland reveal an additional ecohydrological control on DIC speciation.
... This is in line with a previous report based on the same wetland sediment in which sulfate-reducing rates did not account for important AOM activities (Valenzuela et al. 2019). Considering this, sulfate only acted as a methanogenesis suppressor presumably by previously reported mechanisms of competition for organic substrates with methanogens which is in line with previous studies (Figs. 4 and 5) (Lovley and Klug 1983;Gauci et al. 2002Gauci et al. , 2004Pester et al. 2012). These findings imply that even if partially oxidized sulfur products are produced by the electron-accepting capacity in ...
elemental sulfur was the dominant product of sulfide oxidation (~ 50 to 75% of oxidized sulfur), thiosul-fate was the second most abundant product accounting for ~ 20% of the oxidized sulfide. The incorporation of S into PPHS' organic structure was revealed by the formation of methylthio, ethylthio, thiol, and aromatic-disulfide/polysulfide moieties after the reaction , which may compromise the availability of NOM to act as TEA for the oxidation of organic matter or methane. Wetland sediment incubations amended with sulfate and PPHS revealed that PPHS were the preferential TEA for catalyzing AOM (NOM-AOM) while sulfate suppressed methanogenic activities. Considering this and several novel findings concerning sulfate-and NOM-driven AOM, we discuss novel mechanisms by which sulfur/organic matter interactions could impact the microbial processes of CH 4 production and consumption. Abstract Redox-active natural organic matter (NOM) possesses great potential to fuel chemical and biological reactions due to its electron-transferring capacity. Chemical sulfide oxidation with redox-active NOM as the terminal electron acceptor (TEA) has been shown to determine the extent to which organic matter degradation produces CO 2 or CH 4 by suppressing methanogenesis. However, the effect that such S cycling reactions potentially have on CH 4-consuming processes, such as sulfate-and NOM-dependent anaerobic oxidation of methane (AOM), is yet to be disclosed. In this study, bulk Pahokee Peat humic substances (PPHS) were employed as a model source of redox-active NOM to test their role as TEA for the chemical oxidation of dissolved sulfide. While
... Gas samples were stored in a 12-mL pre-evacuated vials (Labco Exetainer, Castleford, England) or a vacuum-sealed 25-mL aluminum foil sampling bag (Delin Ltd., DaLian, China). Methane fluxes were calculated using a linear regression of the changes in the gas concentrations inside the chamber, the measured time, base area (or stem surface area), chamber volume, and the molar volume of CH 4 at ambient temperature (Gauci et al. 2002). The majority (98%) of CH 4 flux measurements had regression coefficients of r 2 > 0.90. ...
Purpose
Increasing evidence indicates that trees could emit methane (CH4) from soils into the atmosphere. However, inter-species and seasonal variations in the magnitude of tree-mediated CH4 fluxes within coastal mangrove wetlands have not yet been clarified.
Methods
We measured CH4 emission fluxes in three independent mangrove communities, Avicennia marina, Aegiceras corniculatum, and Kandelia obovata, within a subtropical mangrove wetland during tree dormancy and growth seasons using static chambers. Tree-stem, pneumatophore, and soil–atmosphere-interface CH4 fluxes were simultaneously measured, thus enabling an estimate of the contributions from each pathway to ecosystem CH4 fluxes.
Results
Pneumatophore and tree-stem CH4 fluxes were much higher than the soil–atmosphere-interface CH4 flux. In mangrove communities with pneumatophores (A. marina), the pneumatophore CH4 fluxes accounted for 84% of the ecosystem CH4 flux, whereas tree-stem and soil–atmosphere-interface CH4 fluxes accounted for 9% and 7%, respectively. In contrast, in mangrove communities without pneumatophores (A. corniculatum and K. obovata), the tree-stem CH4 fluxes dominated (75–79%) the ecosystem CH4 fluxes, whereas the soil–atmosphere-interface CH4 fluxes accounted for 21–25%. Ecosystem CH4 fluxes, as well as pneumatophore, tree-stem, and soil–atmosphere-interface CH4 fluxes were higher during the growth season than the dormancy season. However, the partitioning of ecosystem CH4 fluxes did not significantly change between the two seasons.
Conclusion
Tree stems and pneumatophores are important conduits for CH4 emissions in mangrove wetlands. Ecosystem CH4 emissions may offset 53% (with pneumatophores) or 10–13% (without pneumatophores) of the total C burial rates in global mangrove wetlands.
