Figure 5 - available via license: Creative Commons Attribution 4.0 International
Content may be subject to copyright.
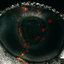
Structure of capsule polysaccharide repeating unit (top) and galactan exopolysaccharide repeating unit (bottom).
Source publication
Recent evidence indicates that Kingella kingae produces a polysaccharide capsule. In an effort to determine the composition and structure of this polysaccharide capsule, in the current study we purified capsular material from the surface of K. kingae strain 269-492 variant KK01 using acidic conditions to release the capsule and a series of steps to...
Citations
... In addition to the lipid-anchored polysaccharides, K. kingae also exhibits the ability of galactan exopolysaccharide secretion, which is involved in biofilm establishment, growth, and architectural remodeling [6]. This expolysaccharide is a polymer of galactofuranose that is present in two recognized structures in K. kingae, based on the link connecting the galactofuranose residues [39]. Interestingly, data from in vivo studies have shown that the galactan exopolysaccharide shares an overlapping effect with the polysaccharide capsule in terms of resistance to opsonization [37]. ...
Infective endocarditis due to Kingella kingae is a rare but serious invasive infection that occurs mostly in children. Recent advances in nucleic acid amplification testing as well as in cardiac imaging have enabled more accurate diagnosis. A good understanding of the epidemiology and virulence factors remains crucial to guide the therapeutic approach. Here, we synthesize the current state of knowledge on epidemiological features, pathophysiological insights, complications, and therapy regarding Kingella kingae endocarditis in children and adults. Finally, throughout this comprehensive review, knowledge gaps and areas for future research are also identified.
... Units of α-Kdo (2) have been found mostly in the LPSs of gram-negative bacteria, where the two α-Kdo residues are attached to the lipid A, [9] whereas the less common β-Kdo glycosides (4) are found in the glycolipid terminus of capsular polysaccharides on many pathogens, including E. coli, N. meningitidis, Kingella kingae, Campylobacter jejuni, and Haemophilus influenzae. [10,11] These glycosides are also present in the extracellular exopolysaccharides (EPSs) of Burkholderia pseudomallei and Burkholderia cepacia ( Figure 1). [12-16a] Among the three parts of LPSs, namely the O-antigen, the core structure, and the Lipid A, most research is dedicated to the Oantigen. ...
... [23] Lou et al. used a similar strategy for the synthesis of α-Kdo glycosides. [24] According to this report, Lou et al. coupled a peracetylated Kdo ortho-hexynylbenzoate 5 donor with various acceptors (7)(8)(9)(10)(11)(12)(13)(14)(15)(16) in the presence of SPhosAuNTf 2 and a modulating molecule (DMF) to produce Kdo glycosides with excellent α-anomeric selectivity (Scheme 1). Finally, the author used this modulating method to create an α-linked di-Kdo glycoside 21 (Scheme 2). ...
Higher carbon saccharide 3‐deoxy‐d‐manno‐oct‐2‐ulosonic acid (Kdo) is a structural unit of bacterial lipopolysaccharides (LPSs) and capsular polysaccharides (CPSs). Kdo is present in the inner core region of LPSs, and this region is structurally conserved. Being non‐mammalian in origin, Kdos are effectively recognized by the native and adaptive immune systems. Therefore, the synthesis of new Kdo derivatives and neoglycoconjugates is highly important for the development of vaccines. This review highlights recent accomplishments related to α‐glycosylations, β‐glycosylations and C‐glycosylations of Kdos and their application to the stereoselective synthesis of inner core oligosaccharides.
... Until today, most of the NMR-characterized polysaccharides and lipopolysaccharides, are not classified as SAAs (Olsthoorn et al., 2000;Starr et al., 2013). This fact may be due to the high complexity and the high molecular weight they exhibit since NMR analysis is a method limited to molecular weights ≤10000 g/mol due to signal to noise ratio. ...
Synthetic surfactants are used in several industries, including manufacturing, pharmaceutical and cosmetic’s, food and feed, agriculture, petroleum and environmental remediation for their ability to adsorb to fluid and solid-water interfaces. However, their widespread use and their synthetic preparation through environmentally unfavorable processes counterbalances the value of this class of reagents. This fact has stimulated new efforts to exploit natural sources of surfactants, such as new classes of bacterial systems or manipulation of existing biological systems, that may produce, through an environmentally friendly process, new biodegradable surfactants and emulsifiers of high commercial value. A downside of microbial production of biobased chemicals such as these types of chemicals, is that their fermentation often yields crude materials consisting of several bioproducts with complex physical and chemical properties. Extraction, identification, and efficient characterization of biosurfactants from a crude mixture of biomolecules requires carefully designed, and detailed analytical processes using state-of-the-art methods. The purpose of this review article is to present the current state-of-the-art and future outlook on the various multidisciplinary biophysical methods applied in the discovery, extraction identification, and in-depth characterization of microbially-produced surface‐active compounds.
