Fig 1 - uploaded by Afshin Derakhshani
Content may be subject to copyright.
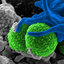
Three different forms of cell death: A) apoptosis is a form of cell death that some molecular mechanisms in a cell lead to its death. It is generally characterized by distinct morphological features including cell shrinkage, blebbing and nuclear fragmentation. B) Necrosis is a type of cell death that is morphologically described by an increasing translucent cytoplasm, swelling of organelles, and increased cell volume. C)
Source publication
Cell death resistance is a key feature of tumor cells. One of the main anti-cancer therapies is increasing the susceptibility of cells to death. Cancer cells have developed a capability of tumor immune escape. Hence, restoring the immunogenicity of cancer cells can be suggested as an effective approach against cancer. Accumulating evidence proposes...
Similar publications
The long non-coding RNA MIR4435-2HG has been confirmed to play a crucial regulatory role in various types of tumors. As a novel type of non-coding RNA, MIR4435-2HG plays a key role in regulating the expression of tumor-related genes, interfering with cellular signaling pathways, and affecting tumor immune evasion. Its unique structure allows it to...
Background
Despite immunotherapies having revolutionized the treatment of advanced cutaneous melanoma, effective and durable responses were only reported in a few patients. A better understanding of the interaction of melanoma cells with the microenvironment, including extracellular matrix (ECM) components, might provide novel therapeutic options....
Myeloid-derived suppressor cells (MDSCs) play a critical role in tumor immune escape because of its remarkable immunosuppressive effect. However, the mechanism of MDSCs migrated into tumor microenvironment remains unclear. In this study, we demonstrated the recruitment of MDSCs can be promoted by exosomes derived from prostate cancer cells, which c...
Human pituitary adenomas are one of the most common intracranial neoplasms. Although most of these tumors are benign and can be treated medically or by transsphenoidal surgery, a subset of these tumors are fast-growing, aggressive, recur, and remain a therapeutic dilemma. Because antibodies against immune checkpoint receptors PD-1 and CLTA-4 are no...
Background:
The bladder cancer is immunogenic, and neoantigens generated by tumor cells trigger a notable immune response in the host. On the other hand, multiple immune escape mechanisms allow for avoiding the recognition by the host immune system. Toll-like receptor type 4 and inflammatory cytokines play major role in the immune response to blad...
Citations
... Both HSP70 and HSP90 is usually located in the intracellular compartment, however, these HSPs translocate to cell surface during ICD. These ecto-HSP70 and HSP90 can interact with receptors on the surface APCs (e.g., CD40 and CD91) and enhance the immunogenicity of dying cells, results in the crosspresentation of cancer cell antigens to major histocompatibility complex (MHC)-I molecules and subsequent activation of CD8 + Tcells (16,25,26). Antigens from dying cancer cells are taken up and processed by APCs, which then present these antigens through their MHC-I molecules to T cells (27). ...
Cancer immunotherapy, such as immune checkpoint blockade (ICB), has emerged as a groundbreaking approach for effective cancer treatment. Despite its considerable potential, clinical studies have indicated that the current response rate to cancer immunotherapy is suboptimal, primarily attributed to low immunogenicity in certain types of malignant tumors. Immunogenic cell death (ICD) represents a form of regulated cell death (RCD) capable of enhancing tumor immunogenicity and activating tumor-specific innate and adaptive immune responses in immunocompetent hosts. Therefore, gaining a deeper understanding of ICD and its evolution is crucial for developing more effective cancer therapeutic strategies. This review focuses exclusively on both historical and recent discoveries related to ICD modes and their mechanistic insights, particularly within the context of cancer immunotherapy. Our recent findings are also highlighted, revealing a mode of ICD induction facilitated by atypical interferon (IFN)-stimulated genes (ISGs), including polo-like kinase 2 (PLK2), during hyperactive type I IFN signaling. The review concludes by discussing the therapeutic potential of ICD, with special attention to its relevance in both preclinical and clinical settings within the field of cancer immunotherapy.
Simple Summary
Modulated electro-hyperthermia (mEHT) is a heating therapy that uses synergized thermal and nonthermal effects to heat and destroy malignant cells selectively without damaging healthy cells. This article presents the clinical validation of mEHT. The therapy is dominantly applied for such advanced malignancies when the conventional oncotherapies fail to apply. Survival results of mEHT were collected and compared with other methods. The results demonstrate the superiority of the mEHT method.
Abstract
The mEHT method uses tissues’ thermal and bioelectromagnetic heterogeneity for the selective mechanisms. The success of the therapy for advanced, relapsed, and metastatic aggressive tumors can only be demonstrated by measuring survival time and quality of life (QoL). The complication is that mEHT-treated patients cannot be curatively treated any longer with “gold standards”, where the permanent progression of the disease, the refractory, relapsing situation, the organ failure, the worsening of blood counts, etc., block them. Collecting a cohort of these patients is frequently impossible. Only an intent-to-treat (ITT) patient group was available. Due to the above limitations, many studies have single-arm data collection. The Phase III trial of advanced cervix tumors subgrouping of HIV-negative and -positive patients showed the stable efficacy of mEHT in all patients’ subgroups. The single-arm represents lower-level evidence, which can be improved by comparing the survival data of various studies from different institutes. The Kaplan–Meier probability comparison had no significant differences, so pooled data were compared to other methods. Following this approach, we demonstrate the feasibility and superiority of mEHT in the cases of glioblastoma multiform, pancreas carcinomas, lung tumors, and colorectal tumors.
