Figure - available from: International Journal of Nanomedicine
This content is subject to copyright. Terms and conditions apply.
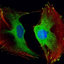
Morphology of human dermal fibroblasts on days 1 and 3 after seeding on nonmodified PLA membranes, or on PLA membranes with a fibrin nanocoating (F). Notes: Cells cultivated in the standard cell culture medium. The PS was used as a control material. Cells stained with Texas Red C2-Maleimide and Hoechst #33258. Olympus IX 51 microscope, obj 10×, DP 70 digital camera. Abbreviations: PLA, polylactide; PS, polystyrene culture dish; obj, objective.
Source publication
Fibrin plays an important role during wound healing and skin regeneration. It is often applied in clinical practice for treatment of skin injuries or as a component of skin substitutes. We prepared electrospun nanofibrous membranes made from poly(l-lactide) modified with a thin fibrin nanocoating. Fibrin surrounded the individual fibers in the memb...
Citations
... It was possibly also because of the cells' mitogenic properties. Collagen I's mRNA expression, the total amount of collagen produced, and its deposition as ECM on the membrane surface were all boosted by fibrin [11,36]. ...
The regeneration of skin because of numerous sorts of injuries such as burns, wounds, tissue damage, and eczema is regarded as vital; nevertheless, the process of healing and remodeling can be impeded by several reasons. The cutting-edge of nanofibrous technology offers the opportunity to repurpose and innovate new therapies and improve the effectiveness of the available medical treatments. There may be less need for skin transplants and skin grafts as regenerative medicine advances using biopolymeric materials. Skin injuries can be difficult to treat, especially when it comes to managing wounds. The fabrication of different dosage forms such as film, foam, sponge, hydrogel, and nanofiber membranes using scaffolding material made from synthetic and natural polymers is considered a treatment method for wounds. Scaffolds have found applicability in tissue engineering, where the materials are fabricated into artificial tissue that stimulates growth factors and enhances tissue regeneration. Among these materials, nanofibers possess a unique structure of small pore size and high porosity, thus protecting wounds from infections and ensuring unrestricted transportation of gas and liquid molecules. We have described several polymers in this study that have been used to create scaffolds made of electrospun nanofibers. These scaffolds are studied and discussed using different polymers to show the effect on skin repair mechanisms and investigate the remodeling abilities aiming to potentially show a foundation for clinical applications and industrial manufacturing. The extracellular matrix (ECM) and the nanofiber structure share many similarities, and the use of different types of polymers, including biopolymers like collagen and chitosan and biodegradable polymers like polycaprolactone, polylactic acid, and polyvinyl alcohol, helps to make the field relevant to skin regeneration and remodeling. Hence, this review summarized and discussed the polymeric nanofibers such as collagen, polycaprolactone, poly vinyl alcohol reporting pre-clinical trials of wound healing and skin regeneration.
... Additionally, the fibrin network links with various cell types like smooth muscle cells and endothelial cells via integrin adhesion receptors. Moreover, it interacts with cell adhesion-mediating ECM proteins like fibronectin and vitronectin, facilitating fibroblast adhesion [53]. ...
The skin is subject to damage from the surrounding environment. The repair of skin wounds can be very challenging due to several factors such as severe injuries, concomitant infections, or comorbidities such as diabetes. Different drugs and wound dressings have been used to treat skin wounds. Tissue engineering, a novel therapeutic approach, revolutionized the treatment and regeneration of challenging tissue damage. This field includes the use of synthetic and natural biomaterials that support the growth of tissues or organs outside the body. Accordingly, the demand for polymer-based therapeutic strategies for skin tissue defects is significantly increasing. Among the various 3D scaffolds used in tissue engineering, hydrogel scaffolds have gained special significance due to their unique properties such as natural mimicry of the extracellular matrix (ECM), moisture retention, porosity, biocompatibility, biodegradability, and biocompatibility properties. First, this article delineates the process of wound healing and conventional methods of treating wounds. It then presents an examination of the structure and manufacturing methods of hydrogels, followed by an analysis of their crucial characteristics in healing skin wounds and the most recent advancements in using hydrogel dressings for this purpose. Finally, it discusses the potential future advancements in hydrogel materials within the realm of wound healing.