... While this work was performed with our prototype strain KK01 expressing a !5)b-Galf-(1! exopolysaccharide, a second exopolysaccharide with the structure !3)b-Galf-(1!6)-b-Galf-(1! has been identified in other clinical isolates of K. kingae (5,9,10). In clinical strain PYKK181, the !3)-b-Galf-(1!6)-b-Galf-(1! galactan has been shown to promote biofilm dispersal (10). ...
... Earlier work characterized the K. kingae galactan and identified the biosynthetic machinery encoded by the pam locus (7,9). In this work, we showed that the pamD and pamE genes encode LPS glycosyltransferases that are required for expression of K. kingae LMW LPS and for surface presence of galactan. ...
Kingella kingae is a leading cause of bone and joint infections and other invasive diseases in young children. A key K. kingae virulence determinant is a secreted exopolysaccharide that mediates resistance to serum complement and neutrophils and is required for full pathogenicity. The K. kingae exopolysaccharide is a galactofuranose homopolymer called galactan and is encoded by the pamABC genes in the pamABCDE locus. In this study, we sought to define the mechanism by which galactan is tethered on the bacterial surface, a prerequisite for mediating evasion of host immune mechanisms. We found that the pamD and pamE genes encode glycosyltransferases and are required for synthesis of an atypical lipopolysaccharide (LPS) O-antigen. The LPS O-antigen in turn is required for anchoring of galactan, a novel mechanism for association of an exopolysaccharide with the bacterial surface.
IMPORTANCE Kingella kingae is an emerging pediatric pathogen and produces invasive disease by colonizing the oropharynx, invading the bloodstream, and disseminating to distant sites. This organism produces a uniquely multifunctional exopolysaccharide called galactan that is critical for virulence and promotes intravascular survival by mediating resistance to serum and neutrophils. In this study, we established that at least some galactan is anchored to the bacterial surface via a novel structural interaction with an atypical lipopolysaccharide O-antigen. Additionally, we demonstrated that the atypical O-antigen is synthesized by the products of the pamD and pamE genes, located downstream of the gene cluster responsible for galactan biosynthesis. This work addresses how the K. kingae exopolysaccharide can mediate innate immune resistance and advances understanding of bacterial exopolysaccharides and lipopolysaccharides.
... While this work was performed with our prototype strain KK01 expressing a !5)b-Galf-(1! exopolysaccharide, a second exopolysaccharide with the structure !3)b-Galf-(1!6)-b-Galf-(1! has been identified in other clinical isolates of K. kingae (5,9,10). In clinical strain PYKK181, the !3)-b-Galf-(1!6)-b-Galf-(1! galactan has been shown to promote biofilm dispersal (10). ...
... Earlier work characterized the K. kingae galactan and identified the biosynthetic machinery encoded by the pam locus (7,9). In this work, we showed that the pamD and pamE genes encode LPS glycosyltransferases that are required for expression of K. kingae LMW LPS and for surface presence of galactan. ...
Kingella kingae is a leading cause of bone and joint infections and other invasive diseases in young children. A key K. kingae virulence determinant is a secreted exopolysaccharide that mediates resistance to serum complement and neutrophils and is required for full pathogenicity. The K. kingae exopolysaccharide is a galactofuranose homopolymer called galactan and is encoded by the pamABC genes in the pamABCDE locus. In this study, we sought to define the mechanism by which galactan is tethered on the bacterial surface, a prerequisite for mediating evasion of host immune mechanisms. We found that the pamD and pamE genes are glycosyltransferases and are required for synthesis of an atypical lipopolysaccharide (LPS) O-antigen. The LPS O-antigen in turn is required for anchoring of galactan, a novel mechanism for association of an exopolysaccharide with the bacterial surface.
