Figure - available from: Advanced Functional Materials
This content is subject to copyright. Terms and conditions apply.
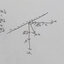
a) Schematic diagrams for the comparison of Na nucleation, deposition in Ti3C2 and CT‐Sn(II)@Ti3C2 matrixes. Binary phase diagrams of b) Na with Sn,59 c) Na with Cu.63
Source publication
Sodium (Na) metal is a promising alternative to lithium metal as an anode material for the next‐generation energy storage systems due to its high theoretical capacity, low cost, and natural abundance. However, dendritic/mossy Na growth caused by uncontrollable plating/stripping results in serious safe concerns and rapid electrode degradation. This...
Similar publications
Lithium (Li) metal has emerged as a viable alternative anode material to address the current energy density shortfalls in Li batteries. However, its integration into widespread implementation remains somewhat constrained due to the substandard reversibility issues and safety concerns arising from erratic Li deposition. To effectively tackle these o...
Lithium is regarded as an ideal anode for next‐generation Li metal batteries (LMB) as it exhibits extraordinarily high theoretical capacity and the lowest electrochemical potential among all anode candidates. However, safety concerns and poor cycling stability of Li induced by uncontrollable dendrite growth and severe side reactions impede its prac...
With the application of smart meters, more information is available from residential buildings for support heat load forecast. Yet, there is still a lack of an effective method to exploit the value of the high spatial granularity information, particularly for residential communities with high randomness in human behaviors. To fill this gap, this pa...
Lithium (Li) metal anode has been considered as the most promising anode for rechargeable batteries with high energy density owing to its extraordinarily high theoretical specific capacity (3860 mAh g−1) and the lowest negative electrochemical potential (–3.040 V vs. the standard hydrogen electrode). However, the practical applications of the Li me...
Citations
... Among the electrochemical ESDs (EESDs) family, rechargeable batteries (especially lithium-ion batteries (LIBs)), supercapacitors (SCs), and their hybrid forms are the most beneficial EESDs-based technology. Still, the same new storage techniques are underway for commercial use in the near future [43]. Batteries are used broadly nowadays, mostly in hybrid electric vehicles (EVs) and portable wearable electronic devices. ...
Unsustainable fossil fuel energy usage and its environmental impacts are the most significant scientific challenges in the scientific community. Two-dimensional (2D) materials have received a lot of attention recently because of their great potential for application in addressing some of society’s most enduring issues with renewable energy. Transition metal-based nitrides, carbides, or carbonitrides, known as “MXenes”, are a relatively new and large family of 2D materials. Since the discovery of the first MXene, Ti3C2 in 2011 has become one of the fastest-expanding families of 2D materials with unique physiochemical features. MXene surface terminations with hydroxyl, oxygen, fluorine, etc., are invariably present in the so far reported materials, imparting hydrophilicity to their surfaces. The current finding of multi-transition metal-layered MXenes with controlled surface termination capacity opens the door to fabricating unique structures for producing renewable energy. MXene NMs-based flexible chemistry allows them to be tuned for energy-producing/storage, electromagnetic interference shielding, gas/biosensors, water distillation, nanocomposite reinforcement, lubrication, and photo/electro/chemical catalysis. This review will first discuss the advancement of MXenes synthesis methods, their properties/stability, and renewable energy applications. Secondly, we will highlight the constraints and challenges that impede the scientific community from synthesizing functional MXene with controlled composition and properties. We will further reveal the high-tech implementations for renewable energy storage applications along with future challenges and their solutions.
... Additionally, we conducted a comparative analysis of the electrochemical performance of the representative MXene electrodes for Na metal anodes ( Figure S17 and Table S2, Supporting Information). [23][24][25][43][44][45][46][47][48][49][50] The findings indicate that our designed 3DP Nb 2 CT x /rGO electrodes demonstrate exceptional performance, particularly in terms of outstanding long-term cycling stability. ...
