Fig 1 - uploaded by Birger Brodin
Content may be subject to copyright.
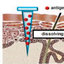
The skin penetration model. Skin biopsies were placed in agar in cell inlets. The inlets were placed in supplemented DMEM medium and incubated at 37°C and 5% CO 2
Source publication
This study aimed to investigate the effect of a novel kind of immune-stimulating complexes (ISCOMs) on human skin penetration
of model compounds in vitro to evaluate their potential as a delivery system, ultimately for transcutaneous vaccination. Special focus was on elucidating
the mechanisms of penetration. Preparation of ISCOMs was done by dialy...
Context in source publication
Context 1
... of elastic vesicles into both human and mice skin in vitro and to study interactions between vesicles and skin (11,13). The results of those studies showed the location of intact elastic vesicles in the upper three layers of the stratum corneum and that the intercellular lipid layer was disturbed by the application of elastic vesicles. TEM has also been used to demonstrate that metallic nanoparticles with a size of 10 nm were able to penetrate the skin (14) and to study changes in ultrastructure in the skin after drug delivery by iontophoresis (15,16). The objectives of the current study were to investigate the effect of Posintro TM nanoparticles on human skin penetration of model compounds and to elucidate the mechanism by which this takes place. The results can be used to evaluate their potential as a transcutaneous delivery system. Quillaja saponaria A (Quil A) is a saponin derivative and was obtained from Brenntag Biosector, Denmark, and mega-10 (decanoyl- N -methylglucamide) was from Bachem, Germany. Cholesterol (>98%), dioleoyl phosphatidylethanol- amine (>99%), and 1-palmitoyl-2-oleoyl- sn -glycero-3-phos- phocholine (POPC, >99%) were obtained from Avanti Polar Lipids, AL, USA. Methyl nicotinate, DC-cholesterol ( ∼ 95%), sucrose (>99.5%), 4 ′ -6-diamidino-2-phenylindole (DAPI), and agarose were purchased from Sigma-Aldrich, Denmark. Acridine (2,8-bis(dimethylamino)-10-dodecyl-acri- dinium bromide) was obtained from ACROS, Belgium. Dulbecco ’ s modi fi ed Eagle ’ s medium (DMEM) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 500 ng/ml amphotericin B, and 10% ( v / v ) fetal calf serum were all purchased from BioWhittaker, Cambrex. Fluorescent mounting medium was purchased from DakoCytomation, Denmark. Tissue-TEK OCT was purchased from Sakura Finetek, Denmark. Phosphate-buffered saline (PBS) pH 7.4 was prepared from distilled, deionized water (Milli-Q Water system, Millipore, Axeb, Denmark). Three percent ( w / v ) glutaraldehyde, glycide ether 100 (Epon), 1% ( w / v ) aqueous osmium tetroxide, methylene blue, and ethanol were all purchased from Roth GmbH, Germany, while copper grids and formvar solution were from Plano, Germany. Uranyl acetate was obtained from SERVA, Germany, and propylene oxide and cacodylate buffer pH 7.4 were from Polysciences Europe, Germany, and lead citrate was from Merck, Germany. Posintro TM nanoparticles were prepared by the standard dialysis method fi rst described by Höglund et al. in 1989 (17). The particles used for the CLSM studies were slightly modi fi ed, as the fl uorophore (acridine) was incorporated into the nanoparticles, which were subsequently named acridine – Posintro TM . Brie fl y, cholesterol, DC-cholesterol, and POPC (and acridine) were dissolved in 20% ( w / v ) mega-10 in Milli- Q water. The saponin, Quil A, was dissolved in Milli-Q water, and appropriate amounts of the components were mixed and stirred for 2 h at 37°C. The theoretical weight ratio before dialysis between Quil A/POPC/total cholesterol was 5:1:1, and the total lipid concentration was 0.2% ( w / v ). The amount of DC-cholesterol was either 25% of the total cholesterol or 50% of the total cholesterol. Where nothing else is men- tioned, the amount of DC-cholesterol