Figure 2 - uploaded by Gloria Tabacchi
Content may be subject to copyright.

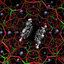
(a) Deuterium power spectrum for pure D2O (dashed line) and for 15 M NaOD solution (solid line). The experimental Raman spectrum 2d is shown in the inset. (b) Deuterium power spectrum (solid line) and IR spectrum (dashed line) of 14 M KOD solution (see text).
Source publication
Car-Parrinello molecular dynamics simulations have been carried out for aqueous NaOH and KOH solutions under ambient conditions over a wide range of concentrations. From these simulations, we have observed a continuous change of the water structure with added hydroxide, characterized by a significant shift of the second peak of the OO radial distri...
Similar publications
This study aims to describe the influence of periodic flow-pulsations on heat transfer of laminar free surface impinging jets (Re = 1000, Pr = 7). Fully resolved two-dimensional numerical simulations were carried out in which flow-pulsation was achieved by periodically altering the velocity of the jet at the nozzle exit with an amplitude of 50% of...
A femtomolar digital homogenous immunoassay is developed based on sensitively distinguishing the immunocomplexes labeled with quantum dot (QD) aggregate from the excessive free monodispersed single QDs. The success in quantifying...
In this paper, the method based on the process of forming a flat blank into the thin-walled conical part is proposed. The forming method is based on two stages. At the first stage, forming of the free portion of the blank is carried out until it fully contacts the conical surface of the punch. During this stage, thickness unevenness is created, whi...
The authors, together with A. del Campo, developed an alternative method for determining the Feynman propagator [1] for a non-relativistic problem. One started with the time dependent Schroedinger equation for the problem. Carried out a Laplace transform with respect to time to get the equation for the energy dependent Green function and derived it...
We have carried out optical and transport measurements of the free carriers and optical measurements of states above the semiconducting energy gap in the layered crystal Bi2Te3. We find that cooling causes a substantial amount of spectral weight to condense from the above-gap states into the metallic states.
Citations
... Water molecules coordinated to Na + ions contribute to a positive signal between 3,200 and 3,800 cm −1 , and the O-H bonds of water molecules coordinated to OH − anions are responsible for the negative <3,500 cm −1 continuum. The latter assignment is consistent with previous studies of NaOH in bulk [35][36][37] and water clusters 38,39 , where the <3,500 cm −1 continuum band is also attributed to the solvation of OH − species. Note that the continuum that extends below 2,500 cm −1 does not arise from the bulk χ (3) contributions, because the bulk χ (3) contributions have only a small contribution below 3,000 cm −1 , unlike the spectra shown in Fig. 2d ref. 40,41. ...
The distribution of ions at the air/water interface plays a decisive role in many natural processes. Several studies have reported that larger ions tend to be surface-active, implying ions are located on top of the water surface, thereby inducing electric fields that determine the interfacial water structure. Here we challenge this view by combining surface-specific heterodyne-detected vibrational sum-frequency generation with neural network-assisted ab initio molecular dynamics simulations. Our results show that ions in typical electrolyte solutions are, in fact, located in a subsurface region, leading to a stratification of such interfaces into two distinctive water layers. The outermost surface is ion-depleted, and the subsurface layer is ion-enriched. This surface stratification is a key element in explaining the ion-induced water reorganization at the outermost air/water interface.
... The latter assignment is consistent with previous studies of NaOH in bulk [37][38][39] and water clusters 40,41 where the <3500 cm -1 continuum band is also attributed to the solvation of OH − species. Note that the continuum that extends below 2500 cm −1 does not arise from the bulk (3) contributions because the bulk (3) contributions have only a small contribution below 3000 cm -1 , unlike the spectra shown in Figure 2d. ...
The distribution of ions at the air/water interface plays a decisive role in many natural processes. It is generally understood that polarizable ions with low charge density are surface-active, implying they sit on top of the water surface. Here, we revise this established hypothesis by combining surface-specific heterodyne-detected vibrational sum-frequency generation with neural network-assisted ab initio molecular dynamics simulations. Our results directly demonstrate that ions in typical electrolyte solutions are, in fact, located in a subsurface region leading to a stratification of such interfaces into two distinctive water layers. The outermost surface is ion-depleted, and the sub-surface layer is ion-enriched. As a result, an effective liquid/liquid interface buried a few {\AA} inside the solution emerges, creating a second water/electrolyte interface, in addition to the outermost air/water interface.
... On the other hand, the structural and dynamic properties of water have significantly changed with the addition of hydroxide, preferring the OH − (H 2 O) 4 structure at low concentrations of KOH. 23 It is observed that the research works about TiO 2 with the KOH electrolyte are mainly carried out through experiments. Herein, we use classical molecular dynamics to simulate the dynamic process of K + and OH − on the TiO 2 surface, which is more conducive to further understand the interfacial behavior from a microscale. ...
