Figure 1 - available via license: Creative Commons Attribution 4.0 International
Content may be subject to copyright.
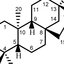
Molecules which inhibit the assembly of HIV-1 viral capsid [16]. (A) The structure of the hexamer of the HIV-1 CA protein (PDB ID: 1VUU). The different monomers are green, red, blue, magenta, cyan, and orange. The NTD and the CTD of each monomer are in different shades of the same color; (B) The structure of HIV-1 p24 NTD in the absence (left) and presence (right) of CAP-1. Phe32 is shown in yellow. CAP-1 is shown in magenta (PDB IDs: 1VUU and 2JPR); (C) Formula of the CA-binding compound described by Lemke et al. [26]; (D) Formula of the CA-binding compounds PF74 and BI-2; (E) Structure of the CA protein of HIV-1 CTD in the absence (left) and presence (right) of the capsid pool inhibitor (CAI, orange). The helix from which the CAC1 peptide was derived is cyan. The sequence of CAI and the structure of the optimized peptide NYAD-1 are given below (PDB IDs: 1AUM and 2BUO); (F) The structure of the Bevirimat [9].
Source publication
Feline immunodeficiency virus (FIV) is a member of the retroviridae family of viruses. It causes acquired immunodeficiency syndrome (AIDS) in worldwide domestic and non-domestic cats and is a cause of an important veterinary issue. The genome organization of FIV and the clinical characteristics of the disease caused by FIV are similar to human immu...
Contexts in source publication
Context 1
... host factors interact with CA, and amino-acid substitutions or chemical inhibitors that disrupt these interactions can inhibit HIV infection [18,19]. For example, inhibitors of HIV-1 PF74 and BI-2 ( Figure 1D) bind to a pocket between the N-terminal (NTD) and C-terminal (CTD) domains of CA and prevent interactions of the viral capsid with cellular proteins CPSF6 and Nup153 [20][21][22][23]. Additionally, compounds or peptides such as CAP-1 or NYAD-1 ( Figure 1B,D), which prevent the formation of the mature functional viral capsid, inhibit the replication of the virus [23][24][25]. ...
Context 2
... example, inhibitors of HIV-1 PF74 and BI-2 ( Figure 1D) bind to a pocket between the N-terminal (NTD) and C-terminal (CTD) domains of CA and prevent interactions of the viral capsid with cellular proteins CPSF6 and Nup153 [20][21][22][23]. Additionally, compounds or peptides such as CAP-1 or NYAD-1 ( Figure 1B,D), which prevent the formation of the mature functional viral capsid, inhibit the replication of the virus [23][24][25]. An interesting feature of the CA protein as a pharmacological target is its highly conserved sequence in the circulating strains of infected individuals, resulting in a low propensity to develop resistance [26,27]. ...
Context 3
... interesting feature of the CA protein as a pharmacological target is its highly conserved sequence in the circulating strains of infected individuals, resulting in a low propensity to develop resistance [26,27]. On the other hand, the inhibitory effect of compound C ( Figure 1C) can be reversed with a simple substitution of an amino acid at the N-terminal compound binding site of CA. Thus, the binding site in CA is important because some areas seem more vulnerable to mutations with the concomitant loss of binding to the compound [17]. ...
Context 4
... the binding site in CA is important because some areas seem more vulnerable to mutations with the concomitant loss of binding to the compound [17]. In another way, inhibition of the capsid assembly acting on DNA control, as done by the drug Bevirimat, produces non-infective viral particles ( Figure 1F) [9]. ...
Context 6
... structural similarities with known HIV capsid inhibitors were estimated using the ChemFinder tool from ChemOffice ® . For the selection process, we used a structural similarity criteria of 80% with the reported inhibitor of CA as a search filter (Supplementary Figure S1). As an example, this resulted in the identification of 18 molecules from our library which harbor 80% similarity with the CAP-1 HIV-1 inhibitor (Figure 1), from which the compound with the best score was selected [9,[22][23][24][31][32][33]. ...
