Figure 5 - uploaded by Emilia Komulainen
Content may be subject to copyright.
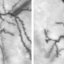
SCG10 has a requisite role in neuronal migration, increasing soluble tubulin pools and forward movement of neurons.(a) Confocal sections of β-III tubulin (TuJ1) staining from intermediate zone of wild-type and Jnk1−/− cortex. Tubulin staining is diffuse in Jnk1−/− cortex, suggesting increased tubulin solubility. (b) Quantitative measurements (mean ±s.e.m.) of tyrosinated tubulin from cortex and hippocampus of wild-type and Jnk1−/− brains. (c) Representative immunoblots from b. (d) Mouse embryonic fibroblasts were co-transfected with GFP-SCG10-WT, GFP-SCG10S62A/S73A (SCG10-AA) or GFP-SCG10S62D/S73D and venus-tubulin. TX100-soluble (S) and insoluble (I) fractions were separated and immunoblotted for tubulin. Taxol (1 μM) was added as positive control. Data are mean ± s.e.m. (e) Representative immunoblots from b. (f) Representative images of MEF cells expressing indicated constructs. Endogenous tubulin and corresponding GFP are shown. Transfected cells are marked with arrows (63×). Cells expressing GFP-SCG10 show enriched filamentous tubulin compared to cells expressing GFP-SCG10-AA. (g) Immunoblot of cells expressing GFP-SCG10 with non-targeting (NT) or SCG10 shRNA. Neurons transfected with GFP (green) with NT- or SCG10-shRNA. SCG10 expression 72 h later detected by immunostaining (red). SCG10 silencing was effective. (h) Neurons were transfected with SCG10- or NT-shRNA with GFP or GFP-JBD. Migration in Transwells was measured 48 h later. SCG10-shRNA increased migration. (i) Migration (mean ± s.e.m.) of neurons in Transwells transfected with GFP and GFP-SCG10-AA, GFP-JBD or venus-tubulin. Significance was tested by one-way ANOVA and post-hoc Tukey HSD test. **P < 0.05; ***P < 0.001.
Source publication
Cell migration is the consequence of the sum of positive and negative regulatory mechanisms. Although appropriate migration of neurons is a principal feature of brain development, the negative regulatory mechanisms remain obscure. We found that JNK1 was highly active in developing cortex and that selective inhibition of JNK in the cytoplasm markedl...
Contexts in source publication
Context 1
... and less structured in Jnk1 −/− telen- cephala than in the wild type (Fig. 5a). To measure microtubule stability more directly, we measured the levels of tyrosinated tubulin, a classical marker of unstable microtubules (Fig. 5b,c). Tyrosinated tubulin was increased in both cortex and hippocampus of Jnk1 −/− mice, which suggests that, as in adults 24 , microtubule stability was disturbed in immature brains from ...
Context 2
... and less structured in Jnk1 −/− telen- cephala than in the wild type (Fig. 5a). To measure microtubule stability more directly, we measured the levels of tyrosinated tubulin, a classical marker of unstable microtubules (Fig. 5b,c). Tyrosinated tubulin was increased in both cortex and hippocampus of Jnk1 −/− mice, which suggests that, as in adults 24 , microtubule stability was disturbed in immature brains from mice lacking JNK1. Phosphorylation of Ser62 and Ser73 by JNK modifies the microtubule regulatory properties of SCG10 (refs. 6,25). Ser-73-phosphorylated ...
Context 3
... of Ser62 and Ser73 by JNK modifies the microtubule regulatory properties of SCG10 (refs. 6,25). Ser-73-phosphorylated SCG10 is enriched in the intermediate zone of E15 telencephalon 6 . We found that expression of GFP-SCG10 S62A/S73A increased micro- tubule solubility, whereas the phospho-mimetic GFP-SCG10 S62D/S73D stabilized microtubules (Fig. 5d,e). Moreover, there were prominent microtubule bundles in cells expressing wild-type GFP-SCG10 or GFP-SCG10 S62D/S73D but not in cells expressing GFP-SCG10 S62A/S73A (Fig. 5f). Thus, in agreement with a previous model 26 , preventing Ser62/Ser73 phosphorylation of SCG10 increases tubulin solubility, whereas phosphorylated SCG10 stabilizes ...