As a promising anode material for Na‐metal batteries, the practical application of Na metal is severely hindered due to the formation of the notorious dendrite and unstable solid‐electrolyte interface (SEI). To address these issues, a direct‐ink writing (DIW) 3D printing technology is proposed to construct an artificial 3D hierarchical porous sodiophilic Nb2CTx/reduced graphene‐oxide (Nb2CTx/rGO) aerogel monolith, which is employed as the matrix of Na metal anode. Benefiting from the homogeneous ion flux and exceptional sodiophilic features, the Nb2CTx nanoflakes embedded within the 3D scaffold regulate the uniform deposition of metallic Na with a stable SEI layer, achieving an ultralong cycle lifespan of 3000 h at 1 mA cm⁻² with 1 mAh cm⁻² and an impressive Coulombic efficiency of 99.68% over extended lifespan of 7064 h. Further, the in‐depth characterization analysis proves that the formed stable SEI layer consists of an inorganic NaF accumulated in the inner layer and loosely‐bound organic species in the outer layer. Remarkably, when integrated into a Na metal full cell, the reversible capacity reaches 90.22 mAh g⁻¹ after 200 cycles at 100 mA g⁻¹. The work provides a promising strategy to utilize Na metal anodes with long cycle lifespan for next‐generation sodium metal batteries.
... In terms of interfacial engineering, "zincophilic" coatings such as Ti 3 C 2 T x MXene (MX) were recently demonstrated to be particularly effective as an interfacial layer to achieve smooth deposition on metal anodes such as Li, Na, K, and Zn [24][25][26][27]. Ti 3 C 2 T x MXene can unlock fast electrochemical kinetics in the plating/stripping process due to its high electronic conductivity and rapid Zn-ion diffusion. ...
Zinc-ion batteries (ZIBs) are currently being studied as an alternative to lithium-ion batteries (LIBs). The nucleation and growth of the zinc deposition mechanism is a critical field of research in ZIBs, as it directly affects the battery efficiency and lifespan. It is of paramount importance in mitigating the formation of porous, dendritic Zn structures that may cause cell inefficiency and, eventually, short-circuiting failures. Interfacial engineering plays a key role in providing reversible plating and stripping of metallic Zn in ZIBs through the proper regulation of the electrode–electrolyte interface. In this work, we investigated the behavior and characteristics of Zn plating on inkjet-printed Ti3C2Tx MXene-coated substrates according to the different electrolyte compositions. Specifically, ZnCl2 and ZnSO4 solutions were employed, evaluating the effect of a relatively low-molecular-weight polyethylene glycol (PEG400) addition to the electrolyte as additive. Electrochemical analyses demonstrated higher deposition kinetics in chloride-based electrolytes rather than sulfate ones, resulting in lower nucleation overpotentials. However, the morphology and microstructure of the plated Zn, investigated via scanning electron microscopy (SEM) and X-ray diffraction (XRD), revealed that the electrolytic solution played a predominant role in the Zn crystallite formation rather than the Ti3C2Tx MXene coating. Specifically, the preferential Zn [002] orientation could be favored when using additive-free ZnSO4 solution, and a PEG addition was found to be an efficient texturing agent only in ZnCl2 solution.
... [35][36][37] Large interlayer spacing is more compatible with Na + (larger than Li + ) and boosts the kinetics of sodium ion. [38] The S-doping can enlarge the interlayer spacing of the materials, conducive to pseudocapacitive capacity. [39] Besides, C-S-S-C bonds situated at the carbon skeleton edge can enlarge the interlayer distance of the carbonaceous materials. ...
Nickel sulfides are promising anode candidates in sodium ion batteries (SIBs) due to high capacity and abundant reserves. However, their applications are restricted by poor cycling stability and slow reaction kinetics. Thus, mesoporous nickel sulfide microsphere encapsulated in nitrogen, sulfur dual‐doped carbon (MNS@NSC) is prepared. The packaged structure and carbon matrix restrain the volume variation together, the N, S dual‐doping improves the electronic conductivity and offers extra active sites for sodium storage. Ex‐situ X‐ray diffraction appeals copper collector adsorbs polysulfide to inhibit the polysulfide accumulation and enhance conductivity. Moreover, the large subsurface attributed to C‐S‐S‐C bonding further boosts pseudocapacitive capacity, conducive to charge transfer. As a result, MNS@NSC delivers a high reversible capacity of 640.2 mAh g⁻¹ after 100 cycles at 0.1 A g⁻¹, an excellent rate capability (569.8 mAh g⁻¹ at 5 A g⁻¹), and a remained capacity of 513.8 mAh g⁻¹ after undergoing 10000 circulations at 10 A g⁻¹. The MNS@NSC|| Na3V2(PO4)3 full cell shows a cycling performance of specific capacity of 230.8 mAh g⁻¹ after 100 cycles at 1 A g⁻¹. This work puts forward a valid strategy of combing structural design and heteroatom doping to synthesize high‐performance nickel sulfide materials in SIBs.