Context 7
... the selection process, we used a structural similarity criteria of 80% with the reported inhibitor of CA as a search filter (Supplementary Figure S1). As an example, this resulted in the identification of 18 molecules from our library which harbor 80% similarity with the CAP-1 HIV-1 inhibitor (Figure 1), from which the compound with the best score was selected [9,[22][23][24][31][32][33]. The random selection of the other compounds was performed based on several criteria: abundance of the compound, solubility, cost-effective synthetic procedures, and compounds with toxicology data. ...
Context 8
... structural similarities with known HIV capsid inhibitors were estimated using the ChemFinder tool from ChemOffice ® . For the selection process, we used a structural similarity criteria of 80% with the reported inhibitor of CA as a search filter (Supplementary Figure S1). As an example, this resulted in the identification of 18 molecules from our library which harbor 80% similarity with the CAP-1 HIV- 1 inhibitor (Figure 1), from which the compound with the best score was selected [9,[22][23][24][31][32][33]. ...
Context 9
... the selection process, we used a structural similarity criteria of 80% with the reported inhibitor of CA as a search filter (Supplementary Figure S1). As an example, this resulted in the identification of 18 molecules from our library which harbor 80% similarity with the CAP-1 HIV- 1 inhibitor (Figure 1), from which the compound with the best score was selected [9,[22][23][24][31][32][33]. The random selection of the other compounds was performed based on several criteria: abundance of the compound, solubility, cost-effective synthetic procedures, and compounds with toxicology data. ...
Citations
... More than 400 compounds from our chemical library described elsewhere have been tested for their ability to interfere with CA assembly. Then from the data previously acquired from those 400 molecules tested in CA from FIV, we selected 10 compounds for their structural relationship with known CA HIV-1 inhibitors [27][28][29][30][31]. From those molecules, 5 compounds demonstrated interference with the assembly of HIV-1 CA in vitro One hit molecule, compound 696, which was recently shown by NMR to bind FIV capsid protein [27], demonstrated an inhibitory effect on HIV-1 replication in cellulo, with an IC 50 of 3 µM. ...
... More than 400 compounds from our chemical library described elsewhere [28][29][30] have been tested for their ability to interfere with CA assembly. As the assembly inhibition assay requires high protein and molecule concentrations, with the concomitant solubility problems, a dose-response curve could not be determined for this assay as the 1:1 protein: compound ratio is unreachable. ...
... However, it is a common first-line screening strategy for anti-assembly molecules to select molecules to be tested in cellulo and/or in vivo [26]. Then from the data previously acquired from those 400 molecules tested in CA from FIV, we selected 10 compounds for their structural relationship with known CA HIV-1 inhibitors [30]. From those molecules, 5 compounds demonstrated interference with the assembly of HIV-1 CA in vitro (Fig. 1), namely compounds 305, 553, 878, 696, and 1310 (Fig. 2). ...
The capsid (CA) subunit of the HIV-1 Gag polyprotein is involved in several steps of the viral cycle, from the assembly of new viral particles to the protection of the viral genome until it enters into the nucleus of newly infected cells. As such, it represents an interesting therapeutic target to tackle HIV infection. In this study, we screened hundreds of compounds with a low cost of synthesis for their ability to interfere with Gag assembly in vitro. Representatives of the most promising families of compounds were then tested for their ability to inhibit HIV-1 replication in cellulo. From these molecules, a hit compound from the benzimidazole family with high metabolic stability and low toxicity, 2-(4-N,N-dimethylaminophenyl)-5-methyl-1-phenethyl-1H-benzimidazole (696), appeared to block HIV-1 replication with an IC50 of 3 µM. Quantitative PCR experiments demonstrated that 696 does not block HIV-1 infection before the end of reverse transcription, and molecular docking confirmed that 696 is likely to bind at the interface between two monomers of CA and interfere with capsid oligomerization. Altogether, 696 represents a promising lead molecule for the development of a new series of HIV-1 inhibitors.
