Figure - available from: Journal of Cell Biology (JCB)
This content is subject to copyright.
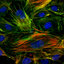
Localization of pY ERK on Golgi complex is specific for cells entering mitosis. G2/M cells were identified by pH3 and DAPI staining in asynchronous Ptk1 cells and the corresponding pY ERK staining was examined. Three separate examples of cells showing progression from late G2 (top) through early (middle) and late prophase of mitosis (bottom) are shown. The arrows in the left panels highlight the perinuclear pY ERK staining in the cells entering mitosis. In all cases where pH3 staining was positive, the cells also showed perinuclear Golgi-like pY ERK staining. Bar, 10 μm.
Source publication
Phosphorylation of the extracellular signal–regulated kinases (ERKs) on tyrosine and threonine residues within the TEY tripeptide
motif induces ERK activation and targeting of substrates. Although it is recognized that phosphorylation of both residues
is required for ERK activation, it is not known if a single phosphorylation of either residue regu...
Citations
... In contrast, it was barely detectable and identical between the genotypes when we used an antibody that requires phosphorylation of both residues (Cell Signaling Technologies 9102; data not shown). Phosphorylation of either residue by the dual-specificity kinase Mek results in partial Erk activation 34,35 , and it appears that Norbin is indirectly required for the phosphorylation of phospho-Thr202 at least. ...
Norbin is an adaptor protein that binds numerous G protein-coupled receptors (GPCRs), is highly expressed in neurons, and is essential for a functioning nervous system in rodent models. Yet, beyond its control of neurite outgrowth and synaptic plasticity, few cellular roles of Norbin have been investigated to date. Furthermore, while Norbin is known to regulate the steady-state cell surface levels of several GPCRs, only in one case has the protein been shown to control the agonist-induced receptor internalisation which serves to attenuate GPCR signalling. Here, we generated a Norbin-deficient PC12 cell line which enabled us to study both the cellular functions of Norbin and its roles in GPCR trafficking and signalling. We show that Norbin limits cell size and spreading, and is required for the growth, viability and cell cycle progression of PC12 cells. We also found that Norbin regulates both the steady-state surface level and agonist-induced internalisation of the GPCR sphingosine-1-phosphate receptor 1 (S1PR1) in these cells, suggesting that its role in agonist-dependent GPCR trafficking is more widespread than previously appreciated. Finally, we show that Norbin limits the S1P-stimulated activation of Akt and p38 Mapk, and is required for the activation of Erk in PC12 cells. Together, our findings provide a better understanding of the cellular functions of Norbin and its control of GPCR trafficking.
... § Based on analysis of previously reported data (Basu et al., 2016(Basu et al., ). et al., 1998Roberts et al., 2002) and fragmentation of Golgi cisternae (Acharya et al., 1998;Cha and Shapiro, 2001;Shaul and Seger, 2006). Therefore, MAPK pathways may have a general role in regulating events that occur throughout the cell cycle, necessitating that the pathways themselves exhibit cell-cycledependent activity Mansour et al., 1994;Wright et al., 1999;Katz et al., 2007). ...
Mitogen-Activated Protein Kinase (MAPK) pathways control cell differentiation and the response to stress. In Saccharomyces cerevisiae, the MAPK pathway that controls filamentous growth (fMAPK) shares components with the pathway that regulates the response to osmotic stress (HOG). Here, we show that the two pathways exhibit different patterns of activity throughout the cell cycle. The different patterns resulted from different expression profiles of genes encoding mucin sensors that regulate the pathways. Cross-pathway regulation from the fMAPK pathway stimulated the HOG pathway, presumably to modulate fMAPK pathway activity. We also show that the shared tetraspan protein, Sho1p, which has a dynamic localization pattern, induced the fMAPK pathway at the mother-bud neck. A Sho1p-interacting protein, Hof1p, which also localizes to the mother-bud neck and regulates cytokinesis, also regulated the fMAPK pathway. Therefore, spatial and temporal regulation of pathway sensors, and cross-pathway regulation, control a MAPK pathway that regulates cell differentiation in yeast.