Significance
Kingella kingae is an emerging pediatric pathogen and produces invasive disease by colonizing the oropharynx, invading the bloodstream, and disseminating to distant sites. This organism produces a uniquely multifunctional exopolysaccharide called galactan that is critical for virulence and promotes intravascular survival by mediating resistance to serum and neutrophils. In this study, we established that at least some galactan is anchored to the bacterial surface via a novel structural interaction with an atypical lipopolysaccharide O-antigen. Additionally, we demonstrated that the atypical O-antigen is synthesized by the pamD and pamE genes, located downstream of the gene cluster responsible for galactan biosynthesis. This work addresses how the K. kingae exopolysaccharide can mediate innate immune resistance and advances understanding of bacterial exopolysaccharides and lipopolysaccharides.
... Investigation into the genetic requirements of encapsulation revealed multiple loci critical for surface presentation of the capsule in K. kingae. The ctrABCD operon is required for capsule export [9,24]; the lipA and lipB genes are both essential for surface localization of capsule, and homologs of these genes in Escherichia coli and N. meningitidis were shown to assemble the β-Kdo linker between the lipid membrane anchor and the capsular polysaccharide [24,25]; and the csaA gene product (encoded in the region called the capsule synthesis locus) is the capsule synthase required for polymerizing the GalNAc-Kdo capsule polymer of prototype strain KK01 [24,26,27]. Site-directed mutagenesis studies targeting predicted active site residues in CsaA identified this protein as a bifunctional glycosyltransferase responsible for catalyzing both linkages in the GalNAc-Kdo capsule polymer. ...
... Investigation of encapsulation in the K. kingae population revealed that there are four capsular polysaccharides represented in a diverse collection of clinical isolates that are termed types a, b, c, and d [26][27][28]. The type a capsule is a polymer of [26][27][28]. ...
... Investigation of encapsulation in the K. kingae population revealed that there are four capsular polysaccharides represented in a diverse collection of clinical isolates that are termed types a, b, c, and d [26][27][28]. The type a capsule is a polymer of [26][27][28]. Of note, the genomic location of the capsule synthesis locus is maintained across the K. kingae population structure, but the gene content varies depending on the capsule type [26]. ...
The emergence of Kingella kingae as an important etiology of pediatric osteoarticular infections over the past three decades has led to significant research efforts focused on understanding the pathogenicity of this fastidious Gram-negative bacterium. This work has uncovered multiple virulence factors that likely play key roles in the ability of the organism to colonize the upper respiratory tract, breach the epithelial barrier, and disseminate to distal sites of infection. Herein the current body of knowledge about K. kingae virulence factors is reviewed in the context of K. kingae disease pathogenesis. The work summarized here has identified multiple targets for therapeutic intervention as well as potential vaccine antigens.
... Similar to other respiratory pathogens such as pneumococci and H. influenzae type b, K. kingae elaborates a polysaccharide capsule and secretes an exopolysaccharide. Both components inhibit the host's immune response [14][15][16], enabling colonization of the upper respiratory tract, protecting the organism from phagocytosis by blood leukocytes and tissue macrophages, and facilitating the invasion of deep tissues. The maturation of the T-cell independent arm of the immune system, which is responsible for producing antibodies to polysaccharide antigens, is delayed in humans until the age of 2-4 years [2], explaining the increased susceptibility of young children to both colonization and disease. ...
With the appreciation of Kingella kingae as a prime etiology of osteoarticular infections in young children, there is an increasing interest in the pathogenesis of these diseases. The medical literature on K. kingae’s colonization and carriage was thoroughly reviewed. Kingella kingae colonizes the oropharynx after the second life semester, and its prevalence reaches 10% between the ages of 12 and 24 months, declining thereafter as children reach immunological maturity. Kingella kingae colonization is characterized by the periodic substitution of carried organisms by new strains. Whereas some strains frequently colonize asymptomatic children but are rarely isolated from diseased individuals, others are responsible for most invasive infections worldwide, indicating enhanced virulence. The colonized oropharyngeal mucosa is the source of child-to-child transmission, and daycare attendance is associated with a high carriage rate and increased risk of invasive disease. Kingella kingae elaborates a potent repeat-in-toxin (RTXA) that lyses epithelial, phagocytic, and synovial cells. This toxin breaches the epithelial barrier, facilitating bloodstream invasion and survival and the colonization of deep body tissues. Kingella kingae colonization and carriage play a crucial role in the person-to-person transmission of the bacterium, its dissemination in the community, and the pathogenesis of invasive infections.
