Fig 1 - available via license: Creative Commons Attribution 4.0 International
Content may be subject to copyright.

Oxidative states pathways of PANI polymer
Source publication
Environmental management demands innovative techniques for its protection and treatment. The essential agreement of the modern world is to overcome every issue in a sustainable way. The two major financial problems in this area are water pollution and material corrosion. Persistent, organic compounds such as pesticides have devastating effects on t...
Contexts in source publication
Context 1
... to its highly branched structure, polyaniline ( Fig. 1) is one of the ECPs that has received the most research attention (Kumari Jangid et al., 2020). Incredible qualities such as good processability, high conductivity, a large surface area, and chemical stability of the PANI ( Ho et al., 2021;Tarawneh et al., 2020;Tran et al., 2022) make it attractive and suitable for environmental ...
Context 2
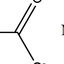
... et al., 2018). PANI can occur in three different oxidation forms, such as pernigraniline, leucoemeraldine, and emeraldine ( Beygisangchin et al., 2021). By employing a different technique to optimize the parameters, polyaniline's oxidizing potential can be changed ( Beygisangchin et al., 2021). The first aniline base is leucoemeraldin (LB) (Fig. 1A), which is in a reduced oxidation state. Even though, leucoemeraldin is unstable and very reactive in atmospheric conditions. The next one is emeraldine (EB) (Fig. 1B), which is partly oxidized and chemically stable. This molecule is the only one that is conductive, besides leucoemeraldine and the fully oxidized perniganiline (PB) (Fig. ...
Context 3
... a different technique to optimize the parameters, polyaniline's oxidizing potential can be changed ( Beygisangchin et al., 2021). The first aniline base is leucoemeraldin (LB) (Fig. 1A), which is in a reduced oxidation state. Even though, leucoemeraldin is unstable and very reactive in atmospheric conditions. The next one is emeraldine (EB) (Fig. 1B), which is partly oxidized and chemically stable. This molecule is the only one that is conductive, besides leucoemeraldine and the fully oxidized perniganiline (PB) (Fig. 1D) (Stejskal & Gilbert, 2002). So, the preparation and synthesis of this polymeric material require careful conditions. The EB doped with inorganic acid (e.g., ...
Context 4
... (LB) (Fig. 1A), which is in a reduced oxidation state. Even though, leucoemeraldin is unstable and very reactive in atmospheric conditions. The next one is emeraldine (EB) (Fig. 1B), which is partly oxidized and chemically stable. This molecule is the only one that is conductive, besides leucoemeraldine and the fully oxidized perniganiline (PB) (Fig. 1D) (Stejskal & Gilbert, 2002). So, the preparation and synthesis of this polymeric material require careful conditions. The EB doped with inorganic acid (e.g., hydrochloric acid) or organic acid (e.g., sulfosalicylic acid) is easily converted into an emeraldine salt (ES) form of PANI. ESs possess an increased conductivity due to the ...
Context 5
... preparation and synthesis of this polymeric material require careful conditions. The EB doped with inorganic acid (e.g., hydrochloric acid) or organic acid (e.g., sulfosalicylic acid) is easily converted into an emeraldine salt (ES) form of PANI. ESs possess an increased conductivity due to the protonation of imine nitrogen in the EB by the acid (Fig. 1C) (Yang et al., 2020(Yang et al., ) et al., 2020. Moreover, the partially oxidated ES form of the PANI is catalytically active compared to LB, and PB and has high electron transfer performance with low E g due to the formation of the polaron and bipolaron band ( Belabed et al., 2013). This formation is due to charge defects created as a ...
Context 6
... the study from Janaki et. al. (Janaki et al., 2012), PANI/chitosan composite was thoughtfully synthesized and structurally characterized. The prepared composite showed high efficiency to adsorb dyes congo red, coomassie brilliant blue, and remazol brilliant blue R. Efficiency removal of mentioned dyes was in the range of 95 -99%. ...
Context 7
... (Asgari et al., 2019) developed a ZnO/PANI composite and tested its ability to degrade metronidazole under UV and visible light. By monitoring degradation kinetics, it was demonstrated that UV light possesses a better capacity to induce faster decomposition (97%) of target antibiotics than visible light (120 and 150 min, respectively). ...
Citations
... The most promising techniques nowadays are advanced oxidative processes (AOPs), where photocatalysis has almost a mandatory role. Different organic and inorganic-based materials are utilized as photocatalysts with the aim to promote the decomposition of various pollutants [13,14]. Rapid production of complex, persistent organic hazardous chemicals has caused an increased demand for enhanced photosensitive materials that can catalyze pollutant decomposition over their surfaces. ...
This work aimed to investigate the influence of modified titanium(IV) oxide by different nanosized particles on photocatalytic capacity to decompose the chosen organic pollutant under simulated sunlight. For that purpose, rutile-phased titanium(IV) oxide (r-TiO2) was decorated with iron vanadate (FeVO4/r-TiO2) and vanadium-substituted goethite (Fe1-xVxOOH/r-TiO2). The obtained composites were characterized by field emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, X ray powder diffraction, Brunauer-Emmett-Teller, Fourier transform infrared spectroscopy - attenuated total reflec-tance and ultraviolet-visible diffuse reflectance spectroscopy techniques. Both synthesized photocatalysts showed higher photoactivity than the base r-TiO2 for the degradation of the target contaminant - thiophanate-methyl (2.5 h vs. 5 h). During the tests, parameters like the irradiation time, catalysts amount, and pesticide concentration were systematically investigated. Furthermore, photocatalysts were applied in multicycle degradation tests for examining their effectiveness during exploitation time. Monitoring of the removal rate was performed both by UV/visible spectrometry and high-performance liquid chromatography (HPLC). In order to prove completion of fungicide degradation chemical oxygen demand was measured in the course of the photocatalytic experiment. The final concentration of the observed contaminant in treated samples was under the prescribed legislative level. The fabricated materials displayed great reliability, durability and photocatalytic activity repre-senting good potentials for implementing this process in real wastewater treatment plants.