FIG 1 - uploaded by Grant S Henderson
Content may be subject to copyright.

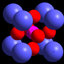
a) Pairing arrangement of lithium cations between NBO atoms, as described by Hannon et al. (1992). This arrangement requires that the Li cations be in pairs in order to be consistent with a coordination number of 2. However, it also assumes that Li bonds exclusively with Q 3 species. b) This alternative arrangement is consistent with Q 2 formation if the coordination number of Li is 2. This confi guration would not require Li cations to be in pairs, although they would need to be near the Q 2 tetrahedra. c) A possible confi guration consistent with Q 2 formation and Li coordination of 4. Again Li pairing would not be required.
Source publication
The addition of alkali oxides to silicate glasses results in the disruption of the silicate network and the formation of non-bridging oxygen atoms and Q n species. Lithium-containing silicate glasses behave differently than other alkali-containing silicate glasses. Addition of Li 2 O to silicate and germanate glasses generates Q 2 species at low Li...
Contexts in source publication
Context 1
... coordination number for sodium, observed to be five in sodium silicate glasses by Greaves et al. (1981) and Greaves (1985). Hannon et al. (1992) interpreted these results to imply that lithium occupies a distinctly different position in alkali silicate glasses, compared to other alkalis, and exists in pairs between two non-bridging oxygen atoms (Fig. ...
Context 2
... lower coordination-number has been attributed to the increased covalency of the Li-O bond and the tendency for lithium to prefer bonding with NBO atoms. Figure 1a shows the bonding arrangement suggested by Hannon et al. (1992). The lithium cation is coordinated to a single oxygen atom on a Q 3 tetrahedron, and the Li cations must be in pairs to satisfy the experimentally determined coordination number of two. ...
Context 3
... coordination number of two. However, Uhlig et al. (1996) and Zhao et al. (1998) found that Li is coordinated to four atoms of oxygen. For this coordination, Li would not be required to form Li pairs. The pronounced Q 2 formation observed by Soltay & Henderson (2005) suggests that the lithium atoms may be bound in two possible confi gurations (Fig. 1) where Q 2 tetrahedra form. Which confi guration is preferred would, however, depend upon whether or not the coor- dination number of Li is two or four. The fi rst confi gu- ration (b of Fig. 1) occurs if each Li atom bonds to a NBO atom on two adjacent Q 2 tetrahedra. The Li atoms have a coordination number of two, and although they ...
Context 4
... Li pairs. The pronounced Q 2 formation observed by Soltay & Henderson (2005) suggests that the lithium atoms may be bound in two possible confi gurations (Fig. 1) where Q 2 tetrahedra form. Which confi guration is preferred would, however, depend upon whether or not the coor- dination number of Li is two or four. The fi rst confi gu- ration (b of Fig. 1) occurs if each Li atom bonds to a NBO atom on two adjacent Q 2 tetrahedra. The Li atoms have a coordination number of two, and although they need not be paired, the confi guration does require that the Li atoms be positioned near two Q 2 tetrahedra. This confi guration is also compatible with an interpretation requiring only the ...
Context 5
... require that the Li atoms be positioned near two Q 2 tetrahedra. This confi guration is also compatible with an interpretation requiring only the formation of Q 3 tetrahedra. Each Li atom would then need to bond to a single NBO atom on adjacent Q 3 tetrahedra, and there would be no need for pairing of the Li cations. A second confi guration (c, Fig. 1) also is possible. Here, the Li bonds to two NBO atoms on two adjacent Q 2 tetrahedra with a coor- dination number of four, and there is no clustering of the Li atoms. Confi guration (c) is consistent with the results of Uhlig et al. (1996) Clearly, it is not possible to distinguish between the two mechanisms, although a detailed ...
