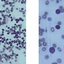
Figure 2 - uploaded by Vin Tangpricha
Content may be subject to copyright.
Pathology from the kidney mass. Tissue from partial nephrectomy revealed renal cell carcinoma, clear cell type, Fuhrman grade 2, magnified at ×200 (above) and ×400 (below).
Source publication
We report a case of a 62-year-old woman with renal cell carcinoma (RCC) presenting with a hypercalcemia-induced coma. A laboratory evaluation indicated nonparathyroid-mediated hypercalcemia with an initial serum calcium level of 18.6 mg/dL. Our patient's parathyroid hormone (PTH)-related peptide level was undetectable. Initial imaging was negative,...
Context in source publication
Context 1
... to cause hypercalcemia such as interleukin-6 (IL-6), prostaglandins, and tumor ne- crosis factor alpha (TNF- α ). This report describes an unusual case of paraneoplastic hypercalcemic coma occurring with renal cell carcinoma (RCC) and a discussion of the differential diagnosis and laboratory evaluation of this condition. A 62-year-old white woman was admitted to an outside medical facility with profound onset of hypercalcemia causing marked mental status changes. The patient had been in good health until the day of her admission when she was found unconscious at her home. She was transported to the emergency department where she was found to have a serum calcium level of 18.6 mg/dL. Her medical history includes chronic bronchiectasis, atrial fibrillation, duodenal ulcer, depression, urolithiasis, polymyalgia rheumatica, and maxillary sinusitis with history of sinus abscess. Her home medications included weekly rotating antibiotics for chronic sinusitis: ciprofloxacin 500 mg bid, followed by cefuroxime axetil 500 mg bid, followed by trimeth- oprim/sulfamethoxazole 160/800 bid, and then followed by doxycycline 100 mg bid; acetaminophen prn; amitriptyline 50 mg qhs; bumetanide 1 mg daily prn; potassium chloride 60 meq bid; spironolactone 25 mg bid; Premarin® 1.25 mg daily; digoxin 0.25 mg bid; trazodone 150 mg prn insomnia; pantoprazole 40 mg daily; docusate 100 mg TID prn constipation; gabapentin 300 mg qhs; metoprolol extended release 100 mg qhs; and prednisolone 15 mg daily. She was treated with approximately 8 L of normal saline; once daily intravenous (IV) furosemide; six doses of subcuta- neous calcitonin at 100 U every 6 hours; 20 mg of solumedrol twice daily; and 90 mg of IV pamidronate. The following morning her serum calcium was 12.9 mg/dL. By the fifth hospital day, her serum calcium was normal at 8.7 mg/dL, and her mental status normalized. Her evaluation revealed a suppressed parathyroid hormone (PTH) level of 2.7 pg/mL, undetectable PTHrP, and low 1,25-dihydroxyvitamin D (1,25 (OH) 2 D). Her hypercalcemia was thought to be because of an underlying malignancy, and she was discharged with plans for outpatient imaging. One month later, she presented again in an unconscious state with a serum calcium level of 11.0 mg/dL. She was treated again with IV fluids and pamidronate. She underwent a comprehensive search for malignancy, which was negative. CT scans of the head, neck, chest, abdomen, and pelvis did not identify suspicious masses. A total body bone scan and skeletal survey x-ray did not identify osteolytic lesions. An upper endoscopy and colonoscopy did not visualize malignant lesions. Mammogram and thyroid uptake scans were normal. The patient was discharged with plans for evaluation at an outside facility. Within 2 months of her initial presentation, the patient was referred to our academic medical center for further evaluation of her hypercalcemia. She denied any history of fevers, night sweats, hematuria, flank pain, or parathyroid disease but did report mild constipation, a history of nephrolithiasis, and 20- lb unintentional weight loss within the previous 3 months. Physical examination revealed a blood pressure of 124/ 64 mmHg, pulse rate of 84/minute, respiratory rate 18/ minute, and temperature at 97.5°F. Head, ear, nose, and throat examination was normal without thyroid enlargement, carotid bruits, or cervical lymphadenopathy. Pulmonary and cardiovascular exams were normal. No costovertebral