Context 4
... telencephalon 6 . We found that expression of GFP-SCG10 S62A/S73A increased micro- tubule solubility, whereas the phospho-mimetic GFP-SCG10 S62D/S73D stabilized microtubules (Fig. 5d,e). Moreover, there were prominent microtubule bundles in cells expressing wild-type GFP-SCG10 or GFP-SCG10 S62D/S73D but not in cells expressing GFP-SCG10 S62A/S73A (Fig. 5f). Thus, in agreement with a previous model 26 , preventing Ser62/Ser73 phosphorylation of SCG10 increases tubulin solubility, whereas phosphorylated SCG10 stabilizes microtubules in intact cells. We propose that SCG10-mediated stabilization acts by increasing the rate of plus end microtubule growth ( Supplementary Fig. 1b), as has been ...
Context 5
... of stathmin-1 in Drosophila, which has only one stathmin gene, results in serious anomalies including migration irregularities in the developing nervous system 28 . To test whether SCG10 influences migration, we transfected neurons with SCG10 short hairpin RNA (shRNA) for 72 h, by which time SCG10 expression was efficiently reduced (Fig. 5g). This knockdown increased the migration rate of neurons to a similar extent as did JNK1 depletion (Fig. 5h), and was consistent with the expected increase in microtubule solubility upon removal of SCG10 S62D/S73D (refs. 6,27; Fig. 5d,e). Moreover, there was no additional increase in migration in these cells when JNK was inhibited with ...
Context 6
... migration irregularities in the developing nervous system 28 . To test whether SCG10 influences migration, we transfected neurons with SCG10 short hairpin RNA (shRNA) for 72 h, by which time SCG10 expression was efficiently reduced (Fig. 5g). This knockdown increased the migration rate of neurons to a similar extent as did JNK1 depletion (Fig. 5h), and was consistent with the expected increase in microtubule solubility upon removal of SCG10 S62D/S73D (refs. 6,27; Fig. 5d,e). Moreover, there was no additional increase in migration in these cells when JNK was inhibited with JBD ( Fig. 5h), indicating that JNK1 and SCG10 regu- late migration by a common ...
Context 7
... neurons with SCG10 short hairpin RNA (shRNA) for 72 h, by which time SCG10 expression was efficiently reduced (Fig. 5g). This knockdown increased the migration rate of neurons to a similar extent as did JNK1 depletion (Fig. 5h), and was consistent with the expected increase in microtubule solubility upon removal of SCG10 S62D/S73D (refs. 6,27; Fig. 5d,e). Moreover, there was no additional increase in migration in these cells when JNK was inhibited with JBD ( Fig. 5h), indicating that JNK1 and SCG10 regu- late migration by a common ...
Context 8
... 5g). This knockdown increased the migration rate of neurons to a similar extent as did JNK1 depletion (Fig. 5h), and was consistent with the expected increase in microtubule solubility upon removal of SCG10 S62D/S73D (refs. 6,27; Fig. 5d,e). Moreover, there was no additional increase in migration in these cells when JNK was inhibited with JBD ( Fig. 5h), indicating that JNK1 and SCG10 regu- late migration by a common ...
Context 9
... data show that deletion of Jnk1 or exogenous expression of SCG10 S62A/S73A increased microtubule plasticity (Fig. 5a-f) and neuronal migration (Fig. 5h). To test whether these phenomena were causally linked, we artificially increased the soluble tubulin pool by expression of epitope-tagged tubulin and again measured migration. Exogenous expression of tubulin in neurons was suffi- cient to induce a twofold increase in migration, suggesting that the ...