Hydrogen can be produced by photoelectrochemical (PEC) water splitting using a KOH solution as an electrolyte and TiO2 as a photoanode. In this work, we fabricated anatase TiO2 nanoring/nanotube arrays and TiO2 nanotube arrays using an anodic oxidation method, confirmed by field-emission scanning electron microscopy (FESEM) and X-ray diffractometry (XRD), and then conducted the photoelectrochemical experiments with 1 M KOH and Na2SO4 solutions. The bias voltage, small impedance, negative flat-band potential, large capacitance, and depletion layer width in the anatase TiO2–KOH system were observed, leading to the stable and large photocurrent density. To understand the effects of KOH on the interface properties of TiO2/H2O, the electric double layers of anatase TiO2(001), (100), (101)/KOH interfaces were further investigated by calculating the ion–surface interaction with molecular dynamics simulations. It is noted that the number density of potassium ions has the same trend as that of oxygen atoms due to the layering effect in liquids and the strongest ionic hydration of K⁺ on anatase (101) is observed by analyzing the radial distribution function and coordination number. In addition, the electrostatic characteristics along the TiO2/KOH interfaces were analyzed based on the Grahame double layer model. The potential drops in the outer Helmholtz layer of anatase (001), (100), and (101) surfaces are 1.08, 0.26, and 0.51 V, respectively. Compared with TiO2–H2O systems, the larger potential drops in the TiO2–KOH system explain the phenomenon that KOH solute contributes substantially to a chemical bias in PEC reactions. At the same time, we estimated the depletion layer widths of anatase TiO2(001), (100), and (101) surfaces as 37.48, 173.25, and 64.49 Å, respectively, which are of similar magnitude to the experimental results. Anatase TiO2(100) with the widest depletion layer is suggested in photocatalysis. These works provide a clear understanding of interfacial behavior of KOH on anatase TiO2 from a microscale, which can be used to explain the promotion effect of the KOH electrolyte and guide the design of TiO2 nanocrystals in the PEC system.
... 55,56 At high concentration, fewer water molecules are available to coordinate OH − , making the OH − (H 2 O) 3 configuration more favorable. 57 We argue that the OH − concentration in the diffuse layer is high, although we have yet to quantify the concentration, and thus the 3coordinated structure dominates. This interpretation is supported by a nuclear magnetic resonance study of the water molecule distribution around TiO 2 NPs, showing strong confinement of water molecules with low mobility and reactivity within the first few layers above the TiO 2 surface. ...
We report an experimental observation of a significant amount of hydroxide OH– created upon water dissociation and subsequently trapped around TiO2 nanoparticles dispersed in NH4OH aqueous solution. The hydroxide species is identified and quantified by a combination of photoemission and photon-emission X-ray spectroscopies conducted on liquid samples using a liquid microjet. Unlike previous X-ray studies that observed only a few monolayers of water coverage on TiO2 surfaces and found maximally sub-monolayer of OH–, the true aqueous environment adopted in this study enables ion mobility and the separation of the water dissociation products H⁺/OH–. This facilitates the formation of a multilayer OH– diffuse layer in which the trapped OH– ions are discovered to coordinate with three water molecules to form a tetrahedral hydration configuration. The negatively charged diffuse layers, together with the positive NH4⁺ Stern layers, constitutes > 0.8-nm-thick electric double layers around the TiO2 nanoparticles. The large observed amount of hydroxide indicates a high efficiency of water dissociation for the TiO2 catalyst, a promising result for H2 generation in true aqueous environments.
... The following are notable insights about the molecular mechanism involving the H 3 O + ion: solvation structure of Zundel (H 5 O 2 + ) 63 and Eigen (H 9 O 4 + ) 64 cation species, [65][66][67] validation of the PT process through interconversion of the two above-mentioned protonated water complexes, 7,68-70 concept of proton rattling 65,71-73 often termed as special pair dance, 70 and coupling of multiple PT events in the concerted manner. 74,75 The corresponding mechanisms for OH − species include coordination patterns of the hydrated structure 71,76-80 and plausibility of the mechanism with a dynamical interplay between three-and four-fold structure motifs 10,11,71,[76][77][78][79][80][81][82] in comparison to other mechanisms supposing mirror image or static solvation complexes individually. [83][84][85] Moreover, the influence of nuclear quantum effects on diffusion properties have been addressed. ...
... This observation supports the widely accepted PT mechanism. 10,11,71,[76][77][78][79][80][81][82] Moreover, these simulation results indicate the importance of treating large and realistic systems. A larger system is beneficial to minimize unphysical mirror image interactions. ...