... Next, we knocked-down each of the identified proteins, as well as GRASP55 and GRASP65, which are wellknown substrates of ERK1/2 (Acharya et al., 1998;Ayala and Colanzi, 2017;Cha and Shapiro, 2001;Jesch et al., 2001). Thus, HeLa cells overexpressing either ERK1 or ERK1c were treated with SiRNAs for the relevant proteins examined and indeed exhibited the expected decrease in protein or mRNA expression (Figures S1B and S1C). ...
ERK1c is an alternatively spliced isoform of ERK1 that specifically regulates mitotic Golgi fragmentation, which allows division of the Golgi during mitosis. We have previously shown that ERK1c translocates to the Golgi during mitosis where it is activated by a resident MEK1b to induce Golgi fragmentation. However, the mechanism of ERK1c functions in the Golgi remained obscure. Here, we searched for ERK1c substrates, and identified HOOK3 as a mediator of ERK1c-induced mitotic Golgi fragmentation, which requires a second phosphorylation by AuroraA for its function. In cycling cells, HOOK3 interacts with microtubules (MTs) and links them to the Golgi. Early in mitosis, HOOK3 is phosphorylated by ERK1c and later by AuroraA, resulting in HOOK3 detachment from the MTs, and elevated interaction with GM130. This detachment modulates Golgi stability, and allows fragmentation of the Golgi. This study demonstrates a novel mechanism of Golgi apparatus destabilization early in mitosis to allow mitotic progression.
... Of note, although only nuclear P-MAPK signals were quantified, a striking accumulation of perinuclear P-MAPK was also apparent in most Tβ4 -/Y but not Tβ4 +/Y VSMCs ( Figure 8D). Association of tyrosine-phosphorylated MAPK with Golgi occurs during the G2/M phase of the cell cycle (49), which is consistent with enhanced proliferation in Tβ4 -/Y VSMCs. ...
Vascular stability and tone are maintained by contractile smooth muscle cells (VSMCs). However, injury-induced growth factors stimulate a contractile-synthetic phenotypic modulation which increases susceptibility to abdominal aortic aneurysm (AAA). As a regulator of embryonic VSMC differentiation, we hypothesised that Thymosin β4 (Tβ4) may function to maintain healthy vasculature throughout postnatal life. This was supported by the identification of an interaction with Low density lipoprotein receptor related protein 1 (LRP1), an endocytic regulator of PDGF-BB signalling and VSMC proliferation. LRP1 variants have been implicated by genome-wide association studies with risk of AAA and other arterial diseases. Tβ4-null mice displayed aortic VSMC and elastin defects, phenocopying LRP1 mutants, and their compromised vascular integrity predisposed to Angiotensin II-induced aneurysm formation. Aneurysmal vessels were characterised by enhanced VSMC phenotypic modulation and augmented platelet-derived growth factor (PDGF) receptor (PDGFR)β signalling. In vitro, enhanced sensitivity to PDGF-BB, upon loss of Tβ4, associated with dysregulated endocytosis, with increased recycling and reduced lysosomal targeting of LRP1-PDGFRβ. Accordingly, the exacerbated aneurysmal phenotype in Tβ4-null mice was rescued upon treatment with the PDGFRβ antagonist, Imatinib. Our study identifies Tβ4 as a key regulator of LRP1 for maintaining vascular health and provides insights into the mechanisms of growth factor-controlled VSMC phenotypic modulation underlying aortic disease progression.
... ; https://doi.org/10.1101/2020.12.18.423530 doi: bioRxiv preprint pathway that itself alters the cell cycle might be critical for its morphogenetic responses to be coordinated. Interestingly, human MEK and ERK are also activated during mitosis in somatic cells to regulate the spindle assembly checkpoint (SHAPIRO et al. 1998;HORNE AND GUADAGNO 2003;ROSNER 2007;CAO et al. 2010), proper entry into anaphase (SHAPIRO et al. 1998;ROBERTS et al. 2002), and fragmentation of Golgi cisternae (ACHARYA et al. 1998;CHA AND SHAPIRO 2001;SHAUL AND SEGER 2006). Therefore, MAP kinases may have a general role in regulating events that occur throughout the cell cycle MANSOUR et al. 1994;WRIGHT et al. 1999;KATZ et al. 2007). ...