Context 10
... data show that deletion of Jnk1 or exogenous expression of SCG10 S62A/S73A increased microtubule plasticity (Fig. 5a-f) and neuronal migration (Fig. 5h). To test whether these phenomena were causally linked, we artificially increased the soluble tubulin pool by expression of epitope-tagged tubulin and again measured migration. Exogenous expression of tubulin in neurons was suffi- cient to induce a twofold increase in migration, suggesting that the level of soluble tubulin is ...
Context 11
... 5h). To test whether these phenomena were causally linked, we artificially increased the soluble tubulin pool by expression of epitope-tagged tubulin and again measured migration. Exogenous expression of tubulin in neurons was suffi- cient to induce a twofold increase in migration, suggesting that the level of soluble tubulin is rate-limiting (Fig. 5i). Thus, conditions that increase soluble tubulin promote migration, whereas conditions that decrease soluble tubulin retard migration. We propose a tentative model to explain the cooperation of JNK1 and SCG10 in regulating neuronal migration (Supplementary Fig. ...
Context 12
... 6a,b). Co-expression of SCG10 S62D/S73D resulted in a substantial restoration of the normal migration phenotype, and these cells entirely failed to reach layers II/III by E19. In addition, neurons that expressed SCG10 S62A/S73A migrated faster than control cells (GFP at E19; Fig. 6a,b), consist- ent with the results obtained with cultured neurons (Fig. 5h,i), and showed a distinct morphology with reproducibly shorter leading process (Fig. 6a) as noted in postnatal cortical neurons 6 ...
Citations
... phosphorylation at Ser73 of STMN2 stabilizes MT in the brain of the mouse embryo [46]. The increased expression of p-STMN2 (Ser73) in the HIP of defeated mice may be a self-repair mechanism against MT damage due to SDS. ...
Objective: Microtubule (MT) stability in neurons is vital for brain development; instability is associated with neuropsychiatric disorders. The present study examined the effects of social defeat stress (SDS) on MT-regulating proteins and tubulin polymerization.
Methods: After 10 days of SDS, defeated mice were separated into susceptible (Sus) and unsusceptible (Uns) groups based on their performance in a social avoidance test. Using extracted brain tissues, we measured the expression levels of α-tubulin, acetylated α-tubulin, tyrosinated α-tubulin, MT-associated protein-2 (MAP2), stathmin (STMN1), phospho stathmin serine 16 (p-STMN1 [Ser16]), phospho stathmin serine 25 (p-STMN1 [Ser25]), phospho stathmin serine 38 (p-STMN1 [Ser38]), stathmin2 (STMN2), phospho stathmin 2 serine 73 (p-STMN2 [Ser73]), 78-kDa glucose-regulated protein (GRP-78), and CCAAT/enhancer binding protein (C/EBP)-homologous protein (CHOP) using Western blot assay. The tubulin polymerization rate was also measured.
Results: We observed increased and decreased expression of acetylated and tyrosinated α-tubulin, respectively, decreased expression of p-STMN1 (Ser16) and increased expression of p-STMN1 (Ser25), p-STMN2 (Ser73) and GRP-78 and CHOP in the prefrontal cortex and/or hippocampus of defeated mice. A reduced tubulin polymerization rate was observed in the Sus group compared to the Uns and Con groups.
Conclusion: Our findings suggest that SDS has detrimental effects on MT stability, and a lower tubulin polymerization rate could be a molecular marker for susceptibility to SDS.
Keywords: Social defeat; Microtubule; Polymerization
... A previous study reported that overexpressing the SCG10-S62A&S73A mutant, which has a constitutive microtubuledepolymerizing ability, decreased neurite length in cultured mouse cortical neurons (Tararuk et al., 2006). Another study using in utero electroporation of the cortex in embryonic day (E) 15.5 mice found that SCG10 knockdown increased the cortical neuron migration rate, and the defects could be rescued by overexpressing SCG10-S62D&S73D, a phospho-mimicking SCG10 mutant lacking the microtubule-regulating function (Westerlund et al., 2011). Recent studies reported the neuroprotective role of SCG10 in motor neurons, as amyotrophic lateral sclerosis-like phenotypes were observed in Scg10 knockout mice (Guerra San Juan et al., 2022;Krus et al., 2022). ...