... Simultaneously, the first week following surgery is crucial for a successful intervention time window and effective clinical adhesion prevention [22][23][24]. Thus, the key to effectively intervening in tissue adhesion is to inhibit fibrin deposition and fibroblast adhesion at the injured site in the early and late stages [25][26][27]. ...
Abdominal adhesion is a frequent clinical issue with a high incidence rate and consequences following intra-abdominal surgery. Although many anti-adhesion materials have been used in surgical procedures, additional research is still needed to determine which ones have the most robust wet tissue adhesion, the best anti-postoperative adhesion, and the best anti-inflammatory properties. We have developed an excellent tissue adhesion and anti-swelling polyvinyl alcohol-chitosan hydrogel (AS hydrogel). According to in vitro cell testing, AS hydrogel significantly decreased inflammation around cells and exhibited good biocompatibility. Further, we assessed how well AS hydrogel prevented intraperitoneal adhesion using a rabbit model with cecum and abdominal wall injuries. According to the data, AS hydrogel has excellent anti-inflammatory and biodegradability properties compared to the control group. It can also prevent intestinal and abdominal wall injuries from occurring during surgery. Based on these results, hydrogel appears to be a perfect new material to prevent postoperative abdominal wall adhesion.
... Skin, the "first line of defense" in the human body, acts as a shield against the external environment and assists in thermal regulation and hydration retention (Bacakova et al., 2016). In addition, it helps to prevent microbial attack via infiltration of immune cells, including neutrophils or macrophages, and restoring damaged tissue function through rapid regeneration (Chaudhari et al., 2016). ...
Skin tissue engineering possesses great promise in providing successful wound injury and tissue loss treatments that current methods cannot treat or achieve a satisfactory clinical outcome. A major field direction is exploring bioscaffolds with multifunctional properties to enhance biological performance and expedite complex skin tissue regeneration. Multifunctional bioscaffolds are three-dimensional (3D) constructs manufactured from natural and synthetic biomaterials using cutting-edge tissue fabrication techniques incorporated with cells, growth factors, secretomes, antibacterial compounds, and bioactive molecules. It offers a physical, chemical, and biological environment with a biomimetic framework to direct cells toward higher-order tissue regeneration during wound healing. Multifunctional bioscaffolds are a promising possibility for skin regeneration because of the variety of structures they provide and the capacity to customise the chemistry of their surfaces, which allows for the regulated distribution of bioactive chemicals or cells. Meanwhile, the current gap is through advanced fabrication techniques such as computational designing, electrospinning, and 3D bioprinting to fabricate multifunctional scaffolds with long-term safety. This review stipulates the wound healing processes used by commercially available engineered skin replacements (ESS), highlighting the demand for a multifunctional, and next-generation ESS replacement as the goals and significance study in tissue engineering and regenerative medicine (TERM). This work also scrutinise the use of multifunctional bioscaffolds in wound healing applications, demonstrating successful biological performance in the in vitro and in vivo animal models. Further, we also provided a comprehensive review in requiring new viewpoints and technological innovations for the clinical application of multifunctional bioscaffolds for wound healing that have been found in the literature in the last 5 years.
... A blood clot, which contains a crosslinked fibrin mesh, activated platelets, erythrocytes, and leukocytes, is formed to stop bleeding and later serves as a temporary scaffold for cells and as a reservoir of GFs. 13 The ability of fibrin to bind and retain GFs activity has been published previously. 14,15 The fibrin coating was also shown to serve as an excellent substrate for cell growth on PLA nanofibers 16 and on PLCL/PCL nanofibrous membranes. 17 We previously reported that NF with fibrin containing 20% of hPL enhanced proliferation and differentiation of human keratinocytes. ...