Similar publications
New Pb, Sr and O isotopic analyses of rocks from the Skaergard intrusion indicate the following: (1) initial 87Sr/86Sr of the gabbroic magma was less than or equal to 0.7041; (2) limited contamination of magma with crustal Sr and Pb may have occurred in a deep reservoir below the presently exposed parts of the intrusion; (3) marked crustal contamin...
The results provided by the microprobe and thermomagnetic studies of native iron in sediments are generalized. It is found that the sediments are abundant in Ni-free iron particles. These particles are shown to have extraterrestrial origin and likely to be parts of cosmic dust. It is suggested that these particles were formed from the mantle and cr...
Glass ceramics in the system 35CuO·(35 − X)MnO·XBi2O3·30SiO2 (mol%), (X = 0–20), were prepared via melting quenching technique. The effect of replacing MnO by Bi2O3 on the crystallization behavior and electrical properties was studied. XRD results revealed crystallization of cubrite (Cu2O), crednerite (CuMnO2) and bismouth silicates phases. Additio...
Th, Sr, Nd, and O isotopes have been determined in a suite of volcanic rocks from Hekla and in a few samples from Askja and Krafla volcanic centers in Iceland. Although 87Sr/86Sr and 143Nd/ 144Nd ratios are nearly the same for all compositions at Hekla, the 230Th/232Th) ratios differ and thus clearly show that the silicic rocks cannot be derived fr...
The effect of MgO and total FeO on ferric/ferrous ratio in model multicomponent silicate melts was investigated experimentally in the temperature range 1300–1500 °C at 1 atm total pressure in air. We demonstrate that the addition of these weak network modifier cations results in an increase of Fe³⁺/Fe²⁺ ratio in both mafic and silicic melts. Based...
Citations
... The 6 Li isotope prefers octahedral site, while the heavier 7 Li isotope favors tetrahedral coordination (e.g., Wunder et al., 2007Wunder et al., , 2011Kowalski and Jahn, 2011). Most Li-bearing minerals such as biotite, muscovite, and alkali feldspar that commonly crystallize from granitic melts contain Li in octahedral coordination (Magna et al., 2016), while in granitic melts, Li is tetrahedrally coordinated (Zhao et al., 1998;Soltay and Henderson, 2005). Therefore, fractional crystallization of biotite, muscovite, and alkali feldspar will lead to an increase in the 7 Li/ 6 Li ratio of the granitic melt with increasing magmatic differentiation, as these minerals will preferentially incorporate 6 Li (Wenger and Armbruster, 1991). ...
... In these minerals, Li is octahedrally coordinated Magna et al., 2016). In contrast, Li is tetrahedrally coordinated in granitic melts (Soltay & Henderson, 2005). Teng et al. (2006) and Magna et al. (2016) proposed that 6 Li favors a high-coordination site, whereas 7 Li preferentially incorporates into a lower-coordination site during equilibrium fractionation. ...
The Xihuashan and Yaogangxian granitic plutons in South China comprise highly evolved multiphase Li‐rich granites and host quartz‐vein‐type tungsten deposits. The δ⁷Li values of Phase A (early stage), B (middle stage), and C (late stage) from the Xihuashan pluton are 1.0–1.2‰, 1.1–3.0‰, and 2.4–2.8‰ respectively, increasing through chemical evolution. The granites from the Yaogangxian pluton also display gradually enriched in heavy Li isotopes in a later stage, although systematically lighter than those of the Xihuashan pluton. In both plutons, the δ⁷Li shows good correlations with SiO2 and Li concentrations as well as Rb/Sr, Nb/Ta, and Zr/Hf ratios, indicating Li isotopic fractionation most likely caused by magmatic differentiation. In situ analyses show that the minerals of Xihuashan pluton record a continuous elemental spectrum, reflecting the results of progressive magmatic differentiation. The δ⁷Li values of quartz, feldspar, mica, and zircon all correlate well with the chemical evolutions of granitic magma, systematically elevated in Phases B and C relative to Phase A. The Li isotope data of the mineral separates further document that the enrichment of ⁷Li in the residual melt was most likely due to the equilibrium fractionation between the mineral and melts. The data are interpreted to reflect that intense magmatic differentiation was responsible for Li isotopic variations coupled with the enrichment in the Li, F, P, and rare metals in the late‐phase granites of the Xihuashan pluton. The lithium isotope behavior documented in this study provides new insights into magmatic differentiation and associated rare‐metal mineralization.