Proton transfer in water‐based environments occurs because of hydrogen‐bond interaction. There are many interesting physicochemical phenomena in this field, causing fast structural diffusion of hydronium and hydroxide ions. During the last few decades, to support experimental observations and measurements, quantum‐mechanical molecular dynamics (QMMD) simulations with reasonable accuracy and efficiency have significantly unraveled structural, energetic, and dynamical properties of excess proton in aqueous environments. This review summarizes the state‐of‐the‐art QMMD studies of proton transfer processes in aqueous solutions and complex systems including bulk liquid water, ice phases, and confined water in nanochannel/nanoporous materials as well as reports on CO2 scrubbing by amine‐based chemical absorption.
This article is categorized under:
• Structure and Mechanism > Reaction Mechanisms and Catalysis
• Molecular and Statistical Mechanics > Molecular Dynamics and Monte‐Carlo Methods
• Electronic Structure Theory > Semiempirical Electronic Structure Methods
• Theoretical and Physical Chemistry > Reaction Dynamics and Kinetics
... These five hydrogen bonds average to 1.733 Å, indicating that the interactions experienced by this five-hydrogen bonded water are particularly strong. Interestingly, a similar square-pyramidal "hypercoordinated" geometry is found in the anionic hydrogen bonded complex (H 9 O 5 − ) [165,166], which is one of the dominant solvation structures for the OH − ion in aqueous hydroxide solutions [167]. ...
Confinement of molecules in one dimensional arrays of channel-shaped cavities has led to technologically interesting materials. However, the interactions governing the supramolecular aggregates still remain obscure, even for the most common guest molecule: water. Herein, we use computational chemistry methods (#compchem) to study the water organization inside two different channel-type environments: zeolite L – a widely used matrix for inclusion of dye molecules, and ZLMOF – the closest metal-organic-framework mimic of zeolite L. In ZLMOF, the methyl groups of the ligands protrude inside the channels, creating nearly isolated nanocavities. These cavities host well-separated ring-shaped clusters of water molecules, dominated mainly by water-water hydrogen bonds. ZLMOF provides arrays of “isolated supramolecule” environments, which might be exploited for the individual confinement of small species with interesting optical or catalytic properties. In contrast, the one dimensional channels of zeolite L contain a continuous supramolecular structure, governed by the water interactions with potassium cations and by water-water hydrogen bonds. Water imparts a significant energetic stabilization to both materials, which increases with the water content in ZLMOF and follows the opposite trend in zeolite L. The water network in zeolite L contains an intriguing hypercoordinated structure, where a water molecule is surrounded by five strong hydrogen bonds. Such a structure, here described for the first time in zeolites, can be considered as a water pre-dissociation complex and might explain the experimentally detected high proton activity in zeolite L nanochannels.
... The tetrahedral coordination of one water molecule by four other H 2 O molecules represents the water network structure. 44 The 4.4 Å peak does not occur in the simulations of the 5.5 m solution at high temperatures (Fig. 3c) and not at any studied temperature in 27 m solution (Fig. 3d). At 27 1C, the second maximum of g OO (r) of the 27 m solution (Fig. 3d) appears at 3.4 Å, which is about one Å shorter compared to the 5.5 m solution. ...
... An increasing association of sodium and hydroxide ions is observed with increasing NaOH concentration of the solution and a decreasing number of hydration water molecules, which is in accordance with Chen et al. 44 Increasing NaOH association is also observed with increasing temperature. However, the intensity increase in the spectra below 3200 cm À1 with P, T and NaOH content cannot be explained solely by the interaction of Na + with OH À groups due to the small variations in the Na-O distance with pressure and temperature (Table 1) and the weak Na-O bonding. ...
Hydrothermal diamond anvil cell experiments in combination with Raman spectroscopy and first principles molecular dynamics simulations were performed to investigate the structure and dynamics of aqueous NaOH solutions for temperatures up to 700 °C, pressures up to 850 MPa and two different solute concentrations. The significant changes observed in the O-H stretching region of the Raman spectra between ambient and supercritical conditions are explained by both dynamic effects and structural differences. Especially important are a Grotthuss-like proton transport process and the decreasing network connectivity of the water molecules with increasing temperature. The observed transfer of Raman intensity towards lower wavenumbers by the proton transfer affects a wide range of frequencies and must be considered in the interpretation of Raman spectra of highly basic solutions. We suggest a deconvolution of the spectra using model with four Gaussian functions, which are assigned to the molecular H2O and OH− vibrations, and one asymmetric exponentially modified Gaussian (EMG) function, which is assigned to [HO(H2O)n ]− vibrations.