... Vertical growth of 2D nanosheets on the substrate by chemical vapor deposition method (CVD) is another approach, which maintains the parallel channels; however, the amount of deposition of 2D nanosheets is insufficient, reducing the capacitive performance [44]. Recently, the most studied and promising approach to enhance the performance of 2D MXenes is by introducing interlayer spacers among the nanosheets [45][46][47]. Remarkably, the capacitance of MXene can be enhanced by intercalating it with transition metal oxide (TMOs) for high-performance electrode materials [48][49][50]. One of the most studied electrode materials among other TMOs is manganese dioxide (MnO 2 ) due to its high capacitive performance, low cost, most abundant, environment-friendly nature, and high theoretical capacity of 1370 F/g. ...
... The most direct strategy is to increase the interlayer distance of MXene (Fig. 3d). By introducing large ions like Sn 2+ and cetyltrimethylammonium cation (CTA + ) as the pillars between the adjacent MXene layers, the electrode can fulfil the ion transport requirement for large-size ions like Na + or K + [55][56][57]. Additionally, changing the 2D layer-like structure in MXenes can offer high electrochemical performance in the beyond-lithium batteries. ...
... Mashtalir et al. found that intercalating hydrazine together with N,N-dimethylformamide molecules greatly increased the c-lattice parameter of Ti3C2Tx from 19.50 Å to 25.48 Å and then to 26.80 Å [71]. Reversible humiditydependent expansion of the Ti3C2Tx MXenes intercalated with cations (e.g., Li + , Na + , K + , Rb + , Mg 2+ or Ca 2+ ) was also observed [72], and the interlayer space range increased from 12. (Fig. 5a-b) [55]. In their initial study, they synthesized a Sn 2+ pillared Ti3C2 MXene matrix by pre-intercalating the pristine MXene with cetyltrimethylammonium bromide (CTAB) to obtain a stable 'pillared' structure and then intercalated the Sn 2+ by ionexchange with the CTA + . ...
With the rapid growth in renewable energy, researchers worldwide are trying to expand energy storage technologies. The development of beyond-lithium battery technologies has accelerated in recent years, amid concerns regarding the sustainability of battery materials. However, the absence of suitable high-performance materials has hampered the development of the next-generation battery systems. MXenes, a family of 2D transition metal carbides and/or nitrides, have drawn significant attention recently for electrochemical energy storage, owing to their unique physical and chemical properties. The extraordinary electronic conductivity, compositional diversity, expandable crystal structure, superior hydrophilicity, and rich surface chemistries make MXenes promising materials for electrode and other components in rechargeable batteries. This report especially focuses on the recent MXene applications as novel electrode materials and functional separator modifiers in rechargeable batteries beyond lithium. In particular, we highlight the recent advances of surface and structure engineering strategies for improving the electrochemical performance of the MXene-based materials, including surface termination modifications, heteroatom doping strategies, surface coating, interlayer space changes, nanostructure engineering, and heterostructures and secondary materials engineering. Finally, perspectives for building future sustainable rechargeable batteries with MXenes and MXene-based composite materials are presented based upon material design and a fundamental understanding of the reaction mechanisms.
... Two-dimensional (2D) MXene materials have aroused the interest of scientists in numerous disciplines [35][36][37][38][39] Ti 3 C 2 T x MXene has the potential to be employed as an anode host for the deposition of metals in energy storage devices on account of its high metallic electrical conductivity (~10 4 S cm − 1 ) and ion-friendly hydrophilic property provided by the surface functional groups (OH, O, and F) [15,29,38,[40][41][42][43][44]. Nonetheless, 2D Ti 3 C 2 T x film is mostly restacked during structure formation and thus has a compact, dense structure with inaccessible sites and inhomogeneous nucleation sites on the surface, which provide hotspots for the development of mossy dendrites during plating and stripping in cycling and, eventually, cell death [15,29,40]. ...
... Two-dimensional (2D) MXene materials have aroused the interest of scientists in numerous disciplines [35][36][37][38][39] Ti 3 C 2 T x MXene has the potential to be employed as an anode host for the deposition of metals in energy storage devices on account of its high metallic electrical conductivity (~10 4 S cm − 1 ) and ion-friendly hydrophilic property provided by the surface functional groups (OH, O, and F) [15,29,38,[40][41][42][43][44]. Nonetheless, 2D Ti 3 C 2 T x film is mostly restacked during structure formation and thus has a compact, dense structure with inaccessible sites and inhomogeneous nucleation sites on the surface, which provide hotspots for the development of mossy dendrites during plating and stripping in cycling and, eventually, cell death [15,29,40]. It is inevitable to tailor the interspace of a nanostructured anode host and exert control over the initial nucleation site to maximize the performance of AFSMBs with excellent sodiophilicity and electrophilicity. ...