... The lighter 6 Li isotope prefers octahedral site, while the heavier 7 Li isotope favors tetrahedral coordination (e.g., Kowalski and Jahn, 2011;Wunder et al., 2007Wunder et al., , 2011. In most Li-rich minerals such as spodumene and mica, Li is octahedrally coordinated (Wenger and Armbruster, 1991), while in granitic melts, it is tetrahedrally coordinated (Soltay and Henderson, 2005;Zhao et al., 1998). ...
Peralkaline granites from the Siwana complex in western India host mineralization of rare earth and other high field strength elements (REE/HFSE). In this study, we use textural relations, major-trace element chemistry and Li-isotope composition of minerals to constrain the magmatic evolution of the granites and the mechanism of hydrothermal REE mineralization. Three facies of peralkaline granites, viz., hypersolvus, transsolvus, and pegmatitic are identified in the Siwana complex. The hypersolvus granites constitute two mineralogical variants: clinopyroxene- and ferrorichterite-bearing. Transsolvus granites contain riebeckite-arfvedsonite and occur as patches/network of dikes in hypersolvus granites. Rayleigh crystal fractionation modelling using incompatible trace element abundances in clinopyroxene and amphibole indicates that the hypersolvus and the transsolvus granites formed after 50-85% and 98% melt crystallization, respectively. Early magmatic clinopyroxenes and ferrorichterites in the hypersolvus granites have fairly heavy δ7Li (+18.5‰ to +20.4‰) suggestive of crystallization from highly evolved magmas. Late magmatic arfvedsonites in the transsolvus granites have even heavier δ7Li (+29.7‰ to +33.8‰). Rayleigh distillation modelling using Li isotopes reveal that the hypersolvus and transsolvus granites crystallized from parental magmas that had undergone >90% fractional crystallization.
The Siwana granitoids are hydrothermally altered as evidenced from pseudomorphic re-equilibration of clinopyroxene, alkali feldspar megacrysts, vein and patch perthite formation, replacement of ferrorichterite/clinopyroxene by hydrothermal aegirine, alteration of primary fluorapatite, monazite, aenigmatite, eudialyte, and vlasovite. Three stages of hydrothermal alteration are identified in the granites: stage-I alteration was brought about by highly-saline high-temperature fluid that scavenged REE, HFSE, Ti, Na, and Fe from clinopyroxene and aenigmatite. This was followed by extensive replacement of ferrorichterite and arfvedsonite by aegirine, which decreased the pH and salinity of the fluid. The third stage of alteration led to the replacement of vlasovite by calciocatapleiite, elpidite, and eudialyte and was accompanied by the replacement of alkali feldspar by aegirine, quartz, and secondary REE minerals. The bulk of the REE mineralization occurs in transsolvus granites as cerite associated with aegirine and quartz. Textural relations indicate that these minerals pseudomorph alkali feldspar. Distinct assemblages of secondary REE minerals are identified in the pyroxene- and amphibole-bearing granites-only hydroxyl-dominated REE minerals like cerite are identified in the former while late-stage replacement of cerite by bastnäsite and chevkinite is observed in the latter, the difference controlled by the extent of ferrorichterite to aegirine transformation. The precipitation of secondary REE minerals was largely triggered by pH increase due to the interaction of alkali feldspar with REE-bearing hydrothermal fluids, resulting in the replacement of alkali-feldspar with Fe-phyllosilicates.