angle tenderness or flank masses were found on abdominal exam. Her neurological exam was normal except difficulty with concentration. Her skin had no bruises or rash. Repeat laboratory tests included normal blood counts, electrolytes, transaminases, and alkaline phosphatase. Calcium was elevated to 12.1 mg/dL. Albumin was 3.8 g/dL. Intact PTH was 8 pg/mL (normal 10 – 65 pg/mL), PTHrP was undetectable, midsegment PTHrP was undetectable, 1,25(OH) 2 D was suppressed at 6.7 pg/mL (normal 25 – 66 pg/mL), and 25- hydroxy vitamin D was 52 ng/mL. Twenty-four-hour urine calcium was elevated at 400 mg (normal 100 – 300 mg), and phosphorus was 310 mg (normal 400 – 1300 mg). Calcitonin, thyrotropin, and serum protein electrophoresis were normal. The initial CT images performed at the outside facility were not available for review. We suspected a paraneoplastic syndrome due to an occult malignancy because of her weight loss, hypercalcemia, and suppressed PTH value. Our patient underwent a PET scan to investigate for a potential malignancy, revealing reactive inflammation in subcentimeter mediastinal lymph nodes and a 2.7×2.3-cm exophytic mass at the superior pole of the left kidney, which was not previously reported in prior radiologic imaging. A repeat abdominal CT scan was performed and confirmed the left-sided kidney mass (Fig. 1). She underwent a partial left nephrectomy 10 weeks after her initial presentation. Surgical pathology demonstrated a 3.9-cm RCC, clear cell type with Fuhrman ’ s nuclear grade 2 (Fig. 2), with staging of T1aN0M0. After surgery, she became hypocalcemic and required IV infusion of calcium. She was discharged home 3 days after surgery. Twenty-six days after surgery, she was seen in our outpatient endocrinology clinic with a serum calcium level of 10.7 mg/dL. Two months after her surgery, our patient ’ s serum calcium level was measured at 10.4 mg/dL with an intact PTH level of 45 pg/mL (Fig. 3). Follow-up CT scan of the abdomen revealed no recurrence of the RCC. We analyzed presurgery serum samples for TNF α and IL-6 levels by ELISA (Alpco Diagnostics, Salem, NH, USA); however, both of these cytokines were undetectable. Immunohistochem- istry for IL-6 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif, USA) and PTH (BioGenex, San Ramon, Calif, USA) was performed on two separate areas of the tumor with adjacent normal kidney. Normal renal parenchyma and parathyroid adenoma served as positive controls for IL-6 and PTH, respectively. Negative controls were performed by replacing the primary antibody with buffer. The RCC was negative for both IL-6 and PTH immunohistochemistry. TNF α and PTHrP stains were not available. Renal cell carcinoma accounts for 2% of cancers worldwide. The incidence of RCC has risen over the past several decades, due largely to incidental detection by imaging modalities such as CT scan, ultrasound, and MRI. 2 Nearly 50% of cases are identified incidentally by imaging, 3 and accuracy of diagnosis with CT scan is greater than 95%. 4 Given this high level of accuracy and the size of her tumor, we must attribute our patient ’ s initial negative CT scan to human error. When we finally obtained the original images, the tumor was, in fact, present. Detection of unknown primary tumors ranges from 33% to 57% with PET-CT, 5 – 7 but there is a paucity of data on the use of PET-CT in paraneoplastic syndromes. The most common clinical presentations of RCC are hematuria (50% to 60% of patients), abdominal pain (40%), and a palpable abdominal mass (30%); this classic triad of symptoms is present in less than 10% of cases. 8 Paraneoplastic manifestations of RCC, including hypercalcemia, polycythemia, hepat- ic dysfunction, amyloidosis, fever, and weight loss, are present in up to 20% of patients. 3,8,9 Unusual presentations of RCC include pancreatitis, 10 life-threatening polyneuropathy, 11 gas- trointestinal bleeding, 12 and polymyalgia rheumatica, 13 but hypercalcemia-induced coma has not been reported. In retrospective studies to determine the etiology of hypercalcemia in all patients, 28 – 36% of cases are attributed to malignancy. 