... The diffusion behavior of the protein is altered later, when it is complexed with an increased number of unlabeled ligand molecules. The binding affinity (K d ) of the ligand can be determined by obtaining the titration curves [31]. By definition, the dissociation constant (K d ) refers to the concentration of ligand at which half of the ligand binding sites on the protein are occupied by the ligand molecules [32]. ...
Ebola virus disease (EVD), a disease caused by infection with Ebola virus (EBOV), is characterized by hemorrhagic fever and a high case fatality rate. With limited options for the treatment of EVD, anti-Ebola viral therapeutics need to be urgently developed. In this study, over 500 extracts of medicinal plants collected in the Lingnan region were tested against infection with Ebola-virus-pseudotyped particles (EBOVpp), leading to the discovery of Maesa perlarius as an anti-EBOV plant lead. The methanol extract (MPBE) of the stems of this plant showed an inhibitory effect against EBOVpp, with an IC50 value of 0.52 µg/mL, which was confirmed by testing the extract against infectious EBOV in a biosafety level 4 laboratory. The bioassay-guided fractionation of MPBE resulted in three proanthocyanidins (procyanidin B2 (1), procyanidin C1 (2), and epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin (3)), along with two flavan-3-ols ((+)-catechin (4) and (−)-epicatechin (5)). The IC50 values of the compounds against pseudovirion-bearing EBOV-GP ranged from 0.83 to 36.0 µM, with 1 as the most potent inhibitor. The anti-EBOV activities of five synthetic derivatives together with six commercially available analogues, including EGCG ((−)-epigallocatechin-3-O-gallate (8)), were further investigated. Molecular docking analysis and binding affinity measurement suggested the EBOV glycoprotein could be a potential molecular target for 1 and its related compounds.
... Interestingly, GS-6207 binds at the CA/CA interface within HIV-1 CA hexamers and has been optimized from lead molecules using the structural data available on these hexamers [14,52,53]. For FIV, assembly inhibitors have recently been identified that bind the same region of CA, which seems to be similarly able to interact with feline nucleoporins [42,54] and thus represent an interesting therapeutic target. Obtaining structural data on FIV CA oligomers will therefore be of prime importance for the optimization of these compounds towards efficient anti-FIV molecules. ...
The Gag polyprotein is implied in the budding as well as the establishment of the supramolecular architecture of infectious retroviral particles. It is also involved in the early phases of the replication of retroviruses by protecting and transporting the viral genome towards the nucleus of the infected cell until its integration in the host genome. Therefore, understanding the structure–function relationships of the Gag subunits is crucial as each of them can represent a therapeutic target. Though the field has been explored for some time in the area of Human Immunodeficiency Virus (HIV), it is only in the last decade that structural data on Feline Immunodeficiency Virus (FIV) Gag subunits have emerged. As FIV is an important veterinary issue, both in domestic cats and endangered feline species, such data are of prime importance for the development of anti-FIV molecules targeting Gag. This review will focus on the recent advances and perspectives on the structure–function relationships of each subunit of the FIV Gag polyprotein.
... Il faut ainsi reporter les attributions de la protéine triplement marquée sur les pics de la protéine non deutérée avant de comparer les différences de déplacements chimiques. Comme on peut le voir sur la Figure 53, les différences de déplacements chimiques entre la protéine marquée au deutérium et la protéine Les composés ont pu être testés sur celui de la protéine FIV p24, selon le protocole d'assemblage in vitro de la capside déjà mis en place (Sierra et al., 2018). Nous avons ainsi identifié un composé interférant avec l'assemblage de la capside du FIV, nommé 696 conformément à son identifiant dans la chimiothèque. ...
... Le composé 696 fait partie de la famille des benzimidazoles (Figure 54). J'ai fait le test d'assemblage sur la protéine tronquée p24 CPT et la protéine entière p24E P1T (notée p24E dans la suite du manuscrit), à deux concentrations différentes, d'après le protocole mis en place au préalable (Sierra et al., 2018). Brièvement, un échantillon de protéine purifiée, stockée dans un tampon NaPi 50 mM pH 7,4, NaCl 50 mM a été mélangé avec un même volume de tampon NaCl 1 M. La forte salinité déclenche l'assemblage de la protéine. ...