... 19−22 Various authors have shown that OH − prefers to be hypercoordinated by the surrounding water molecules, 6,21,23 and one of the mirror species (H 3 O 2 − ) could only be found as a short-lived transient solvation complex. 24 At the viewpoint of theoretical studies, the diffusion of H 3 O + and OH − in aqueous solution involves both vehicular and Grotthuss hopping mechanisms, where the latter refers to oxygen−hydrogen bond cleavage and formation. Therefore, traditional classical molecular dynamics (MD) simulations are insufficient. ...
... 52 For the solvation shell of OH − , two differences are observed from the comparison of RDFs of H*−H (Figure 6a Similar findings were also reported in other works. 24 Another remarkable difference is the first peak of the O*−O RDF (Figure 6d), as well as its coordination number. The water-2017 force field predicts a stronger correlation and larger coordination number (around 5 and 4 predicted with the water-2017 and water-2010 force fields, respectively) compared to water-2010, indicating more water molecules are involved in the first solvation shell of OH − . ...
Hydronium (H3O+) and hydroxide (OH-) ions have anomalously large diffusion constants in aqueous solutions due to their combination of vehicular and Grotthuss hopping diffusion mechanisms. An improvement of ReaxFF reactive water force field on the basis of our previous first-generation ReaxFF water force field (water-2010) is presented to describe the proton transfer (PT) mechanisms of H3O+ and OH- in water. Molecular dynamics simulation studies with water-2017 force field support the Eigen-Zundel-Eigen mechanism for PT in acidic aqueous solution and reproduce the hyper-coordinated solvation structure of the OH- in basic environment. In particular, it predicts the correct order of the diffusion constants of H2O, H3O+ and OH- and their values are in agreement with the experimental data. Another interesting observation is that the diffusion constants of H3O+ and OH- are close to each other at the high concentration due to the strong correlation between OH- ions in basic aqueous solution. Based on our results, it is shown that ReaxFF provides a novel approach to study the complex acid-base chemical reactions in aqueous solution with any pH value.
... Such phenomenon has also been reported by EPSR (the Empirical Potential Structure Refinement code) simulations and ab initio simulations in the study of NaOH and KOH aqueous solutions. 17,33 However, it is worth mentioning that the solute concentrations investigated are very high that a large number of water molecules are engaged in the hydration shell of the cation or the anion, and none of them can be truly considered as belonging to the second or third hydration shell of water H-bonding network. Fig.2 shows the water-water partial radial distribution functions at the concentration of 1:5 (solute:solvent, molar ratio) in NaOH and KOH solutions under different temperatures. ...
Molecular dynamics simulations of oxygen molecules in NaOH and KOH solutions at different temperatures (25-120 ) and concentrations (1:100-1:5, molar ratios) were performed in this study. The interactions of oxygen molecules with the surrounding solvent and solute were clarified by considering the solvent-solvent, oxygen-solvent, and oxygen-solute radial distribution functions. The self-diffusion coefficients of the oxygen molecules and the solute were both determined by analyzing the mean-squared displacement (MSD) curves, using Einstein's relationship. It was concluded that at all concentrations, the diffusion coefficient of oxygen in NaOH solution is smaller than that in the corresponding KOH solution. The diffusion coefficients for hydroxide, Na+, and K+ decrease with increasing solute concentration, following similar trends to those of oxygen. The oxygen diffusion coefficient obtained in this study is in good agreement with the reported experimental value, suggesting that MSD is an attractive approach to study the oxygen diffusion behavior in strong alkaline solutions at elevated temperatures, which are experimentally extremely challenging.
... In alkali solution, the network structure is destroyed by the addition of metal ions, since water molecules form stable hydrated ions with metal ions. For the OHions, the traditional proton transfer mechanism of OHwas questioned, recent research re-examined the water structure, the OH --water interactions, and the mechanism of proton transfer using different methods [8][9][10][11][12][13][14][15][16][17][18]. Experiments and molecular modeling showed that NaOH destroyed the tetrahedral hydrogen bond network structure of the water, and there were a variety of OH --H 2 O complex (H 7 O 4 -, H 9 O 5 -, H 11 O 6 -) in NaOH aqueous solution [18]. ...
NaOH/urea aqueous solution is a novel, green solvent for cellulose. To explain why cellulose just be dissolved in this solvent under −13 °C, we studied and discussed the dissolving process of cellobiose in water, urea solution, NaOH solution and NaOH/urea aqueous solution. Dissolving cellobiose in water and the urea solution absorb heat, which is an entropy-driven process. Dissolving cellobiose in NaOH solution and mixed NaOH/urea solution is exothermic, which is an enthalpy-driven process. OH− plays an important role in the dissolving process by forming a hydrogen-bonding complex. From the thermodynamic point of view, negative entropy can well interpret why cellulose must be dissolved in cold NaOH/urea aqueous solution.