... In particular, the main role in the formation of silicate structures belongs to network-modifying cations, while silica-oxygen anion groups is only adjusted to them (Belov, 1976). Their concentrations define the number of non-bridging oxygens and, correspondingly, the degree of polymerization and the density of silicate and germanate glasses and melts (Mysen and Frantz, 1994;Soltay and Henderson, 2005a;Soltay and Henderson, 2005b;Anfilogov et al., 2005;Ashton-Patton, 2008;Rossano and Mysen, 2012). In addition, the type and content of networkmodifying cations determine also the coordination number of Ge in the germanate systems (Henderson and Wang, 2002;Yiannopoulos et al., 2002;Amos and Henderson, 2003;Ivanova, 2013). ...
Glasses of the three-component Li2O-K2O-GeO2 system are studied by Raman spectroscopy. Spectra are curve-fitted and the main structural units formed in the system are established. Conditions of formation of highly coordinated germanium atoms are determined and non-statistic distribution of network-modifying cations is found. It is shown that lithium for potassium substitution is accompanied by a change of coordination number of germanium, which could cause the destruction of Ge-O-Ge bonds with formation of non-bridging oxygens.
... In particular, the main role in the formation of silicate structures belongs to network-modifying cations, while silica-oxygen anion groups is only adjusted to them (Belov, 1976). Their concentrations define the number of non-bridging oxygens and, correspondingly, the degree of polymerization and the density of silicate and germanate glasses and melts (Mysen and Frantz, 1994;Soltay and Henderson, 2005a;Soltay and Henderson, 2005b;Anfilogov et al., 2005;Ashton-Patton, 2008;Rossano and Mysen, 2012). In addition, the type and content of networkmodifying cations determine also the coordination number of Ge in the germanate systems (Henderson and Wang, 2002;Yiannopoulos et al., 2002;Amos and Henderson, 2003;Ivanova, 2013). ...
Glasses of the three-component Li 2 O-K 2 O-GeO 2 system are studied by Raman spectroscopy. Spectra are curve-fitted and the main structural units formed in the system are established. Conditions of formation of highly coordinated germanium atoms are determined and non-statistic distribution of network-modifying cations is found. It is shown that lithium for potassium substitution is accompanied by a change of coordination number of germanium, which could cause the destruction of Ge-O-Ge bonds with formation of non-bridging oxygens.
... 7 Li is preferentially incorporated into a lower-coordinated phase, while 6 Li favors a more highly coordinated site (e.g., Wunder et al., 2007). Essentially, lithium is octahedrally coordinated in mica (Brigatti et al., 2000(Brigatti et al., , 2003 but is tetrahedrally coordinated in silicate melts (Wenger and Armbruster, 1991;Soltay and Henderson, 2005;Maloney et al., 2008), indicating that mica incorporates 6 Li over 7 Li in felsic melts (Deveaud et al., 2015). Therefore, residual melts have a heavier Li isotope composition if mica acts as the dominant fractionating phase during differentiation. ...
Lithium isotope composition is potentially an effective geochemical tracer for hydrothermal processes and magmatic differentiation associated with rare-metal granitic rocks. The Yashan and Xihuashan plutons in South China are extraordinarily Li-F rich rare-metal granites that contain niobium-tantalum and tungsten deposits, respectively. As a moderately incompatible trace element in a felsic melt system, Li notably increases from protolithionite granite and Li-mica granite (88.7‒175 µg/g) to topaz-lepidolite granite (7430‒8080 µg/g) in the Yanshan pluton. Despite a large variation in Li concentrations, the δ7Li values of the Yashan pluton vary within in a narrow range from -1.5‰ to 1.5‰. In contrast, the δ7Li values of the Xihuashan pluton notably increase from biotite granite and two-mica granite (-0.2‰ to +0.7‰) to muscovite granite (+1.9‰ to +4.4‰) with much less variation in the Li concentrations (37.8‒209 µg/g), which is best explained by the high diffusion rate of 6Li relative to 7Li during disequilibrium fluid-rock interaction. The Xihuashan greisen has negative δ7Li values (from -2.7‰ to -2.1‰), which are attributed to extensive fluid-rock interaction in an open system.