... With Ti 3 C 2 T x :CNT ratio of 8:2, better electrochemical performance is achieved (detailed in the supplementary data, Fig. S4b-d), and subsequent experiments were conducted using this sample. The initial Na nucleation overpotentials might provide insights into the sodiophilicity of various substrates [11,40] The overpotentials are calculated to be 38.5, 10.6, 12.4, 40.6 mV and 9.0 mV for pristine Cu foil, Ti 3 C 2 T x NAFs, CNT FSEs, Ti 3 C 2 T x film, and Ti 3 C 2 T x /CNT NAFs, respectively (Fig. 2a). Ti 3 C 2 T x /CNT NAFs have the lowest overpotential among the five substrates, indicating the lowest Na nucleation energy barrier and uniform Na nucleation sites across the entire surface and interstices. ...
Anode-free sodium-metal batteries are considered one of the most promising alternatives for developing high-end batteries due to their high theoretical capacity, low cost, and high natural abundance. However, they have severe drawbacks in the form of inferior long-term cyclic stability. We engineered mechanically resilient MXene/CNT nano-accordion frameworks (NAFs) to host significant Na without dendrite development, even at high currents. The microcellular structures of MXene/CNT-NAFs possess numerous micro-sized pores and sodium nucleation sites. The synergetic effects of strong adhesion and charge transfer between MXene and CNT reduce overpotential during plating/stripping and facilitate uniform Na deposition. Resilient nano-accordion structures are compressed by capillary of Na nucleation during plating and expand during stripping, allowing long-term plating/stripping with little volume change. These benefits allow the MXene/CNT-NAFs/Na asymmetric cell to maintain its average CE at 99.7 % with capacities of 1.0 mAh⋅cm−2 at 1.0 mA⋅cm−2 for 900 h. Furthermore, MXene/CNT-NAFs symmetric cell exhibits a very low overpotential of 12.0 mV after 1,500 h with a capacity of 3.0 mAh⋅cm−2 at 3.0 mA⋅cm−2 and stores high capacities of 20.0 mAh⋅cm−2 at 5.0 mA⋅cm−2 for 1,200 h. The anodefree MXene/CNT-NAFs//Na3V2(PO4)3@C full-cell demonstrates exceptional long-term cyclic stability over 5,000
cycles at 5.0 C and 10.0 C without cell failure.
... The XRD pattern in Figure 2a shows that the (104) peak of Ti 3 AlC 2 disappeared and the (002) peak of Ti 3 C 2 T x shifted toward a lower range, indicating the formation of a uniform, ordered layer structure in the Ti 3 C 2 T x . 27,28 The 2θ of the (002) peak was shifted from 6.2°for Ti 3 C 2 T x to 5.5°for h-Ti 3 C 2 T x (24 h), indicating the expansion of the d-spacing from 1.42 to 1.60 nm based on Bragg's law. The increase in d-spacing was favorable for the rapid diffusion of phosphate anions along the nanoscale channels between the nanosheets. ...
The discharge of wastewater contaminated with low concentrations of phosphate can have serious consequences, including environmentally disastrous blooms of algae and harmful concentrations of phosphate in public water sources. Herein, we demonstrate an electrofiltration system with a flow-through configuration that uses an electrified hydroxyl-terminated Ti 3 C 2 T x MXene (h-Ti 3 C 2 T x) filter to achieve near complete removal of ultralow concentration phosphate (5 mg P/L). Increasing either the applied voltage or the flow rate increases the phosphate sorption kinetics of the electrified h-Ti 3 C 2 T x filter. With a 6 mL/min recirculating flow rate, the h-Ti 3 C 2 T x filter exhibits a sorption kinetics of 1.97 h −1 and sorption capacity of 91.8 mg P/g, which was 2.6-and 4.3-fold higher than that of a pristine Ti 3 C 2 T x filter, respectively. The enhanced electrofiltration kinetics and capacities are attributed to the synergistic effects of plentiful sites for sorption, electrochemical enhancement, and flow-through design. The mechanism for phosphate sorption combined interlayer diffusion with surface complexation at the outer level in terms of electrostatic attraction and at the inner level in terms of Ti−O−P interactions. Overall, our research elucidates the utilization of a flow-through electrified filter incorporating −OH groups that can boost the sorption kinetics and capacity for phosphate, offering a promising paradigm for efficient water purification.