... G. Mise en évidence du composé 6 696 ciblant la protéine de capside du FIV L'ensemble des travaux présentés a permis de caractériser la fixation de 696 sur la protéine monomérique, son site d'interaction et son affinité. L'étude par criblage de différents composés sur la cinétique de l'assemblage a permis une identification de plusieurs molécules interférant avec l'assemblage de la capside (Sierra et al., 2018). Le test permet de rapidement cribler un grand nombre de composés de la chimiothèque en utilisant des plaques 96 ou 384 puits. ...
Le virus de l’immunodéficience féline (Feline immunodeficiency virus, FIV) est un lentivirus, comme le virus de l’immunodéficience humaine. Il représente un enjeu de la médecine vétérinaire, aussi bien pour les animaux domestiques que sauvages. Il infecte entre 5 et 10 % des chats domestiques dans le monde, et l’ensemble des espèces félines est concerné par le FIV, dont les différents types sont chacun adaptés à une espèce hôte. L’infection au virus conduit à une immunodéficience similaire au syndrome d’immunodéficience acquise induite par le virus de l’immunodéficience humaine chez l’Homme. Il n’existe à ce jour aucun traitement efficace contre le FIV. Le FIV est constitué de structures supramoléculaires, protégeant son génome sous forme d’ARN génomique. La capside est une de ces structures et contient le génome viral. La capside protège le génome jusqu’à la réalisation de la rétrotranscription et participe à l’entrée du génome dans le noyau et à l’intégration du génome viral dans le génome cellulaire. La protection de l’ARN génomique avant la rétrotranscription prévient la détection de l’ARN viral par les protéines de restriction antivirale et sa dégradation. La capside virale est un assemblage d’une protéine sous-unitaire, la protéine de capside, CA ou p24, qui s’assemble en pentamères ou hexamères qui s’assemblent ultimement pour former la capside. La capside interagit avec de nombreux partenaires cellulaires. Ces interactions sont très bien décrites pour le VIH-1, l’espèce virale la plus étudiée du genre des lentivirus, mais sont moins bien caractérisées chez le FIV. Cette capacité à s’assembler de cette façon est requise au moment du bourgeonnement des virions à la membrane plasmique, et lors du réassemblage des sous-unités lors de la maturation du virion. La stabilité de la capside à l’intérieur de la cellule infectée a une ambivalence, entre la protection du génome viral, et la nécessité du désassemblage de la capside pour la libération du génome viral sous sa forme ADN. Ce double comportement montre en quoi la capside est une bonne cible thérapeutique. Perturber l’assemblage ou le stabiliser sont deux approches possibles pour interférer avec la capside et mettre au point des composés actifs contre l’infection au FIV. Pour le traitement du VIH-1, des composés interagissant avec la capside virale sont en cours d’essais cliniques. Dans le cadre d’une collaboration avec une équipe Uruguayenne , nous avons identifié une molécule interférant avec l’assemblage de la capside du FIV in vitro. Nous avons mis en évidence une fixation spécifique de la molécule sur FIV p24, sous sa forme monomérique. Nous avons mesuré l’affinité du composé pour FIV p24 et caractérisé son site de fixation. Pour la caractérisation de ce site de fixation, nous avons choisi d’utiliser la RMN et pour cela, nous avions besoin de l’attribution RMN de la protéine qui n’avait pas été faite préalablement, que nous avons ainsi réalisée. Nous avons montré que le mécanisme de fixation de notre composé est similaire à celui des traitements en cours d’essais cliniques contre VIH-1 p24. Ces molécules agissent par une compétition pour la fixation sur VIH 1 p24 avec des protéines cellulaires interagissant avec la capside du VIH-1, telles les nucléoporines, et cette compétition inhiberait l’entrée du génome viral dans le noyau. Avec ces données décrites chez le VIH-1 et nos résultats expérimentaux sur la capside du FIV, nous avons cherché si les nucléoporines félines, homologues aux nucléoporines humaines se liant à la capside du VIH-1, pouvaient se lier à la capside du FIV et avons montré une affinité de ces nucléoporines pour FIV p24. Cela soutient l’idée d’un mécanisme commun d’entrée des lentivirus dans le noyau des cellules infectées via leur capside. À terme, le criblage de molécules dérivées de notre molécule guide pourrait permettre l’identification de composés efficaces et peu chers à produire pour la médecine vétérinaire.