14,15 Of patients with known malignancy who develop hypercalcemia, the most common malignancies are lung and kidney. 15,16 Hypercalcemia is the most common paraneoplastic complication of RCC, and approximately 17% of all patients with RCC develop hypercalcemia during the course of their disease. 17 – 19 Albright 20 first reported hypercalcemia occurring in a patient with RCC in 1941. He postulated that the hypercalcemia was because of a humoral factor secreted by the tumor. Later reports confirmed that a protein was secreted from RCC, which could increase urinary cyclic adenosine monophosphate levels similar to PTH. 21,22 In the late 1980s, the substance was identified and named PTHrP. 23 Parathyroid hormone-related peptide is present in several isoforms. The N-terminal sequence is identical in 8 of the first 13 amino acids compared to PTH and binds to the PTH/PTHrP receptor. 24 Parathyroid hormone-related peptide undergoes posttranscriptional modification, dividing itself into the N- terminal fragment, midsection, and C-terminal fragment. 25 The C-terminal fragment is cleared by the kidney and is present in higher serum concentrations, particularly in kidney failure. There is some evidence that the C-terminal fragment inhibits RCC growth, whereas the N-peptide stimulates growth. 24,26 In addition, the C-peptide plays an inhibitory role in calcium metabolism. 26 It is reported that PTHrP levels are elevated in up to 47% of patients with malignancy and hypercalcemia. 27 Parathyroid hormone-related peptide is elevated in up to 15% of all patients with RCC, 19 but it is also expressed in normal tissue. 28 This peptide binds to the common PTH/PTHrP receptor to cause increased resorption of bone and increased renal calcium absorption. 29 – 31 Other humoral factors that have been associated with hypercalcemia in RCC include IL-6, IL-1, TNF α , and trans- forming growth factor alpha and beta. 32,33 Many studies have attempted to determine the exact role of IL-6 in hypercalcemia of RCC. Interleukin-6 has been shown to activate osteoclastic bone resorption, and it acts synergistically when coexpressed with PTHrP. Some studies suggest that IL-6 stimulates tumor growth. 34,35 It is not clear whether this cytokine causes hypercalcemia either directly or indirectly by increasing the effect of PTHrP on bone resorption. 36 – 38 At this time, IL-6- mediated ...
Citations
... Some studies have reported that IL-6 acts synergistically when expressed with PTHrP to activate osteoclasts resulting in bone resorption. Prostaglandins have also been implicated in the pathogenesis of hypercalcemia in patients with RCC by stimulating bone resorption [4]. However, IL-6 and prostaglandins were not measured in our patient due to a lack of available testing. ...
Hypercalcemia of malignancy frequently occurs in patients with solid tumors as a paraneoplastic syndrome known as humoral hypercalcemia of malignancy (HHM), caused by the secretion of parathyroid hormone-related peptide (PTHrP). On the other hand, 1,25-dihydroxyvitamin D [1,25(OH)2D]-mediated hypercalcemia is a less common cause of hypercalcemia of malignancy and is mostly observed in lymphoma patients. Here, we report an interesting case of a 77-year-old male nursing home resident with suspected renal cell carcinoma (RCC) presenting with severe hypercalcemia (18.7 mg/dL), which was initially presumed to be HHM. However, workup revealed nonsuppressed parathyroid hormone, low PTHrP, and elevated 25-hydroxyvitamin D and 1,25(OH)2D levels. Steroids were initiated due to an inadequate response to bisphosphonate therapy and elevated vitamin D metabolites, resulting in further reduction in serum calcium levels. This case highlights the need to consider multiple concurrent etiologies in the differential diagnosis of severe hypercalcemia, including the possible role of calcitriol-mediated hypercalcemia in RCC.
... This study proposes that the extracellular Ca 2+ contributes to ccRCC progression by stimulating autophagy. Interestingly, hypercalcemia has been developed in patients with ccRCC (46,47). Specific Ca 2+ -signaling pathways have been implicated in multidrug resistance and tumor microenvironments (12). ...