... Finalement, les archées sont concurrencées par les bactéries sulfato-réductrices. La présence de sulfate diminue la méthanogenèse (Gauci et al., 2002). Il existe 2 réactions formant du CH4 : la réduction du CO2 par du dihydrogène (H2) et l'acétoclastie (la fermentation de l'acétate) (Corbett et al., 2015;Sugimoto and Wada, 1993).Une large portion du carbone minéralisé du catotelme l'est sous forme de CH4 (Wieder et al., 2006). ...
The main objective of this thesis is to assess the factors that could influence the organic matter (OM) degradation within a carbon-rich permafrost peatland in a global warming context. Field studies and laboratory experiments were carried out in discontinuous permafrost areas of Eastern Siberia, Sweden and NE Europe. The main results of this thesis highlight: i) the importance of night-time in the CO2 production from thermokarst lakes; ii) the decrease of the OM degradation rate and the increase of the biodegradability of waters along the hydrological continuum; iii) that repeated freeze-thaw cycles do not influence the degradation of carbon but favor the OM-metals complexes in large lakes and rivers; iv) that low temperatures exhibit the same effect on biodegradation than the summer ones; and v) that the thaw of permafrost OM does not release a significantly different amount of CH4 and CO2 from biodegradation processes compared to the active layer.
... Finalement, les archées sont concurrencées par les bactéries sulfato-réductrices. La présence de sulfate diminue la méthanogenèse (Gauci et al., 2002). Il existe 2 réactions formant du CH4 : la réduction du CO2 par du dihydrogène (H2) et l'acétoclastie (la fermentation de l'acétate) (Corbett et al., 2015;Sugimoto and Wada, 1993).Une large portion du carbone minéralisé du catotelme l'est sous forme de CH4 (Wieder et al., 2006). ...
Dans le contexte actuel du réchauffement climatique et de son amplification dans les régions nordiques, l'objectif principal de cette thèse est d'évaluer les facteurs pouvant influencer la dégradation de la matière organique (MO) contenue dans les tourbières à pergélisol. Dans ce but, nous avons étudié l'effet des paramètres, directement ou indirectement influencés par le changement climatique, sur la dégradation de la MO. Des études de terrains et des expérimentations ont été effectuées dans des zones à pergélisol discontinu de la Suède et de l'Est Sibérien, associées à des expériences en laboratoire utilisant des substrats de ces régions et du Nord-Est de l'Europe. Ces études ont eu pour but de déterminer l'influence des paramètres physico-chimiques suivant sur la dégradation de la MO: la température, la biomasse microbienne, l'origine de la MO, le type de végétation, l'hétérogénéité des plans d'eau, les cycles de gel-dégel (CGD), les conditions anoxiques et la profondeur des sols. L'originalité de ce travail réside dans la combinaison d'analyses (bio)géochimiques variées, utilisées à la fois lors des missions de terrain et en laboratoire, liant la microbiologie et la géochimie, pour comprendre la transformation de la MO dans les régions arctiques. Après une introduction générale présentant l'impact et la rétroaction positive que peut avoir le dégel du pergélisol sur le réchauffement climatique, le premier chapitre de cette thèse présente le contexte général du sujet d'étude, traitant de la MO et des problèmes actuels des régions arctiques face à ce réchauffement. Il contient aussi les techniques, analyses et méthodes utilisées durant cette thèse. Le second chapitre décrit les 3 sites d'étude et les caractéristiques générales des travaux réalisés sur le terrain, dont l'étude du comportement de la MO durant les cycles nycthéméraux. Le troisième chapitre traite de l'influence de l'origine de la MO et de l'hétérogénéité des plans d'eau sur la biodégradation de la MO. Le chapitre quatre s'intéresse aux effets des CGD subis par les eaux des tourbières durant les périodes de transition (début du printemps et fin de l'automne). Finalement, le cinquième chapitre traite d'expérimentations aérobies et anaérobies de dégradation de la MO. Les expérimentations aérobies traitent de l'effet de la température et des bactéries hétérotrophes sur la biodégradation, et les expérimentations anaérobies ont été effectuées pour déterminer le potentiel de production en gaz à effet de serre d'un profil de sol issu d'une tourbière. Parmi les résultats les plus importants de cette thèse, on retiendra que : i) durant la nuit les petits lacs thermokarstiques relarguent jusqu'à 3 fois plus de CO2 que durant la journée et que les grands lacs passent de puits à source de CO2 ; ii) le long d'un continuum hydrologique (des dépressions jusqu'aux rivières en passant par les lacs thermokarstiques), la vitesse de biodégradation de la MO diminue et la biodégradabilité des eaux augmente ; iii) les CGD fréquents durant les périodes de transition n'influencent pas la dégradation de la MO mais favorisent la complexation MO-métaux dans les eaux à pH neutres des grands lacs et rivières; iv) les températures de ces périodes (4°C environ) ont le même impact sur la biodégradation de la MO que celles observées en été (25°C) ; v) enfin, lors du dégel anaérobie du pergélisol, la biodégradation de la MO de ce dernier ne produit pas plus de CH4 ni de CO2 que celle de la couche active. Les études menées lors de cette thèse devraient préciser les effets, directs et/ou indirects, du changement climatique sur la dégradation de la MO dans les régions à pergélisol discontinu. De plus, l'étude du devenir du carbone contenu dans les sols et les eaux des tourbières ainsi que les émissions de CO2 et de CH4, permettra d'appréhender l'influence du changement climatique sur les interactions entre le carbone biodégradé de la pédo- et de l'hydrosphère pouvant être émis vers l'atmosphère.