... Therefore, we chose a supporting layer based on electrospun PLCL/PCL nanofibers for the fibrin coating. We have already reported that the fibrin coating serves as a great substrate for cell growth on PLA nanofibers 16 and PLCL/PCL nanofibrous membranes. 17 In addition, the fibrin coating (referred as NF0) was enriched with hPL at different concentrations (v/v): 1% (NF1), 5% (NF5), 10% (NF10), 20% (NF20), 50% (NF50), and 100% (NF100); and by the addition of growth factors FGF and/or VEGF (Figure 1). ...
Introduction
The formation of diabetic ulcers (DU) is a common complication for diabetic patients resulting in serious chronic wounds. There is therefore, an urgent need for complex treatment of this problem. This study examines a bioactive wound dressing of a biodegradable electrospun nanofibrous blend of poly(L-lactide-co-ε-caprolactone) and poly(ε-caprolactone) (PLCL/PCL) covered by a thin fibrin layer for sustained delivery of bioactive molecules.
Methods
Electrospun PLCL/PCL nanofibers were coated with fibrin-based coating prepared by a controlled technique and enriched with human platelet lysate (hPL), fibroblast growth factor 2 (FGF), and vascular endothelial growth factor (VEGF). The coating was characterized by scanning electron microscopy and fluorescent microscopy. Protein content and its release rate and the effect on human saphenous vein endothelial cells (HSVEC) were evaluated.
Results
The highest protein amount is achieved by the coating of PLCL/PCL with a fibrin mesh containing 20% v/v hPL (NF20). The fibrin coating serves as an excellent scaffold to accumulate bioactive molecules from hPL such as PDGF-BB, fibronectin (Fn), and α-2 antiplasmin. The NF20 coating shows both fast and a sustained release of the attached bioactive molecules (Fn, VEGF, FGF). The dressing significantly increases the viability of human saphenous vein endothelial cells (HSVECs) cultivated on a collagen-based wound model. The exogenous addition of FGF and VEGF during the coating procedure further increases the HSVECs viability. In addition, the presence of α-2 antiplasmin significantly stabilizes the fibrin mesh and prevents its cleavage by plasmin.
Discussion
The NF20 coating supplemented with FGF and VEGF provides a promising wound dressing for the complex treatment of DU. The incorporation of various bioactive molecules from hPL and growth factors has great potential to support the healing processes by providing appropriate stimuli in the chronic wound.
... Traditional approaches to skin tissue engineering mostly use scaffold-free strategies. Scaffold-free 3D engineering constructs are aggregates of cells resulting from the self-assembly of one or more cell types [11,[16][17][18]. Researchers usually cultivate tissues or organs in cell culture vessels. ...
Tissue engineering is an enabling technology that can be used to repair, replace, and regenerate different types of biological tissues and holds great potential in various biomedical applications. As the first line of defense for the human body, the skin has a complex structure. When skin is injured by trauma or disease, the skin tissues may regenerate under natural conditions, though often resulting in irreversible and aesthetically unpleasant scarring. The development of skin tissue engineering strategies was reviewed. Although the traditional approaches to skin tissue engineering have made good progress, they are still unable to effectively deal with large-area injuries or produce full-thickness grafts. In vitro three-dimensional (3D) skin constructs are good skin equivalent substitutes and they have promoted many major innovative discoveries in biology and medicine. 3D skin manufacturing technology can be divided into two categories: scaffold-free and scaffold-based. The representatives of traditional scaffold-free approaches are transwell/Boyden chamber approach and organotypic 3D skin culture. Because of its low cost and high repeatability, the scaffold-free 3D skin model is currently commonly used for cytotoxicity analysis, cell biochemical analysis, and high-throughput cell function. At present, many drug experiments use artificial skin developed by traditional approaches to replace animal models. 3D bioprinting technology is a scaffold-based approach. As a novel tissue manufacturing technology, it can quickly design and build a multi-functional human skin model. This technology offers new opportunities to build tissues and organs layer by layer, and it is now used in regenerative medicine to meet the increasing need for tissues and organs suitable for transplantation. 3D bioprinting can generate skin substitutes with improved quality and high complexity for wound healing and in vitro disease modeling. In this review, we analyze different types of conventional techniques to engineer skin and compare them with 3D bioprinting. We also summarized different types of equipment, bioinks, and scaffolds used in 3D skin engineering. In these skin culture techniques, we focus on 3D skin bioprinting technology. While 3D bioprinting technology is still maturing and improvements to the techniques and protocols are required, this technology holds great promise in skin-related applications.