is 25% of the total cholesterol. The mixture was dialyzed (Slide-A-Lyzer® cassette, 10,000 MW cutoff, Thermo Scienti fi c, Denmark) against PBS for 48 h at 26°C and 48 h at 5°C with buffer changes every 24 h in order to remove the detergent. Acridine – Posintro TM particles were ultracentrifuged (UC) in a continuous sucrose gradient prepared by the freeze – thaw method described previously (18). Brie fl y, 4.5 ml 25% ( w / v ) sucrose solution was placed in an ultraclear centrifuge tube (Beckman Coulter, CA, USA) and frozen at − 20°C. The day before centrifugation, the tube was placed at 4°C for slow thawing. Acridine – Posintro TM suspension of 0.7 ml was placed on top of the sucrose gradient and centrifuged for 4 h at 15°C and 50,000 rpm (112,000× g ). The visible band was collected by gradient harvesting (Auto-Densi Flow®, MO, USA) and dialyzed against PBS at room temperature for 48 h with two buffer changes in order to remove the sucrose. Size distribution was measured by dynamic light scattering (DLS), and zeta potential was measured by laser Doppler electrophoresis (LDE; Zetasizer Nano ZS, Malvern, UK) in small volume cuvettes before and after UC. TEM images were recorded of the prepared Posintro TM and acridine – Posintro TM by negative staining. Brie fl y, Posintro TM was placed on a formvar-coated copper grid and negatively stained using a fi ltered aqueous solution of 1% ( w / v ) uranyl acetate. Fresh human abdominal skin was obtained after cosmetic surgery from Frederiksborg Klinikken, Denmark (approved by the local ethical committee, no. H-Ø-2001-1-39G) and processed the same day. Subcutaneous fat was removed, and skin biopsies with a diameter of 8 mm were punched out. The skin penetration model (Fig. 1) was prepared by pouring heated liquid agar into the bottom of cell culture inserts (Becton Dickinson Biosciences, Denmark). The inserts were transferred to deep six-well plates (Becton Dickinson Biosciences, Denmark) and incubated at 4°C for 1 h. The skin biopsies were placed in the hardened agar with the stratum corneum facing upwards, and 11 ml of supplemented DMEM medium was added to each well. The skin biopsies were cultured and maintained at 37°C and 5% CO 2 in a humidi fi ed atmosphere. CLSM was used to visualize the penetration of acridine – Posintro TM nanoparticles by localization of the fl uorescent label (acridine) in human skin. Samples of 20 μ l were applied on the surface of the skin biopsies, which were subsequently incubated as described above for 24, 48, or 72 h. The applied samples were composed of 0.05 mg/ml acridine in 5% ( w / v ) mega-10 in PBS and acridine – Posintro TM nanoparticles in PBS, corresponding to 0.05 mg/ml acridine. After 24, 48, or 72 h of exposure, skin biopsies were removed from the penetration model setup, washed in PBS, and quick-frozen in Tissue-Tek. Subsequently, the frozen samples were cut in cryo-dermatome (Leica CM1100, Germany) into 4- μ m-thin sections, which were incubated at room temperature for 30 min with DAPI nuclear stain (1 μg/ml in PBS). The sections were mounted in fl uorescent mounting medium and visualized on a CLSM (Leica TCS SPE, Germany). The excitation wavelength was 405 nm for DAPI and 488 nm for acridine. The emission was visualized in the ranges 410 – 490 and 500 – 535 nm for DAPI and acridine, respectively. Each treatment was repeated three times, and representative images obtained with the same settings were recorded. The potential of the Posintro TM nanoparticles to penetrate into the stratum corneum upon cutaneous application was examined using TEM. Samples of 20 μl Posintro TM were applied to human skin in vitro , either in the absence or presence of a cross-linked polyvinyl-pyrrolidone-based hydrogel containing 65% water (Coloplast A/S, Denmark, patent no. WO/2004/031253) covering the skin surface. The hydrogel was applied in order to study the effect of hydration. After 24 and 