Advanced melanoma is an aggressive form of skin cancer characterized by low survival rates. Less than 50% of advanced melanoma patients respond to current therapies, and of those patients that do respond, many present with tumor recurrence due to resistance. The immunosuppressive tumor-immune microenvironment (TIME) remains a major obstacle in melanoma therapy. Adjuvant treatment modalities that enhance anti-tumor immune cell function are associated with improved patient response. One potential mechanism to stimulate the anti-tumor immune response is by inducing immunogenic cell death (ICD) in tumors. ICD leads to the release of damage-associated molecular patterns within the TIME, subsequently promoting antigen presentation and anti-tumor immunity. This review summarizes relevant concepts and mechanisms underlying ICD and introduces the potential of non-ablative low-intensity focused ultrasound (LOFU) as an immune-priming therapy that can be combined with ICD-inducing focal ablative therapies to promote an anti-melanoma immune response.
Immunotherapy has remarkably revolutionized the management of advanced HCC and prompted clinical trials, with therapeutic agents being used to selectively target immune cells rather than cancer cells. Currently, there is great interest in the possibility of combining locoregional treatments with immunotherapy for HCC, as this combination is emerging as an effective and synergistic tool for enhancing immunity. On the one hand, immunotherapy could amplify and prolong the antitumoral immune response of locoregional treatments, improving patients’ outcomes and reducing recurrence rates. On the other hand, locoregional therapies have been shown to positively alter the tumor immune microenvironment and could therefore enhance the efficacy of immunotherapy. Despite the encouraging results, many unanswered questions still remain, including which immunotherapy and locoregional treatment can guarantee the best survival and clinical outcomes; the most effective timing and sequence to obtain the most effective therapeutic response; and which biological and/or genetic biomarkers can be used to identify patients likely to benefit from this combined approach. Based on the current reported evidence and ongoing trials, the present review summarizes the current application of immunotherapy in combination with locoregional therapies for the treatment of HCC, and provides a critical evaluation of the current status and future directions.
Breast cancer is the most common cancer in women, with a high incidence estimated to reach 2.3 million by 2030. Triple-Negative Breast Cancer (TNBC) is the greatest invasive class of breast cancer with a poor prognosis, due to the side-effects exerted by the chemotherapy used and the low effectivity of novel treatments. In this sense, copper compounds have shown to be potentially effective as antitumor agents, attracting increasing interest as alternatives to the usually employed platinum-derived drugs. Therefore, the aim of this work is to identify differentially expressed proteins in MDA-MB-231 cells exposed to two copper(II)-hydrazone complexes using label-free quantitative proteomics and functional bioinformatics strategies to identify the molecular mechanisms through which these copper complexes exert their antitumoral effect in TNBC cells. Both copper complexes increased proteins involved in endoplasmic reticulum stress and unfolded protein response, as well as the downregulation of proteins related to DNA replication and repair. One of the most relevant anticancer mechanisms of action found for CuHL1 and CuHL2 was the down-regulation of gain-of-function-mutant p53. Moreover, we found a novel and interesting effect for a copper metallodrug, which was the down-regulation of proteins related to lipid synthesis and metabolism that could lead to a beneficial decrease in lipid levels.
The effects of mild-temperature hyperthermia (MTH) and metformin, alone or in combination, on the efficacy of high-dose hypofractionated radiation against experimental tumors were investigated. FSaII fibrosarcoma grown subcutaneously in the hind legs of C3H mice was irradiated with a single 15 Gy dose using a 60Co irradiator. The radio frequency capacitive method was used to heat the tumors at 41.0°C for 30 min. Metformin was intraperitoneally (i.p.) administered daily to tumor-bearing mice at a dose of 150 mg/kg. The expression levels of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and programmed cell death-ligand 1 (PD-L1) were determined by immunohistochemical staining of the excised tumor tissues. The apoptosis of tumor cells in vivo was quantified by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and cleaved caspase-3 staining of the excised tumor tissues. Irradiation of tumors markedly increased the expression of HIF-1α, VEGF, and PD-L1, and MTH and metformin used either alone or in combination significantly abrogated the radiation-induced upregulation of these proteins. MTH and metformin alone or combined increased the radiation-induced apoptosis in tumor cells and enhanced the radiation-induced suppression of tumor growth. The findings indicated that the increased tumor response to 15 Gy irradiation by MTH and metformin alone or in combination was due, in part, to the abrogation of the radiation-induced upregulation of HIF-1α and its downstream targets VEGF and PD-L1.