Lithium isotope fractionations are consistent with a diversity of mineralization in the rare-metal granitic rocks. Tungsten mineralization is likely associated with an open hydrothermal process. Fluid-rock interaction has a much stronger effect on Li isotope fractionation than does magmatic differentiation in a highly evolved magmatic system. Ta-Nb mineralization is related to the magmatic differentiation in a closed magmatic-hydrothermal system. The exsolution of a supercritical fluid during magmatic differentiation and fluid-rock interaction in a closed magmatic-hydrothermal system is insufficient for producing notable Li isotope fractionation.
... It is well established that, in addition to temperature, the differences in coordination among coexisting phases are the first-order control on equilibrium Li isotopic fractionation, the lighter isotope preferentially occupying the more highly coordinated site (Sartbaeva et al., 2004;Teng et al., 2006;Wunder et al., 2006;Magna et al., 2016). Lithium is believed to occupy the 8-fold coordinated site in garnet by substituting for Mg or undergoing coupled substitution with Y (or REE) ions (Halama et al., 2011;Cahalan et al., 2014;Sun et al., 2016), which is consistent with the low δ 7 Li values (−1.5‰-−0.1‰) of garnet (Sun et al., 2016) that preferentially takes 6 Li into its 8-fold-coordinated sites and leaves 7 Li relatively enriched in the felsic melt (4-fold coordination; Soltay and Henderson, 2005). ...
... Q n stands for a SiO 4 group with n bridging oxygen atoms, where n can change between 0 and 4 [22]. According to refs [23,24], we marked out the locations of Q 2 to Q 4 with dashed lines to help read the changes along with lithium content. In 10% Li 2 O sample, Q 3 and Q 4 are dominant species. ...
... We, therefore, measured the absorption spectra of these glasses before and after annealed at 250°C for 30 min, respectively, which consists with the expectation (see Fig. 2(d)). The different impacts lithium has on the thermal degradation of Bi doped germanate and silicate glasses should be partially due to the lower polymerization in germanate because of the preference for Q 2 formation and presence of GeO 6 units [24]. ...
For application of bismuth laser glasses in either fiber amplifier or laser, their performance stability in long run should be understood especially in extreme conditions. However, so far, there are few reports on it. Here, we found, after the cycle experiments on heating and cooling, that the proper increase of lithium content in lithium tantalum silicate laser glass can lead to unusual anti-thermal degradation of bismuth NIR luminescence, which completely differs from the scenario in germanate glass. FTIR, ²⁹Si MAS NMR spectra, absorption and dynamic photoluminescence spectra are employed to unravel how this happens. The results illustrate that it should be due to the decrease of polymerization of silicate glass network, which in turn allows the regeneration at 250°C, and therefore, the content increase of bismuth NIR emission centers. In the meanwhile, we noticed though Bi luminescence can be thermally quenched its peak does not shift along with temperature, which seldom appears in laser materials. The unique property might guarantee the unshift of Bi fiber laser wavelength once such glass was made into fiber devices even as the environmental temperature changes. The role of lithium is discussed in the evolution of glass structures, the suppression of glass heterogeneity, and the thermal stability of Bi luminescence, and it should be helpful to design homogeneous silicate laser glass with outstanding thermal stability.
... The coordinate number of Li in water is generally between 3-6 and it may increase with the increasing of fluid density (Jahn and Wunder, 2009). For the reaction product, Li may substitute for Mg or undergo coupled substitution with Y (or REE) ions at the 8-fold coordinated site in garnet (Halama et al., 2011;Cahalan et al., 2014), and it occupies the 4-fold coordinated site in silicate melt (Soltay and Henderson, 2005). The δ 7 Li values of minerals from residual enclaves show that garnet (−1.5 to −0.1‰) do exhibit much lighter Li isotopic compositions than coexisting biotite (+ 3.2 to + 7.5‰) and quartz (+15.0 to +16.6‰). ...