... Unlike stathmin knockout mice, which live normally (Schubart et al., 1996), most of the Scg10 knockout mice died at or shortly after birth, illustrating the importance of the neuronal-specific SCG10 for survival, although the reason for the death needs to be further studied. SCG10 knockdown, using in utero-electroporated E15.5 mice, has been reported to increase the cortical neuron migration rate (Westerlund et al., 2011); however, we detected no significant layer formation defects in Scg10 knockout mouse brain sections at P0, and the brain structures were generally normal. This discrepancy might be due to compensation by other microtubule dynamics regulators, a possibility that needs further investigation. ...
Proper microtubule dynamics is critical for neuronal morphogenesis and functions, and its dysregulation results in neurological disorders and regeneration failure. Superior cervical ganglion-10 (SCG10, also known as Stathmin-2) is a well-known regulator of microtubule dynamics in neurons, but its functions in the peripheral nervous system (PNS) remain largely unknown. Here, we show that Scg10 knockout mice exhibit severely progressive motor and sensory dysfunctions with significant sciatic nerve myelination deficits and neuromuscular degeneration. Furthermore, increased microtubule stability, shown by a significant increase in tubulin acetylation and decrease in tubulin tyrosination, and decreased axonal transport occur in Scg10 knockout DRG neurons. Meanwhile, SCG10 depletion impairs axon regeneration in both injured mouse sciatic nerve and cultured DRG neurons following replating, and the impaired axon regeneration was found to be induced by a lack of SCG10-mediated microtubule dynamics in the neurons. Thus, our results highlight the importance of SCG10 in peripheral axon maintenance and regeneration.
... A previous study suggested that the REST pathway and miR-124 may form a negative feedback regulatory mechanism [36]. In addition to DCX, other verified target genes of REST, including L1CAM and Superior Cervical Ganglion10 (SCG10) [37], are also essential for axon growth and polarity establishment [38,39]. Therefore, further downregulation of CoREST/REST expression at the late MP stage increases the expression of many genes essential for neuronal polarization so that neurons achieve the intrinsic state necessary to generate neuronal polarity when under the stimulation of intracellular and extracellular polarity-related factors. ...
... Therefore, microtubules in the growth cone need to maintain a certain degree of unstable properties. The microtubule destabilization protein SCG10 is located in the C-domain in growth cones and is activated by the GTPase Rnd1 downstream of Rac1 to depolymerize microtubules and, thus, promote axon growth [39,105]. ...
The establishment and maintenance of neuronal polarity are important for neural development and function. Abnormal neuronal polarity establishment commonly leads to a variety of neurodevelopmental disorders. Over the past three decades, with the continuous development and improvement of biological research methods and techniques, we have made tremendous progress in the understanding of the molecular mechanisms of neuronal polarity establishment. The activity of positive and negative feedback signals and actin waves are both essential in this process. They drive the directional transport and aggregation of key molecules of neuronal polarity, promote the spatiotemporal regulation of ordered and coordinated interactions of actin filaments and microtubules, stimulate the specialization and growth of axons, and inhibit the formation of multiple axons. In this review, we focus on recent advances in these areas, in particular the important findings about neuronal polarity in two classical models, in vitro primary hippocampal/cortical neurons and in vivo cortical pyramidal neurons, and discuss our current understanding of neuronal polarity.
... However, an increased thickness of the E15/E18 telencephalon in Jnk1 KO mice was also reported. Importantly, two differently localized JNK1 forms were described: the cytoplasmic form, which delays neuronal migration, and the nuclear form of JNK1, which enhances migration [32]. In addition, it was reported that JNK1 works as a positive regulator of migration of interneurons, given that JNK antagonists resulted in reduced migration speed at E12 [33]. ...
... SCG10 is a tubulin-sequestering protein that controls MT catastrophe events. SCG10 function is required for growth cone extension, and phosphorylation of SCG10 on serine 62 and serine 73 by JNK1 stabilizes MTs and promotes multipolar stage exit and neuronal migration rate [30,32]. ...