... Even so, both structures are still considered Pan-Assay Interference Compounds (PAINS) because they also have groupings that can function as Michael acceptors with these able to nonspecific toxicity. However, despite extensive efforts, we were unable to identify what these nonspecific activities are for the compounds used here [14,[27][28][29]. . Clear boxes and nd represent compounds whose activity was not determined. ...
The trypanosomatid parasites Trypanosoma brucei, Trypanosoma cruzi and Leishmania are the causative agents of human African trypanosomiasis, Chagas Disease and Leishmaniasis, respectively. These infections primarily affect poor, rural communities in the developing world, and are responsible for trapping sufferers and their families in a disease/poverty cycle. The development of new chemotherapies is a priority given that existing drug treatments are problematic. In our search for novel anti-trypanosomatid agents, we assess the growth-inhibitory properties of >450 compounds from in-house and/or “Pathogen Box” (PBox) libraries against L. infantum, L. amazonensis, L.braziliensis, T. cruzi and T. brucei and evaluate the toxicities of the most promising agents towards murine macrophages. Screens using the in-house series identified 17 structures with activity against and selective toward Leishmania: Compounds displayed 50% inhibitory concentrations between 0.09 and 25 μM and had selectivity index values >10. For the PBox library, ~20% of chemicals exhibited anti-parasitic properties including five structures whose activity against L. infantum had not been reported before. These five compounds displayed no toxicity towards murine macrophages over the range tested with three being active in an in vivo murine model of the cutaneous disease, with 100% survival of infected animals. Additionally, the oral combination of three of them in the in vivo Chagas disease murine model demonstrated full control of the parasitemia. Interestingly, phenotyping revealed that the reference strain responds differently to the five PBox-derived chemicals relative to parasites isolated from a dog. Together, our data identified one drug candidate that displays activity against Leishmania and other Trypanosomatidae in vitro and in vivo, while exhibiting low toxicity to cultured mammalian cells and low in vivo acute toxicity.
... However, the identification of compounds inhibiting the function of FIV p24 is still anticipated. To identify molecules that interfere with FIV p24 capsid assembly, we performed in vitro assembly assays and screened approximately 400 compounds from our in-house chemical library, as previously described 54 . This screening led to the identification of one promising lead compound, 696. ...
... In vitro assembly/disassembly assay. The assembly of p24 was performed and monitored, as previously described 54 . Assembly was triggered using a 1:1 (v/v) mix of 7 mg/mL purified protein in 50 mM NaPi buffer pH 7.4 containing 1M NaCl in a final volume of 50 µL. ...
... The optical density at 345 nm was measured every 40 sec using the Infinite M1000 multiplate reader (Tecan) at 38°C for 35 min. Compounds from our in-house library, which have been described elsewhere 54 Compound 696 was synthesized, as described in the supporting information (supplementary scheme S1). End-point samples from the in vitro assembly assay in the presence of either compound 696 or 1% DMSO (control) were used for the disassembly assay. ...
... This behavior is the combination of two effects: the fast, local environment-dependent responses of the fluorophore to the temperature jump and the slower diffusive thermophoresis fluorescence changes. It will be modified when the protein is complexed with increasing amounts of unlabeled partners, leading to titration curves that can be fit for K d estimation 36 . In practice, FhTIM was labeled with the Monolith His-Tag Labeling Kit RED-tris-NTA (NanoTemper Technologies, München, Germany), as described by the manufacturer. ...