Lithium (Li) elemental and isotopic compositions of the Jurassic Jingshan leucogranites, including garnet-rich mafic enclaves and wall rock Wuhe gneisses from the southeast margin of North China Craton (NCC) were investigated to understand the behavior of Li isotopes during post-collisional magmatism. The Jingshan leucogranites have distinct U-shape REE patterns with Y and REE concentrations significantly lower yet Sr/Y ratios higher than their presumed source rocks, i.e., the Dabie-Sulu gneisses. Trace element modeling of REE and Sr/Y suggests these elemental signatures of the Jingshan leucogranites can be consistently explained by a fluid-present crustal incongruent partial melting: Bt + Qz + Pl + H2O = Grt + melt, leaving mainly Grt + Bt with minor allanite in the residuum. The mafic enclaves show identical Sr-Nd isotopic compositions with their host leucogranites, contrasting with the Wuhe gneiss and the exposed regional lower crust. The garnet-rich mafic enclaves are thus interpreted as entrained residual phases formed by this incongruent partial melting.
... 14 36 Q speciation in the glasses of our study was analyzed by Raman spectroscopy following the procedure developed by Zakaznova-Herzog et al. 37,38 and Malfait et al. 39,40 for alkali and earth-alkali silicate glasses. The mode analyses and assignment given by this approach stand in agreement with the analyses given in earlier Raman studies 34,41,42 as well as the NMR Q species quantification. 13,36 Furthermore, this approach offers an improved understanding of the vibrational mode origins. ...
... The Li trisilicate with NBO/T of ∼0.67 is significantly more depolymerized than Li hexasilicate, with NBO/T of ∼0.33. The number of NBOs per SiO 4 tetrahedral unit defines the type of Q i species,34,35 that is, Q 4 contains only BO, Q 3 one NBO, Q 2 two NBOs, and so forth. According to NMR studies of Schramm et al.36 and Voigt, 13 the average abundance is 48− 55% of Q 4 , 33−37% of Q 3 , and 6−12% of Q 2 in Li 2 Si 6 O 13 glass, while in Li 2 Si 3 O 7 , the average distribution is 30−35% of Q 4 , 56−58% of Q 3 , and 7−11% of Q 2 . ...
Aimed to improve the understanding of lithium migration mechanisms in ion conductors, this study focuses on Li-dynamics in binary Li-silicate glasses. Isotope exchange experiments and conductivity measurements were carried out to determine self-diffusion coefficients and activation energies for Li-migration in Li2Si3O7 and Li2Si6O13 glasses. Samples of identical composition but different isotope content were combined for diffusion experiments in couples or triples. Diffusion profiles developed between 511 and 664 K were analyzed by femtosecond laser ablation combined with multiple collector inductively coupled plasma mass spectrometry (fs LA-MC-ICP-MS) and secondary ion mass spectrometry (SIMS). Analyses of diffusion profiles and comparison of diffusion data reveal that isotope effect of lithium diffusion in silicate glasses is rather small, consistent with classical diffusion behavior. Ionic conductivity of glasses was measured between 312 and 675 K. The experimentally obtained self-diffusion coefficient, DLi,IE, and ionic diffusion coefficient, Dσ derived from specific DC conductivity provided information about correlation effects during Li-diffusion. The DLi,IE/Dσ is higher for the trisilicate (0.27 ± 0.05) than for the hexasilicate (0.17 ± 0.02) implying that increasing silica content reduces the efficiency of Li-jumps in terms of long range movement. This trend can be rationalized by structural concepts based on nuclear magnetic resonance (NMR) and Raman spectroscopy as well as molecular dynamic simulations, i.e., lithium is percolating in low-dimensional, alkali-rich regions separated by silica-rich matrix.