The precise development of the neocortex is a prerequisite for higher cognitive and associative functions. Despite numerous advances that have been made in understanding neuronal differentiation and cortex development, our knowledge regarding the impact of specific genes associated with neurodevelopmental disorders on these processes is still limited. Here, we show that Taok2, which is encoded in humans within the autism spectrum disorder (ASD) susceptibility locus 16p11.2, is essential for neuronal migration. Overexpression of de novo mutations or rare variants from ASD patients disrupts neuronal migration in an isoform-specific manner. The mutated TAOK2α variants but not the TAOK2β variants impaired neuronal migration. Moreover, the TAOK2α isoform colocalizes with microtubules. Consequently, neurons lacking Taok2 have unstable microtubules with reduced levels of acetylated tubulin and phosphorylated JNK1. Mice lacking Taok2 develop gross cortical and cortex layering abnormalities. Moreover, acute Taok2 downregulation or Taok2 knockout delayed the migration of upper-layer cortical neurons in mice, and the expression of a constitutively active form of JNK1 rescued these neuronal migration defects. Finally, we report that the brains of the Taok2 KO and 16p11.2 del Het mouse models show striking anatomical similarities and that the heterozygous 16p11.2 microdeletion mouse model displayed reduced levels of phosphorylated JNK1 and neuronal migration deficits, which were ameliorated upon the introduction of TAOK2α in cortical neurons and in the developing cortex of those mice. These results delineate the critical role of TAOK2 in cortical development and its contribution to neurodevelopmental disorders, including ASD.
... TTL might be directly activated, although no drug was approved yet, or indirectly, via the inhibition of its inhibitors, since TTL activity is decreased through phosphorylation [35]. Another approach is to activate or inhibit, in synergy, signaling pathways that are known to modulate depolymerizing factors and involved in the modulation of the tyrosination cycle such as PKC, BDNF/TrkB, JNK, Stathmin pathways [66][67][68][69][70]. It is worth noting that these pathways are known to be dysregulated in neurodegenerative diseases [71][72][73][74]. ...
Microtubules and their post-translational modifications are involved in major cellular processes. In severe diseases such as neurodegenerative disorders, tyrosinated tubulin and tyrosinated microtubules are in lower concentration. We present here a mechanistic mathematical model of the microtubule tyrosination cycle combining computational modeling and high-content image analyses to understand the key kinetic parameters governing the tyrosination status in different cellular models. That mathematical model is parameterized, firstly, for neuronal cells using kinetic values taken from the literature, and, secondly, for proliferative cells, by a change of two parameter values obtained, and shown minimal, by a continuous optimization procedure based on temporal logic constraints to formalize experimental high-content imaging data. In both cases, the mathematical models explain the inability to increase the tyrosination status by activating the Tubulin Tyrosine Ligase enzyme. The tyrosinated tubulin is indeed the product of a chain of two reactions in the cycle: the detyrosinated microtubule depolymerization followed by its tyrosination. The tyrosination status at equilibrium is thus limited by both reaction rates and activating the tyrosination reaction alone is not effective. Our computational model also predicts the effect of inhibiting the Tubulin Carboxy Peptidase enzyme which we have experimentally validated in MEF cellular model. Furthermore, the model predicts that the activation of two particular kinetic parameters, the tyrosination and detyrosinated microtubule depolymerization rate constants, in synergy, should suffice to enable an increase of the tyrosination status in living cells.
... L'enzyme TTL pourrait être activée directement, bien qu'aucun composé n'ait encore été approuvé, ou indirectement, via l'inhibition de ses inhibiteurs, puisque son activité est diminuée par phosphorylation [134]. Une autre approche consisterait à moduler, en synergie, les voies de signalisation impliquant des facteurs de dépolymérisation telles que les voies PKC, BDNF/TrkB, JNK, Stathmin [176][177][178][179][180]. Il est intéressant de noter que ces voies de signalisation sont dérégulées dans les maladies neurodégénératives [181][182][183][184]. ...