72 h of incubation under the conditions described above, skin biopsies exposed to Posintro TM with or without hydration were cut in half and divided into seven small skin pieces of approximately 1 mm 3 . The small skin pieces were fi xed in 3% ( v / v ) glutaraldehyde in Milli-Q water overnight at 4°C, followed by fi xation in 1% ( w / v ) osmium tetroxide in 0.1 M cacodylate buffer pH 7.4 for 1 h at room temperature. After fi xation, the small pieces were dehydrated in a series of graded ethanol solutions (50%, 70%, 80%, 96%, and 100% ( v / v )) and in propylene oxide with gradually increasing amounts of Epon. The tissue samples were embedded in Epon overnight at 60°C for polymerization. Semithin sections (1 μm) were cut and stained with methylene blue for visualization with light microscopy to verify the integrity of the skin. Several ultrathin (approximately 90 nm) sections were cut on a microtome (Ultracut E, Reichert-Jung, Austria), collected on formvar-coated grids and counterstained with uranyl acetate and lead citrate. The sections were examined in a Philips TEM (EM400, Eindhoven, The Netherlands). Each treatment was repeated three times, and representative micrographs were collected. Human abdominal or mammary skin obtained from cosmetic surgery (same approval as described previously) was frozen at − 20°C immediately after receipt and removal of the subcutaneous fat. Prior to the experiment, the skin was thawed and hydrated overnight in PBS at 5°C. The skin was then placed in a 60°C water bath for 1 min, after which the epidermis was carefully separated from the dermis and placed on top of a cellulose membrane in Franz diffusion cells. The donor solution consisted of 10 mg/ml of the model substance, methyl nicotinate, in the absence or presence of Posintro TM or the different components of the nanoparticles in similar concentrations as theoretically present in the intact nanoparticles. Methyl-nicotinate-containing donor solutions com- prised: PBS, 10% ( w / v ) Tween 80 in PBS, Posintro TM with 25% ( w / w ) DC-cholesterol in PBS, Posintro TM with 50% ( w / w ) DC-cholesterol in PBS, Quil A in PBS, cholesterol in PBS with 10% ( w / v ) Tween 80, DC-cholesterol in PBS with 10% ( w / v ) Tween 80, POPC in PBS with 10% ( w / v ) Tween 80, and a mixture of the above-mentioned components in PBS with 10% ( w / v ) Tween 80. A test volume of 200 μl was applied to a skin diffusion area of 0.2 cm 2 , and the 3 ml stirred receptor phase (PBS) was kept at 37°C, maintaining a skin surface temperature of 32°C. Three-hundred-microliter samples were withdrawn from the receptor compartment after 1/2, 1, 3, 5, 8, 12, and 24 h and immediately replaced by 300 μ l PBS. For each test solution, eight replicates were performed on skin from eight different skin ...
Citations
... According to Kahlon and Dutz ( 2003 ), LPS and its derivatives can activate TLR4 expressed by LCs and DCs. Additionally, Quil A (QA) has been incorporated into TCI formulations to enhance skin penetration and immune responses (Madsen et al. 2009 ). Combining adjuvants that act through different pathways can be used to further optimize immune responses (Garçon et al. 2007 ). ...
Transcutaneous immunization (TCI) is a relatively new and promising minimally invasive technique for vaccination. It involves topical delivery of vaccines to immune cells residing in the skin. The skin is the largest immune organ (total surface area of 1.8 m2) and contains a diverse array of immune cells including Langerhans cells (LCs) and dermal dendritic cells (DDCs). This creates the potential for TCI to be an excellent alternative to traditional vaccination methods. Due to the promise of this approach numerous preclinical animal studies and limited human studies have been carried out utilizing TCI (some of which will be summarized in this review) and it is surely only a matter of time until such products reach the market.