Deregulation of Ca²⁺ signaling has been regarded as one of the key features of cancer progression. Lysine‐deficient protein kinase 1 (WNK1), a major regulator of renal ion transport, regulates Ca²⁺ signaling through stimulating the phosphatidylinositol 4‐kinase IIIα (PI4KIIIα) to activate Gαq‐coupled receptor/PLC‐β signaling. However, the contribution of WNK1‐mediated Ca²⁺ signaling in the development of clear‐cell renal‐cell carcinoma (ccRCC) is yet unknown. We found that the canonical transient receptor potential channel (TRPC)6 was widely expressed in ccRCC tissues and functioned as a primary Ca²⁺ influx mechanism. We further identified that the expressions of WNK1, PI4KIIIcα, TRPC6, and the nuclear factor of activated T cells cytoplasmic 1 (NFATc1) were elevated in the tumor tissues compared with the adjacent normal tissues. WNK1 expression was directly associated with the nuclear grade of ccRCC tissues. Functional experiments showed that WNK1 activated TRPC6‐mediated Ca²⁺ influx and current by stimulating PI4KIIIα. Notably, the inhibition of WNK1‐mediated TRPC6 activation and its downstream substrate calcineurin attenuated NFATc1 activation and the subsequent migration and proliferation of ccRCC. These findings revealed a novel perspective of WNK1 signaling in targeting the TRPC6‐NFATc1 pathway as a therapeutic potential for renal‐cell carcinoma.—Kim, J.‐H., Hwang, K.‐H., Eom, M., Kim, M., Park, E. Y., Jeong, Y., Park, K.‐S., Cha, S.‐K. WNK1 promotes renal tumor progression by activating TRPC6‐NFAT pathway. FASEB J. 33, 8588–8599 (2019). www.fasebj.org
... Elevated levels of TNF-α have been identified in patients with HHM in the absence of PTHrP, suggesting it should be further investigated as a single mediator of HHM. However, synergistic and/or cooperative actions with PTHrP and other cytokines are primarily thought to result in TNF-α-associated HHM [15,68]. ...
Cancer-associated hypercalcemia (CAH) is a frequently-occurring paraneoplastic syndrome that contributes to substantial patient morbidity and occurs in both humans and animals. Patients with CAH are often characterized by markedly elevated serum calcium concentrations that result in a range of clinical symptoms involving the nervous, gastrointestinal and urinary systems. CAH is caused by two principle mechanisms; humorally-mediated and/or through local osteolytic bone metastasis resulting in excessive calcium release from resorbed bone. Humoral hypercalcemia of malignancy (HHM) is the most common mechanism and is due to the production and release of tumor-associated cytokines and humoral factors, such as parathyroid hormone-related protein (PTHrP), that act at distant sites to increase serum calcium concentrations. Local osteolytic hypercalcemia (LOH) occurs when primary or metastatic bone tumors act locally by releasing factors that stimulate osteoclast activity and bone resorption. LOH is a less frequent cause of CAH and in some cases can induce hypercalcemia in concert with HHM. Rarely, ectopic production of parathyroid hormone has been described. PTHrP-mediated hypercalcemia is the most common mechanism of CAH in human and canine malignancies and is recognized in other domestic species. Spontaneous and experimentally-induced animal models have been developed to study the mechanisms of CAH. These models have been essential for the evaluation of novel approaches and adjuvant therapies to manage CAH. This review will highlight the comparative aspects of CAH in humans and animals with a discussion of the available animal models used to study the pathogenesis of this important clinical syndrome.
... Paraneoplastic manifestations are present in 10-40 % of patients with renal cell carcinoma (RCC) [1,2]. A wide range of PNS has been associated with RCC including metabolic syndromes, such as hypercalcemia [3], hyperglycemia [4], renin production [5], prolactin production [6], hepatic syndromes (Stauffer's syndrome) [7], hematologic syndromes (anemia [8], polycythemia [9]), and neuromuscular syndromes (polyneuromyopathy) [10]. The identification of such syndromes is essential, given that they can lead to misdiagnosis, they may be a clue for the diagnosis of otherwise asymptomatic malignancy, and they may be useful for monitoring cancer progression (as a tumor marker) [1]. ...