Le processus de découverte de nouveaux médicaments est long, coûteux et très risqué. L’objectif de cette thèse de doctorat est d’améliorer la pertinence des phases primaires de recherche pharmaceutique en développant des méthodes computationnelles. La première contribution porte sur le développement du graphe de connaissances Pegasus afin de capitaliser sur les données pharmaco-biologiques hétérogènes et de provenances multiples du secteur pharmaceutique. Les applications industrielles de Pegasus répondent à des problématiques de projets thérapeutiques et permettent de caractériser des effets hors cibles de perturbateurs, de concevoir une nouvelle expérience, et d’identifier des librairies de criblage focalisées. La deuxième contribution porte sur le développement d’un algorithme d’identification de composés contrôles positifs et d’un algorithme de normalisation afin d’améliorer la conception et l’analyse d’expériences de criblage phénotypiques à haut contenu. Ces algorithmes permettent de normaliser les signatures phénotypiques obtenues à partir de campagnes de criblage et d’intégrer des similarités phénotypiques informatives dans le graphe de connaissances Pegasus. La troisième contribution porte sur le développement d’un modèle mathématique du cycle de tyrosination des microtubules qui explique, d’une part, l’inactivité de composés chimiques dans les cellules montrés actifs hors cellule, et d’autre part, suggère la nécessité d’activer deux réactions de ce cycle, en synergie, pour obtenir un effet dans les modèles cellulaires. Ceci illustre l’apport de la modélisation mathématique pour, d’une part, prédire et comprendre la dynamique contre-intuitive de processus biochimiques qui n’est pas représentable par des graphes de connaissances statiques comme dans Pegasus, et d’autre part, guider la conception de nouvelles expériences de criblage. Les contributions scientifiques et les applications industrielles de cette thèse sont développées dans le cadre des phases primaires de recherche de nouveaux médicaments et ont vocation à s’étendre aux phases cliniques du processus pharmaceutique.
... Stmn2 is an important cytokine, which can regulate the stability of microtubule protein. Stmn2 can stabilize microtubule by MAPK8 phosphate activation (Westerlund et al. 2011). The role of Stmn2 in the process of vascular repair still needs further verification. ...
This study examined the effects of Stmn2 on phenotype transformation of vascular smooth muscle in vascular injury via RNA sequencing and experimental validation. Total RNA was extracted for RNA sequencing after 1, 3 and 5 days of injury to screen the differentially expressed genes (DEGs). Western blot was used to detect the protein expression of Stmn2 and its associated targets. The morphological changes of carotid arteries in rats were examined by hematoxylin and eosin (H&E) staining. The expression of vascular smooth muscle cell (VSMC) phenotype markers smooth muscle alpha-actin (α-SMA), vimentin and OPN were detected by immunohistochemistry. DEGs were related to the extracellular matrix and other cell components outside the plasma membrane. They were associated with protein binding, cytoskeleton protein binding, signal receptor binding and other molecular functions, actin cytoskeleton regulation and other Kyoto Encyclopedia of Genes and Genomes pathways. Stmn2 was identified as the hub gene of actin cytoskeleton pathway and vascular disease, and its expression followed the trend of decreasing initially and increasing afterwards during the progress of vascular injury. Western blot assay showed that the expression of Stmn2 and Tubulin decreased immediately after vascular injury; Stmn2 overexpression significantly up-regulated the expression of osteopontin and α-SMA and vimentin in VSMCs. The results of morphology analysis and immunostaining also showed that Stmn2 overexpression promoted the intima thickening and enhanced the proliferating cell nuclear antigen expression in the injured vascular tissues. In conclusion, our results implied that Stmn2 may play a potential role in vascular injury, which may be associated with VSMC phenotype transformation. Further studies are warranted to determine detailed molecular mechanisms of Stmn2 in vascular injury.
... Furthermore, JNK activity down regulation likewise gives rise to cortical defects. The inhibition of JNK accelerates radial migration and leads to ill-defined cellular organization (Westerlund et al., 2011). ...