... These modified ISCOMs ® could potentially be used to immunize the organism through a transdermal patch applied to the skin. 99 Cucumarioside A2 from marine macrophytes forms tubular nano-objects (called "tubular ISCOMs ® "), which improve the immunogenicity by a factor of four. 75 In addition to the formation of ISCOM ® -like structures, other types of nano-objects can be prepared from mannosylated saponins based on oleanolic and glycyrrhizic acids. ...
Saponins, amphiphiles of natural origin with numerous biological activities, are widely used in the cosmetic and pharmaceutical industry. Some saponins exhibit relatively selective cytotoxic effects on cancer cells but the tendency of saponins to induce hemolysis limits their anticancer potential. This review focused on the effects of saponin activity on membranes and consequent implications for red blood and cancer cells. This activity seems to be strongly related to the amphiphilic character of saponins that gives them the ability to self-aggregate and interact with membrane components such as cholesterol and phospholipids. Membrane interactions of saponins with artificial membrane models, red blood and cancer cells are reviewed with respect to their molecular structures. The review considered the mechanisms of these membrane interactions and their consequences including the modulation of membrane dynamics, interaction with membrane rafts, and membrane lysis. We summarized current knowledge concerning the mechanisms involved in the interactions of saponins with membrane lipids and examined the structure activity relationship of saponins regarding hemolysis and cancer cell death. A critical analysis of these findings speculates on their potential to further development of new anticancer compounds.
... Recently, Madsen et al. [58,59] has designed a modified ISCOMs, so-called Posintro TM nanoparticles, and investigated the interaction between modified ISCOMs and stratum corneum lipid model systems. The Posintro TM nanoparticles was demonstrated to be advantageous in providing an opportunity for altering the surface charge of the particles, which influences their affinity for the negatively charged antigen sites, cell membranes and lipids in the skin. ...
Transcutaneous immunization represents an attractive alternative to vaccine delivery via topical administration and has received wide attention due to its easy-to-use, needle-free and noninvasive delivery. However, the development of transcutaneous vaccine was kept a challenge because of the barrier function of stratum corneum which inhibits the transport of antigen and adjuvant. Nowadays, pharmaceutical methods and novel physical devices are extensively investigated to overcome the penetration barrier of the stratum corneum for transcutaneous vaccine. In this article, these pharmaceutical methods and novel devices used for the enhancement of transcutaneous immunization were reviewed. In addition, chemokines promoted the migration of Langerhans cells and the transcutaneous adjuvants enhancing the immune responses at certain levels are also discussed for the development of novel transcutaneous vaccines.
... Some strategies aim at developing a drug delivery system which transiently increases the permeability of the skin, others are designed to bypass or even remove the outermost skin layer [13]. Cutaneous application of Posintro™ nanoparticles has been shown to enhance transcutaneous delivery of hydrophobic model compounds [14]. It was shown that a model compound incorporated into the Posintro™ nanoparticles penetrates into the epidermis through the intercorneocyte space in the stratum corneum, thus passing through the intercorneocyte lipids. ...
... However, the current consensus is that despite their small size, the nanoparticles are not able to squeeze between the corneocytes while staying intact. The hypothesis is therefore that the modified ISCOMs disturb the intercorneocyte lipid lamellae in the stratum corneum and thus enhance the penetration of the model compounds into the epidermis where the target cells reside [14]. The objective of the current study was to investigate interaction between modified ISCOMs with different surface charge and the lipids in the intercorneocyte space of the stratum corneum. ...
... The current investigation was initiated as a follow up on previous studies [14], from which we hypothesize that the Posintro™ nanoparticles disturb the intercellular lipid lamellae in the stratum corneum and thereby enhance the penetration of other compounds. In those studies, it was observed that the Posintro™ nanoparticles seemed to penetrate into the stratum corneum through the intercellular space. ...