Objectives
To analyze the association of paraneoplastic syndromes (PNS) with progression-free (PFS) and cancer-specific survival (CSS) among patients with renal cell carcinoma (RCC) undergoing nephrectomy. Methods
We performed a retrospective analysis of 2865 patients undergoing nephrectomy for localized RCC at Mayo Clinic from 1990 to 2010. PNS analyzed were anemia, polycythemia, hypercalcemia, recent-onset hypertension, and liver dysfunction. PFS and CSS were estimated using Kaplan–Meier method and compared with Cox proportional hazard models, unadjusted and adjusted for clinicopathologic features. ResultsA total of 661 (23 %) patients had anemia, 37 (1 %) had polycythemia, 177 (9 %) had hypercalcemia, 51 (2 %) had recent-onset hypertension, and 224 (10 %) had liver dysfunction at time of nephrectomy. Patients with PNS were more likely to have high-grade tumors and advanced disease stages. A total of 675 (24 %) patients developed progression and 1171 (41 %) died of RCC, over a median follow-up of 8.2 years. On univariable analysis, the presence of any PNS was associated with inferior CSS [hazard ratio (HR) = 1.86, p = 0.007] and a trend toward shorter PFS (HR = 1.33, p = 0.07) compared with patients without PNS. Specifically, anemia, polycythemia, hypercalcemia, and liver dysfunction were each associated with inferior CSS and PFS (all p < 0.05). However, on multivariable analysis PNS (overall or each individual syndrome) did not remain independently associated with CSS or PFS. Conclusions
Patients with RCC undergoing nephrectomy presenting with PNS have worse oncologic outcome than those with incidentally found tumors. However, the adverse outcome among PNS patients seems to be largely explained by adverse pathologic features of these tumors.
... In the present study, multivariate analysis revealed that PS, hypercalcemia and MVI are the independent prognostic factors. Hypercalcemia is the most frequent paraneoplastic complication of mRCC, being manifested in up to 20% of RCC patients during the course of their disease (20,21) and known to imply a poor prognosis because of its frequent association with disseminated disease. Previous reports suggested that humoral factors such as PTHrP, (22) IL-6 (23) and prostaglandins (24) may play roles in hypercalcemia either directly or indirectly by increasing bone resorption. ...
The RCC-SELECT study showed the correlation between single nucleotide polymorphisms (SNPs) in STAT3 gene and survival in metastatic renal cell carcinoma (mRCC) patients with first-line interferon-α (IFN-α). In that study, even patients with STAT3 SNPs linked to shorter overall survival (OS) exhibited remarkably improved prognosis. All 180 patients evaluated in the above study were further analysed for correlation between OS and demographics/clinicopathological parameters. OS was estimated using the Kaplan-Meier method. Associations between OS and potential prognostic factors were assessed using the log-rank test and the Cox proportional hazards model. The median OS was 42.8 months. Univariate analysis showed that worse Eastern Cooperative Oncology Group-performance status (ECOG-PS), high T stage, regional lymph node metastasis, distant metastasis, higher grade, infiltrative growth pattern, the presence of microscopic vascular invasion (MVI), hypercalcemia, anemia, thrombocytopenia and elevated C-reactive protein were significantly associated with OS. Multivariate analysis revealed that ECOG-PS [hazard ratio (HR) = 3.665, P = 0.0004], hypercalcemia [HR = 6.428, P = 0.0005] and the presence of MVI [HR = 2.668, P = 0.0109] were jointly significant poor prognostic factors. This is the first study analysing prognostic factors of mRCC patients with first-line IFN-α using large cohort of the prospective study. The present study suggests that first-line IFN-α is still a useful therapy for mRCC even in the era of molecular targeted therapy. This article is protected by copyright. All rights reserved.
... This peptide binds to the common PTH/PTHrP receptor to cause increased resorption of bone and increased renal calcium absorption. Other humoral factors that have been associated with hypercalcemia in RCC include interleukin-6 (IL-6), IL-1, tumor necrosis factor-α, and transforming growth factor alpha and beta [4]. ...
... The most notable among these are dearanged kidney function with or without renal arteriovenous malformation, pathological fractures from bony metastasis, anemia secondary to decreased erythropoietin, hypertension from secretion of renin by the tumor cells, hypercalcemia and paraneoplastic disease. 5 Complete obstruction of IVC, though not a common presentation, can manifest with clinical features, like recurrent pulmonary emboli, pedal edema, renal or hepatic dysfunction, malabsorption and engorgement of abdominal veins. These patients can tolerate clamping of IVC without significant hemodynamic compromise. ...