... Natural fibrin was selected as the scaffold material, owing to its well-known biocompatibility and biodegradability. [21][22][23][24] Fibrin is also an essential material of hemostatic plugs to assist skin regeneration during wound healing. [25][26][27][28][29] In a typical experiment, 1 Â 10 6 cells mL À1 of HaCaT cells were first encapsulated in a 1.5 μL fibrin hydrogel at a concentration of 10 mg mL À1 . ...
A current challenge in 3D bioprinting of skin equivalents is to recreate the distinct basal and suprabasal layers and promote their direct interactions. Such a structural arrangement is essential to establish 3D stratified epidermis disease models, such as for the autoimmune skin disease pemphigus vulgaris (PV), which targets the cell–cell junctions at the interface of the basal and suprabasal layers. Inspired by epithelial regeneration in wound healing, a method that combines 3D bioprinting and spatially guided self‐reorganization of keratinocytes is developed to recapture the fine structural hierarchy that lies in the deep layers of the epidermis. Herein, keratinocyte‐laden fibrin hydrogels are bioprinted to create geographical cues, guiding dynamic self‐reorganization of cells through collective migration, keratinocyte differentiation, and vertical expansion. This process results in a region of self‐organized multilayers (SOMs) that contain the basal‐to‐suprabasal transition, marked by the expressed levels of different types of keratins that indicate differentiation. Finally, the reconstructed skin tissue as an in vitro platform to study the pathogenic effects of PV is demonstrated, illuminating a significant difference in cell–cell junction dissociation induced by PV antibodies in different epidermis layers, which indicates their applications in the preclinical test of possible therapies. A stepwise biofabrication method that combines 3D bioprinting and postprint cell self‐organization is developed for epidermal reconstruction. Skin‐cell‐laden tissue architectures are created to display the multilayered structures seen in vivo. These 3D models recapitulate the basal‐to‐suprabasal transition, providing an in vitro tool to mimic the pathological microenvironment of the disease pemphigus vulgaris.
... (SI- figure S7). These results are in good agreement with the expected effect of adding a thin hydrogel layer coat on top of the hydrophobic surface of PLLA mats and can enhance the adhesion and interaction of cells [31] with first layer of the scaffold for AC tissue engineering. ...
Developing an engineered scaffold inspired by structural features of healthy articular cartilage (AC) has attracted much attention. In this study, the design and fabrication of a three-layered fiber/hydrogel scaffold in which each layer replicates the organization of a pertinent layer of AC tissue is aimed. To this end, electrospun poly L-lactic acid (PLLA) nanofibers are prepared and fragmented into nano/micro cylinders via aminolysis. Three-layers of the scaffold in which continuous fibrous layer, fibrin gel incorporated by chopped fibers and fibrin gel embedded by cylindrical aligned fibrous mat perpendicular to articulating surface, respectively served as an upper, middle and bottom layers, are prepared. The layers’ physicomechanical characteristics are comprehensively evaluated. Results show that optimized electrospinning set up results in the smallest fibers diameter of 367±317 nm and successful aminolysis provides amine-functionalized chopped nanofibers with a mean length of 1.65±1.2 µm. Static mechanical analysis of the layers demonstrates that Young tensile modulus of the upper layer is 152± 17 MPa while compressive moduli of the middle and bottom layers are 38±4 and 79± 6 KPa, respectively. Assessing mechanical parameters under dynamic loading also shows that adding fibrous part in the composite scaffold layers enhances viscoelastic behavior of fibrin gel. Also, incorporation of 0.25% chopped fibers into the fibrin matrix notably enhances the equilibrium water content; however, it increases in-vitro weigh loss rate from 6% to 10.5% during a seven-day period. cytocompatibility analysis confirms that all layers possess acceptable cytocompatibility. In a conclusion, the designed three-layered composite structure successfully mimics the physicomechanical as well as microstructural features of AC and could be suggested as a potential scaffold for this tissue regeneration.