... Acid rain sulfate (SO 2− 4 ) deposition on peatlands and wetlands from natural sources (volcanoes), or anthropogenic sources (fossil fuel combustion) is a known suppressant of CH 4 production (Nedwell and Watson, 1995;Watson and Nedwell, 1998) and emissions (Gauci et al., 2002(Gauci et al., , 2004(Gauci et al., , 2005 and may be an important process in terms of global climate. The importance of the Fe input associated with anthropogenic aerosol deposition in terrestrial biogeochemistry deserves further investigation as do the possible impacts of a drastic diminution of anthropogenic iron and sulfate emissions from combustion processes expected by 2050 to satisfy the Paris climate agreement. ...
Power stations, ships and air traffic are among the most potent greenhouse gas emitters and are primarily responsible for global warming. Iron salt aerosols (ISAs), composed partly of iron and chloride, exert a cooling effect on climate in several ways. This article aims firstly to examine all direct and indirect natural climate cooling mechanisms driven by ISA tropospheric aerosol particles, showing their cooperation and interaction within the different environmental compartments. Secondly, it looks at a proposal to enhance the cooling effects of ISA in order to reach the optimistic target of the Paris climate agreement to limit the global temperature increase between 1.5 and 2 °C.
Mineral dust played an important role during the glacial periods; by using mineral dust as a natural analogue tool and by mimicking the same method used in nature, the proposed ISA method might be able to reduce and stop climate warming. The first estimations made in this article show that by doubling the current natural iron emissions by ISA into the troposphere, i.e., by about 0.3 Tg Fe yr⁻¹, artificial ISA would enable the prevention or even reversal of global warming. The ISA method proposed integrates technical and economically feasible tools.
... Ozone exposure has also been observed to make stomata sluggish, increasing nocturnal transpiration and O 3 uptake (Davison and Barnes, 2002;Hoshika et al., 2013). Although the air temperature was on average 3.8°C higher in the OTCs than at the Scottish field site, the mean summer CH 4 emission rates of 2-12 mg·C·m −2 h −1 were within the range of field flux rates in other sedge-dominated peatlands within the UK (Greenup et al., 2000;Gauci et al., 2002;McNamara et al., 2008;Toet et al., 2011;Levy et al., 2012: 0.1-14 mg m −2 h − 1 ) and Northern Europe (Granberg et al., 2001, Rinnan et al., 2003, Mörsky et al., 2008: 0.7-16 mg m −2 h −1 ). However, the results from both this and our previous study in opentop chambers (OTCs) (Toet et al., 2011) were not consistent with the findings of the four-year mire open-air fumigation study of Mörsky et al. (2008), who reported no overall long-term responses of CH 4 emissions to elevated O 3 , which were comparable to our NFA + 35/10 treatment. ...
... When groundwater discharge was high, substantial quantities of H 2 SO 4 and Fe oxides would likely have been released into the surface water (Sammut et al., 1996; Wilson et al., 1999; Burton et al., 2006). It is well known that sulphate reducing microbes compete with methanogens for organic substrates and H 2 , preferentially inhibiting methanogenesis (Gauci et al., 2000; Dowrick et al., 2006; Baldwin and Mitchell, 2012 ). The depleted δ 13 C-CH 4 values (−69‰ to −63‰) during the groundwater excess stage are in the range of the groundwater CH 4 endmember (− 66.6 ± 4.9‰), suggesting that groundwater may be contributing the majority of surface water CH 4 . ...