The modified ISCOMs, so-called Posintro nanoparticles, provide an opportunity for altering the surface charge of the particles, which influences their affinity for the negatively charged antigen sites, cell membranes and lipids in the skin. Hypothetically, this increases the passage of the ISCOMs (or their components) and their load through the stratum corneum. The subsequent increase in the uptake by the antigen-presenting cells results in enhanced transcutaneous immunization. To understand the nature of penetration of Posintro nanoparticles into the intercorneocyte space of the stratum corneum, the interaction between the nanoparticles and lipid model systems in form of liposomes and/or supported lipid bilayer was studied. As a lipid model we used Stratum Corneum Lipid (SCL), a mixture similar in composition to the lipids of the intercorneocyte space. By Förster Resonance Energy Transfer (FRET), Atomic Force Microscopy (AFM), Electrochemical Impedance Spectroscopy (EIS) and cryo-Transmission Electron Microscopy (cryo-TEM) it was shown that application of nanoparticles to the SCL bilayers results in lipid disturbance. Investigation of this interaction by means of Isothermal Titration Calorimetry (ITC) confirmed existence of an enthalpically unfavorable reaction. All these methods demonstrated that the strength of electrostatic repulsion between the negatively charged SCL and the nanoparticles affected their interaction, as decreasing the negative charge of the Posintro nanoparticles leads to enhanced disruption of lipid organization.
Immune stimulating complexes (ISCOMs) are lipid-based particles that have shown potential as adjuvants and carriers for antigens aiming at prophylactic or therapeutic vaccination upon injection as well as via mucosal and cutaneous administration. Both cellular and humoral immune responses have been reported after vaccination with antigens using ISCOM adjuvants, and some are in clinical trials. The adjuvant particles are formed by self-assembly of phospholipid, saponin, and cholesterol at well-defined ratios from mixtures of the components. In aqueous dispersion, they appear as cage-like structures with a hollow center and approximately 40–60 nm in size. The present chapter discusses state-of-the-art with regards to formulation design, characterization, and assessment of the mechanisms of action for ISCOMs with examples from our own research, along with addressing the different routes of administration referring to the clinical status of ISCOMs as adjuvants. The future perspectives of using ISCOMs as vaccine adjuvants are presented.
Immune stimulating complexes (ISCOMs) belong to the group of particulate vaccine delivery systems. These particles have received considerable attention in the field of vaccine delivery systems, especially for subunit vaccines. ISCOMs have a spherical, open and cage-like structure and a particle size of around 40nm. They contain an adjuvant (Quil A or QS 21) and an antigen incorporated into or associated with their colloidal structure, making ISCOMs particulate antigen delivery systems which allow co-delivery of antigen and adjuvant. In this chapter we initially describe the components, microstructures and preparation methods of ISCOMs followed by their mechanism of immune stimulation and their use as vaccines.
Transcutaneous immunization (TCI) is a promising route of vaccine delivery through skin due to
many well documented advantages. The main obstacle in TCI is the skin’s top dead layer i.e.
stratum corneum which is difficult to penetrate. Efficiently delivery of antigen to the immune
competent cells of epidermis or dermis in TCI might elicit an effective immune response. In this
review, skin immunology with a particular focus on potential of immunological active receptors
in influencing adaptive immune responses is highlighted. The challenges with TCI and methods
to improve it using different adjuvants, chemical and physical approaches, delivery systems, and
combination of above methods to further improve immune response following skin application
of antigen are elaborately discussed. Nanoparticulate vaccine delivery systems with reference to
their applications in TCI are classified according to their chronological development.
Conclusively, clinical translations of above methods are also briefly reviewed.
In this paper, we provide an overview an extensive range of colloidal drug delivery systems with special focus on vesicular and particulates systems that are being used in research or might be potentially useful as carriers systems for drug or active biomolecules or as cell carriers with application in the therapeutic field. We present some important examples of commercially available drug delivery systems with applications in research or in clinical fields. This class of systems is widely used due to excellent drug targeting, sustained and controlled release behavior, higher entrapment efficiency of drug molecules, prevention of drug hydrolysis or enzymatic degradation, and improvement of therapeutic efficacy. These characteristics help in the selection of suitable carrier systems for drug, cell, and gene delivery in different fields.