... Ascorbic acid rich skin constructs Heart valve Human dermal fibroblasts n/a n/a n/a Promoted collagen production in the cells [118] Thrombin/fibrinogen embedded skin explants Skin substitute Skin explants in wound repair Skeletal muscle explants n/a n/a n/a Excellent cell outgrowth from skin explants onto dermal substitute [119] Table 1 clearly indicates that fibrin-based material systems are among the newest autologous clinical treatments that can accelerate the wound healing process, particularly wound epithelialization. Although different animal models have been usefully implemented, many of these developments still need to be tested under clinical conditions. ...
The first bioprocess that occurs in response to wounding is the deterrence of local hemorrhage. This is accomplished by platelet aggregation and initiation of the hemostasis cascade. The resulting blood clot immediately enables the cessation of bleeding and then functions as a provisional matrix for wound healing, which begins a few days after injury. Here, fibrinogen and fibrin fibers are the key players, because they literally serve as scaffolds for tissue regeneration and promote the migration of cells, as well as the ingrowth of tissues. Fibrin is also an important modulator of healing and a host defense system against microbes that effectively maintains incoming leukocytes and acts as reservoir for growth factors. This review presents recent advances in the understanding and applications of fibrin and fibrin-fiber-incorporated biomedical materials applied to wound healing and subsequent tissue repair. It also discusses how fibrin-based materials function through several wound healing stages including physical barrier formation, the entrapment of bacteria, drug and cell delivery, and eventual degradation. Pure fibrin is not mechanically strong and stable enough to act as a singular wound repair material. To alleviate this problem, this paper will demonstrate recent advances in the modification of fibrin with next-generation materials exhibiting enhanced stability and medical efficacy, along with a detailed look at the mechanical properties of fibrin and fibrin-laden materials. Specifically, fibrin-based nanocomposites and their role in wound repair, sustained drug release, cell delivery to wound sites, skin reconstruction, and biomedical applications of drug-loaded fibrin-based materials will be demonstrated and discussed.
... Human dermal fibroblasts on fibrinogen containing nanofiber matrices had faster migration and higher expression of the differentiated phenotype α-smooth muscle actin with the aid of exogenous TGF-β1. The electrospun PLA nanofibers coated with fibrin significantly increased the type I collagen expression and synthesis, spreading, population density, and mitochondrial activity of human dermal fibroblasts (Table 5) [273]. ...
Nanoparticles are the gateway to the new era in drug delivery of biocompatible agents. Several products have emerged from nanomaterials in quest of developing practical wound healing dressings that are nonantigenic, antishear stress, and gas-exchange permeable. Numerous studies have isolated and characterised various wound healing nanomaterials and nanoproducts. The electrospinning of natural and synthetic materials produces fine products that can be mixed with other wound healing medications and herbs. Various produced nanomaterials are highly influential in wound healing experimental models and can be used commercially as well. This article reviewed the current state-of-the-art and briefly specified the future concerns regarding the different systems of nanomaterials in wound healing (i.e., inorganic nanomaterials, organic and hybrid nanomaterials, and nanofibers). This review may be a comprehensive guidance to help health care professionals identify the proper wound healing materials to avoid the usual wound complications.