Many coastal floodplains have been artificially drained for agriculture, altering
hydrological connectivity and the delivery of groundwater-derived solutes including carbon
dioxide (CO2) and methane (CH4) to surface waters. Here, we investigated the drivers of CO2
and CH4 within the artificial drains of a coastal floodplain under sugarcane plantation and
quantify the contribution of groundwater discharge to CO2 and CH4 dynamics over a flood
(290 mm of rainfall). High temporal resolution, in situ observations of dissolved CO2 and
CH4, carbon stable isotope for CH4 (δ13C-CH4), and the natural groundwater tracer radon
(222Rn) allowed us to quantify CO2, CH4 and groundwater dynamics during the rapid
recession of a flood over a five day period. Extreme super-saturation of free CO2 ([CO2*]) up
to 2,951 µM (25,480% of atmospheric equilibrium) was driven by large groundwater input
into the drains (maximum 87 cm day-1), caused by a steep hydraulic head in the adjacent
groundwater. Groundwater input sustained between 95-124% of the surface [CO2*] flux
during the flood recession by delivering high carbonate alkalinity groundwater (DIC = 10,533
µM, ~pH = 7.05) to acidic surface water (pH <4), consequently transforming all
groundwater-derived DIC to [CO2*]. In contrast, groundwater was not a major direct driver
of CH4 contributing only 14% of total CH4 fluxes. A progressive increase in CH4
concentrations of up to ~2,400 nM day-1 occurred as a combination of increased substrate
availability delivered by post-flood drainage water and longer residence times, which allowed
for a biogenic CH4 signal to develop. The progressive enrichment in δ
13C-CH4 values (-70‰ to -48‰) and increase in CH4 concentrations (46-2,460 nM) support coupled production-oxidation, with concentrations and δ13C values remaining higher (-47‰ and 2,798 nM) than
pre-flood conditions (-55‰ and 534 nM) three weeks after the flood. Our findings demonstrate how separate processes can drive the aquatic CO2 and CH4 response to a flood event in a drained coastal floodplain, and the key role groundwater had in post-flood [CO2*] evasion to the atmosphere, but not CH4.
... However, this importance is threatened by widespread evidence of degradation. Peatland degradation can be caused by many factors including erosion (Yeloff et al. 2006), overgrazing (Worrall & Clay 2012), prescribed burning (Worrall et al. 2007, Muller et al. 2012), climate change (Hogg et al. 1992, Heijmans et al. 2013), acid rain (Nedwell & Watson 1995, Watson & Nedwell 1998, Gauci et al. 2002), peat cutting (Cooper et al. 2001, Charman & Pollard 1995) and drainage (Ramchunder et al. 2009). Blanket mires are particularly vulnerable to degradation and this has become widespread in parts of the UK uplands. ...
Actively growing mires have high conservation value and the potential to sequester carbon. However, drainage, burning, overgrazing and atmospheric pollution have led to depauperation of native flora and loss of peat at many peatland sites. In order to counteract such degradation, palaeoecological techniques can be applied and the data then used to inform nature conservation practice. The present study exemplifies this approach and was conducted on degraded blanket mire in Yorkshire, UK, in collaboration with a field-based moorland restoration agency. High-resolution, multiproxy palaeoecological analyses on a peat core from Oxenhope Moor were used to reconstruct Holocene vegetation changes spanning approximately the last 7000 years. Humification, pollen, plant macrofossil and charcoal analyses show distinct changes in species composition and indicate their potential causes. Human-induced changes identified at 2100 cal. BP are most likely to reflect deliberate clearance by fire. Sphagnum imbricatum disappears and is subsequently replaced by S. papillosum at ca. 1000 cal. BP, possibly due to drier conditions and competition between the two species. Increased human activity is identified since the Industrial Revolution where monocots and Eriophorum vaginatum increase, interpreted as a result of managed burning. It is intended that the long-term ecological history of the site, derived using palaeoecological techniques, will be used to inform conservation practice and can help set feasible targets for restoration and conservation. Specifically, encouraging a species mix that has pre-19th century longevity is suggested, including the specific recommendation that translocation of S. imbricatum be explored experimentally at this site, with a view to ascertaining likely success elsewhere. © 2016 International Mire Conservation Group and International Peatland Society.