The c-Jun N-terminal kinase (JNK) is highly evolutionarily conserved and plays important roles in a broad range of physiological and pathological processes. The WD40-repeat protein 62 (WDR62) is a scaffold protein that recruits different components of the JNK signaling pathway to regulate several human diseases including neurological disorders, infertility, and tumorigenesis. Recent studies revealed that WDR62 regulates the process of neural stem cell mitosis and germ cell meiosis through JNK signaling. In this review we summarize the roles of WDR62 and JNK signaling in neuronal and non-neuronal contexts and discuss how JNK-dependent signaling regulates both processes. WDR62 is involved in various human disorders via JNK signaling regulation, and may represent a promising therapeutic strategy for the treatment of related diseases.
... Originally, JNK was postulated to be the transducer of apoptotic signals in multiple cell types (Hibi et al., 1993;Bogoyevitch et al., 2010); in neurons, this pathway was shown to induce axon degeneration (Shin et al., 2012). Three isoforms of the kinase (JNK1, 2, and 3) have been long been known to be related to cell death; recently, however, there is accumulating evidence that JNK has positive roles in neuronal development in the brain (Waetzig et al., 2006;Tararuk et al., 2006), including neurogenesis (Amura et al., 2005;Xu et al., 2014;Lim et al., 2015), neuronal migration (Kawauchi et al., 2003;Westerlund et al., 2011;Myers et al., 2014Myers et al., , 2020Kawauchi, 2015), polarization (Slater et al., 2013), and axon growth and guidance (Oliva et al., 2006;Shafer et al., 2011;Feltrin et al., 2012;Qu et al., 2013;Sun et al., 2013). As has been hypothesized for other kinases (e.g., PKA, Akt, GSKβ, Cdk5, and Rho), JNK activation and phosphorylation of other substrates (Kawasaki et al., 2018;Ishikawa et al., 2019) is physiologically necessary for axon growth in the developing brain (Yamasaki et al., 2011). ...
Neuronal polarization and growth are developmental processes that occur during neuronal cell differentiation. The molecular signaling mechanisms involved in these events in in vivo mammalian brain remain unclear. Also, cellular events of the neuronal polarization process within a given neuron are thought to be constituted of many independent intracellular signal transduction pathways (the “tug-of-war” model). However, in vivo results suggest that such pathways should be cooperative with one another among a given group of neurons in a region of the brain. Lipid rafts, specific membrane domains with low fluidity, are candidates for the hotspots of such intracellular signaling. Among the signals reported to be involved in polarization, a number are thought to be present or translocated to the lipid rafts in response to extracellular signals. As part of our analysis, we discuss how such novel molecular mechanisms are combined for effective regulation of neuronal polarization and growth, focusing on the significance of the lipid rafts, including results based on recently introduced methods.
... The loss of self-renewal produces premature differentiation and early departure of migratory neurons, observed with supernumerary accumulation in the intermediate zone [8]. These defects resemble those seen in the developing cortices of Jnk1 -/mice [56]. Importantly, an active constituent form of JNK1 rescues the phenotype [8,57]. ...
... Also, at E18 in Jnk1-/mice, multipolar neurons accelerated their transition to the bipolar phase with subsequent pial-directed migration. Finally, at the E18 stage of development, the ventricular zone of Jnk1 -/mice was thinner, and layers 2, 3 and 4 were disarranged ( Figure 4C, bottom panel) [56]. ...
The c-Jun N-terminal Kinases (JNKs) are a group of regulatory elements responsible for the control of a wide array of functions within the cell. In the central nervous system (CNS), JNKs are involved in neuronal polarization, starting from the cell division of neural stem cells and ending with their final positioning when migrating and maturing. This review will focus mostly on isoform JNK1, the foremost contributor of total JNK activity in the CNS. Throughout the text, research from multiple groups will be summarized and discussed in order to describe the involvement of the JNKs in the different steps of neuronal polarization. The data presented support the idea that isoform JNK1 is highly relevant to the regulation of many of the processes that occur in neuronal development in the CNS.