... The most advantageous electro-chemical ESDs-based technologies are secondary/rechargeable batteries, particularly Lithium-Ion Batteries (LIBs), supercapacitors (SCs), and their hybrid forms (Fig. 1). However, similar new storage techniques are currently being developed for commercial application or will do so soon [118]. Batteries are widely employed in modern society, namely portable wearable electronic gadgets and hybrid electric vehicles (EVs) [119]. ...
Due to ultralow defect formation energy, borophene differs significantly from other 2D (two-dimensional) materials in that it is difficult to distinguish between its crystal and boron (B) vacancy defect. In contrast to other 2D materials like graphene, borophene does not form layers when it is in its bulk state. In addition, borophene NM’s atomic structure is different from graphene’s in that it consists of connected triangles rather than hexagons. This
atomic configuration has gaps where atoms are missing, resulting in a flaw called a "hollow hexagon" (HH). In borophene phases, these HHs can be found in a variety of ratios. The phase intermixing of borophene is a brandnew example of an ’ordered’ defect discovered in 2D materials.
The majority of 2D materials have flaws or disruptions to the atom arrangement at the boundaries between various domains or phases. Defects play a major influence in determining the properties of materials in a 2D system, because all atoms are virtually on the surface. For instance, the line defects along phase boundaries in borophene have no effect on the material’s electrical characteristics at ambient temperature, in contrast to insulating flaws in metallic graphene. The atoms at the borders of borophene easily fit along line faults and adopt the configuration of their neighbors, causing no disruption. Additionally, the line flaws do not disrupt the seamless structure of borophene and maintain its stability and metallic properties.
Experimentally, all four borophene phases have been synthesized, and they are all metallic. A list of borophene NM’s special characteristics, including its negative Poisson’s ratio and extremely anisotropic Young’s modulus, is discussed. Here we also emphasized on B’s conductive and superconductive qualities. An overview of borophene NM’s uses in the energy sectors, including metal ion batteries, and supercapacitors (SCs), is covered in great
length at the very end.
... MXene is an emerging two-dimensional (2D) transition metal carbide/nitride with high conductivity, abundant functional groups, good hydrophilicity, and high mechanical modulus [143]. The sodiophilic function groups (e.g., -O and -F) on the surface of MXene play important roles in reducing nucleation overpotential and inducing uniform Na nucleation [144]. Moreover, MXene has high electron and ion transport dynamics due to its high electronic conductivity and open cross-linked network. ...
Sodium metal is one of the ideal anodes for high-performance rechargeable batteries because of its high specific capacity (~ 1166 mAh·g ⁻¹ ), low reduction potential (−2.71 V compared to standard hydrogen electrodes), and low cost. However, the unstable solid electrolyte interphase, uncontrolled dendrite growth, and inevitable volume expansion hinder the practical application of sodium metal anodes. At present, many strategies have been developed to achieve stable sodium metal anodes. Here, we systematically summarize the latest strategies adopted in interface engineering, current collector design, and the emerging methods to improve the reaction kinetics of sodium deposition processes. First, the strategies of constructing protective layers are reviewed, including inorganic, organic, and mixed protective layers through electrolyte additives or pretreatments. Then, the classification of metal-based, carbon-based, and composite porous frames is discussed, including their function in reducing local deposition current density and the effect of introducing sodiophilic sites. Third, the recent progress of alloys, nanoparticles, and single atoms in improving Na deposition kinetics is systematically reviewed. Finally, the future research direction and the prospect of high-performance sodium metal batteries are proposed.











![a) The mosaic model of SEI films. Reproduced with permission.[¹⁵]...](publication/377626578/figure/fig3/AS:11431281251518552@1718246503273/a-The-mosaic-model-of-SEI-films-Reproduced-with-permission-Copyright-2018_Q320.jpg)












![Fig. 4. DN learning rules [38]](profile/Tianyu-Hu-5/publication/367329191/figure/fig2/AS:11431281114286219@1674398611361/DN-learning-rules-38_Q320.jpg)
![Fig. 5. China's urban centralized heating area over the years [32]](profile/Tianyu-Hu-5/publication/367329191/figure/fig3/AS:11431281114286220@1674398611399/Chinas-urban-centralized-heating-area-over-the-years-32_Q320.jpg)