... This residue links the core oligosaccharide to lipid A in the LPS. Downfield C-1 (179.2 ppm) and upfield C-2 (99.2) chemical shifts indicated α-anomeric configuration of this residue [30,31], and a downfield displacement of the C-5 chemical shift (+9.4 ppm) [32] gave evidence of it being glycosylated on O-5. ...
Huanglongbing (HLB) disease, also known as citrus greening disease, was first reported in the US in 2005. Since then, the disease has decimated the citrus industry in Florida, resulting in billions of dollars in crop losses and the destruction of thousands of acres of citrus groves. The causative agent of citrus greening disease is the phloem limited pathogen Candidatus Liberibacter asiaticus. As it has not been cultured, very little is known about the structural biology of the organism. Liberibacter are part of the Rhizobiaceae family, which includes nitrogen-fixing symbionts of legumes as well as the Agrobacterium plant pathogens. To better understand the Liberibacter genus, a closely related culturable bacterium (Liberibacter crescens or Lcr) has attracted attention as a model organism for structural and functional genomics of Liberibacters. Given that the structure of lipopolysaccharides (LPS) from Gram-negative bacteria plays a crucial role in mediating host-pathogen interactions, we sought to characterize the LPS from Lcr. We found that the major lipid A component of the LPS consisted of a pentaacylated molecule with a β-6-GlcN disaccharide backbone lacking phosphate. The polysaccharide portion of the LPS was unusual compared to previously described members of the Rhizobiaceae family in that it contained ribofuranosyl residues. The LPS structure presented here allows us to extrapolate known LPS structure/function relationships to members of the Liberibacter genus which cannot yet be cultured. It also offers insights into the biology of the organism and how they manage to effectively attack citrus trees.
... Capsule isolation and staining. Capsule production was visualized as previously described (74). Briefly, bacteria were pelleted from 1 ml of saturated cultures grown overnight in LB broth. ...
Extraintestinal pathogenic Escherichia coli (ExPEC) are major causes of urinary and bloodstream infections. ExPEC reservoirs are not completely understood. Some mastitis-associated E. coli (MAEC) strains carry genes associated with ExPEC virulence, including metal scavenging, immune avoidance, and host attachment functions. In this study, we investigated the role of the high-affinity zinc uptake (znuABC) system in the MAEC strain M12. Elimination of znuABC moderately decreased fitness during mouse mammary gland infections. The ΔznuABC mutant strain exhibited an unexpected growth delay in the presence of bile salts, which was alleviated by the addition of excess zinc. We isolated ΔznuABC mutant suppressor mutants with improved growth of in bile salts, several of which no longer produced the K96 capsule made by strain M12. Addition of bile salts also reduced capsule production by strain M12 and ExPEC strain CP9, suggesting that capsule synthesis may be detrimental when bile salts are present. To better understand the role of the capsule, we compared the virulence of mastitis strain M12 with its unencapsulated ΔkpsCS mutant in two models of ExPEC disease. The wild type strain successfully colonized mouse bladders and kidneys and was highly virulent in intraperitoneal infections. Conversely, the ΔkpsCS mutant was unable to colonize kidneys and was unable to cause sepsis. These results demonstrate that some MAEC may be capable of causing human ExPEC illness. Virulence of strain M12 in these infections is dependent on its capsule. However, capsule may interfere with zinc homeostasis in the presence of bile salts while in the digestive tract.
... Bendaoud et al. [15], have shown that K. kingae strain PYKK181 synthesizes an exopolysaccharide composed of linear galactan homopolymer, which acts as a biofilm formation tool. It is well known that biofilms play a critical function in bacterial adherence and colonization and prevent host defence mechanisms in diseases such as chronic osteomyelitis and lung cystic fibrosis [12,16]. ...
Kingella kingae is a Gram-negative coccobacilli and it is a member of the HACEK (Haemophilus species, Aggregatibacte actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and K. kingae). HACEK organisms are typically oropharyngeal commensals and have long been recognized as a cause of infective endocarditis in children and adults. K. kingae in difficult to be recovered from cultured pharyngeal samples due to its slow growth and the high presence of resident bacterial flora, however, the organism can be better detected using PCR tests. Based on our search in PubMed and other sources, we couldn't discover any study about K. kingae originated from any Arab country. Therefore, we wrote this review to draw the attention of our physicians and clinical microbiologists on the importance of this neglected group of organisms in clinical medicine. However, this review article aims to cover the most important diseases of K. kingae in the pediatric population.