Renal cell carcinoma (RCC) has a tendency to invade inferior vena cava and thereby reach the right heart. This may necessitate a combined surgical procedure. These procedures impose a challenge to the anesthesiologist and may require the use of veno-venous or cardiopulmonary bypass (CPB). Among the serious and feared complication is embolization of the thrombus during mobilization of the tumor causing a massive pulmonary embolism. Transesophageal echocardiography (TEE) not only provide accurate identification and definition of the cranial extent of the tumor, but may also provide continuous monitoring of the hemodynamic status and cardiac complications during surgical manipulation of tumor. In this case report, we have described TEE helped in recognizing not only the extent of the tumor but also (the adequacy of removal of the tumor thrombus) diagnose the residual tumor after removal during right radical nephrectomy.
How to cite this article
Negi SL, Dutta V, Puri GD, Madhavan S, Mohan R. Role of Transesophageal Echocardiography in Detection of Residual Thrombus in Renal Cell Carcinoma extending into Right Atrium. J Perioper Echocardiogr 2015;3(1):35-38.
... Hypercalcemia is a well documented paraneoplastic syndrome that also occurs in ccRCC. Parathyroid hormone-related protein (PTHrP) produced by cancer cells is considered to be the predominant cause (Pepper et al., 2007). PTHrP levels are higher in ccRCC compared with other histological types, and ccRCCs express PTHrP in 95% of cases (Gotoh et al., 1993). ...
Autophagy promotes tumor growth by generating nutrients from the degradation of intracellular structures. Here we establish, using shRNAs, a dominant-negative mutant, and a pharmacologic inhibitor, mefenamic acid (MFA), that the Transient Receptor Potential Melastatin 3 (TRPM3) channel promotes the growth of clear cell renal cell carcinoma (ccRCC) and stimulates MAP1LC3A (LC3A) and MAP1LC3B (LC3B) autophagy. Increased expression of TRPM3 in RCC leads to Ca(2+) influx, activation of CAMKK2, AMPK, and ULK1, and phagophore formation. In addition, TRPM3 Ca(2+) and Zn(2+) fluxes inhibit miR-214, which directly targets LC3A and LC3B. The von Hippel-Lindau tumor suppressor (VHL) represses TRPM3 directly through miR-204 and indirectly through another miR-204 target, Caveolin 1 (CAV1).
Copyright © 2014 Elsevier Inc. All rights reserved.
... Other paraneoplastic manifestations of RCC include hypercalcemia, polycythemia, hepatic dysfunction, amyloidosis, fever, and weight loss. [5,6] RCC can be detected on intravenous pyelography, CT, ultrasound. [4] CCCs are associated with loss of genetic material on 3p chromosome. ...
Clear cell carcinomas (CCCs) are the common renal cell tumors. Sarcomatoid change in the clear cell tumors is uncommon. Not only this sarcomatoid change raises the grade of the tumors thereby adversely affecting the prognosis but it also may pose diagnostic problems. We discuss a case of diabetic female who presented with abdominal mass and polycythemia. She presented with grade IV and stage I CCC with sarcomatoid change. The tumor belonged to stage I. Left sided nephrectomy and splenectomy was done to treat the tumor. Postoperative radiotherapy was done. The patient was followed-up for a period of 2 years, no recurrence was noted. We report this case due to its unusual clinical and histological presentation.
... Hypercalcaemia is a common complication of RCC, occurring in 20% of cases [1]. The aetiology is related to the secretion of humoral factors includ-ing parathyroid hormone-related peptide, IL-6, IL-1 and TNF␣ by the tumour [1]. ...
... Hypercalcaemia is a common complication of RCC, occurring in 20% of cases [1]. The aetiology is related to the secretion of humoral factors includ-ing parathyroid hormone-related peptide, IL-6, IL-1 and TNF␣ by the tumour [1]. In advanced metastatic disease these effects may be compounded by the osteoclast mediated destruction of bone [2]. ...