Trematode infections such as schistosomiasis and fascioliasis cause significant morbidity in an estimated 250 million people worldwide and the associated agricultural losses are estimated at more than US$ 6 billion per year. Current chemotherapy is limited. Triosephosphate isomerase (TIM), an enzyme of the glycolytic pathway, has emerged as a useful drug target in many parasites, including Fasciola hepatica TIM (FhTIM). We identified 21 novel compounds that selectively inhibit this enzyme. Using microscale thermophoresis we explored the interaction between target and compounds and identified a potent interaction between the sulfonyl-1,2,4-thiadiazole (compound 187) and FhTIM, which showed an IC50 of 5 µM and a Kd of 66 nM. In only 4 hours, this compound killed the juvenile form of F. hepatica with an IC50 of 3 µM, better than the reference drug triclabendazole (TCZ). Interestingly, we discovered in vitro inhibition of FhTIM by TCZ, with an IC50 of 7 µM suggesting a previously uncharacterized role of FhTIM in the mechanism of action of this drug. Compound 187 was also active against various developmental stages of Schistosoma mansoni. The low toxicity in vitro in different cell types and lack of acute toxicity in mice was demonstrated for this compound, as was demonstrated the efficacy of 187 in vivo in F. hepatica infected mice. Finally, we obtained the first crystal structure of FhTIM at 1.9 Å resolution which allows us using docking to suggest a mechanism of interaction between compound 187 and TIM. In conclusion, we describe a promising drug candidate to control neglected trematode infections in human and animal health.
... The main significance of this study is the ample proof that viruses are natural multivalent biotechnological platforms for drug delivery applications [119]. As mentioned before, there are certain drawbacks in utilizing virus-like nanoparticle for drug delivery [120] and extensive research to overcome these challenges will help to use them as a potential natural drug nanocarriers in future. ...
Viruses are considered as natural nanomaterials as they are in the size range of 20-500 nm with a genetical material either DNA or RNA, which is surrounded by a protein coat capsid. Recently, the field of virus nanotechnology is gaining significant attention from researchers. Attention is given to the utilization of viruses as nanomaterials for medical, biotechnology and energy applications. Removal of genetic material from the viral capsid creates empty capsid for drug incorporation and coating the capsid protein crystals with antibodies, enzymes or aptamers will enhance their targeted drug deliver efficiency. Studies reported that these virus-like nanoparticles have been used in delivering drugs for cancer. It is also used in imaging and sensory applications for various diseases. However, there is reservation among researchers to utilize virus-like nanoparticles in targeted delivery of genes in gene therapy, as there is a possibility of using virus-like nanoparticles for targeted gene delivery. In addition, other biomedical applications that are explored using virus-like nanoparticles and the probable mechanism of delivering genes.
![Molecules which inhibit the assembly of HIV-1 viral capsid [16]. (A) The structure of the hexamer of the HIV-1 CA protein (PDB ID: 1VUU). The different monomers are green, red, blue, magenta, cyan, and orange. The NTD and the CTD of each monomer are in different shades of the same color; (B) The structure of HIV-1 p24 NTD in the absence (left) and presence (right) of CAP-1. Phe32 is shown in yellow. CAP-1 is shown in magenta (PDB IDs: 1VUU and 2JPR); (C) Formula of the CA-binding compound described by Lemke et al. [26]; (D) Formula of the CA-binding compounds PF74 and BI-2; (E) Structure of the CA protein of HIV-1 CTD in the absence (left) and presence (right) of the capsid pool inhibitor (CAI, orange). The helix from which the CAC1 peptide was derived is cyan. The sequence of CAI and the structure of the optimized peptide NYAD-1 are given below (PDB IDs: 1AUM and 2BUO); (F) The structure of the Bevirimat [9].](publication/326310866/figure/fig1/AS:647016308805632@1531272117132/Molecules-which-inhibit-the-assembly-of-HIV-1-viral-capsid-16-A-The-structure-of-the.png)