Mitogen-Activated Protein Kinase (MAPK) pathways control cell differentiation and the response to stress. MAPK pathways can share components with other pathways yet induce specific responses through mechanisms that remain unclear. In Saccharomyces cerevisiae, the MAPK pathway that controls filamentous growth (fMAPK) shares components with the MAPK pathway that regulates the response to osmotic stress (HOG). By exploring temporal regulation of MAPK signaling, we show here that the two pathways exhibited different patterns of activity throughout the cell cycle. The different patterns resulted from different expression profiles of genes encoding the mucin sensors (MSB2 for fMAPK and HKR1 for HOG). We also show that positive feedback through the fMAPK pathway stimulated the HOG pathway, presumably to modulate fMAPK pathway activity. By exploring spatial regulation of MAPK signaling, we found that the shared tetraspan protein, Sho1p, which has a dynamic localization pattern, induced the fMAPK pathway at the mother-bud neck. A Sho1p-interacting protein, Hof1p, which also localizes to the mother-bud neck and regulates cytokinesis, also regulated the fMAPK pathway. Therefore, spatial and temporal regulation of pathway sensors, and cross-pathway feedback, regulate a MAPK pathway that controls a cell differentiation response in yeast.
... In addition, while phosphorylation of both Thr and Tyr is required for full ERK activation (7), monophosphorylated ERK species have been shown to exhibit intermediate activity in vitro (73). While pTyr ERK2 has been shown to associate with the Golgi complex and influence its structure during the G 2 -M-phase of the cell cycle in HeLa cells, the biological role of monophosphorylated ERK species remains unclear (74). In addition, previous work has shown that dimerization is critical to upstream RAF function and MEK activation by RAF (70,75), yet significantly less studies have focused on the MEK/ERK interaction. ...
The RAS–RAF–MEK–ERK pathway is the most well-studied of the MAPK cascades and is critical for cell proliferation, differentiation, and survival. Abnormalities in regulation resulting from mutations in components of this pathway, particularly in upstream proteins, RAS and RAF, are responsible for a significant fraction of human cancers and nearly all cutaneous melanomas. Activation of receptor tyrosine kinases by growth factors and various extracellular signals leads to the sequential activation of RAS, RAF, MEK, and finally ERK, which activates numerous transcription factors and facilitates oncogenesis in the case of aberrant pathway activation. While extensive studies have worked to elucidate the activation mechanisms and structural components of upstream MAPK components, comparatively less attention has been directed toward the kinases, MEK and ERK, due to the infrequency of oncogenic-activating mutations in these kinases. However, acquired drug resistance has become a major issue in the treatment of RAS- and RAF-mutated cancers. Targeting the terminal kinases in the MAPK cascade has shown promise for overcoming many of these resistance mechanisms and improving treatment options for patients with MAPK-aberrant cancers. Here, we will describe the role of MEK and ERK in MAPK signaling and summarize the current understanding of their interaction and activation mechanisms. We will also discuss existing approaches for targeting MEK and ERK, and the benefits of alternative strategies. Areas requiring further exploration will be highlighted to guide future research endeavors and aid in the development of alternative therapeutic strategies to combat surmounting drug resistance in treating MAPK-mediated cancers.
Visual Overview
http://mcr.aacrjournals.org/content/molcanres/19/3/361/F1.large.jpg.
... In addition, although ERK1/2 proteins seem to function on the outer surface of the Golgi membrane under some conditions (Torii et al., 2004), they do not seem to accumulate at the Golgi at any stage (Shaul and Seger, 2006). However, specific antibody against ERK that is monophosphorylated at Tyr204 (in human ERK1) did stain the Golgi at the G2 to M phase transition, but the identity of the stained molecules was initially obscure (Cha and Shapiro, 2001). The nature of these stained ERK molecules, as well as the identity of the MEK involved in the process were later resolved by the identification of an alternative ERK cascade, composed of the spliced variants of MEK1b, as well as the alternatively spliced ERK1 variant ERK1c (Aebersold et al., 2004;Shaul and Seger, 2006;Shaul et al., 2009). ...
... Interestingly, it has been noticed that this effect is generated independently of ERK1/2, the main ERK isoforms downstream of MEK1/2 (Acharya et al., 1998;Colanzi et al., 2000Colanzi et al., , 2003a. However, it has been shown that mono-phosphorylated ERK does exist in the mitotic Golgi, suggesting that the effect of MEK1/2 might be mediated by other ERK-like proteins (Cha and Shapiro, 2001). Indeed, our group has identified ERK1c, a unique primate splicing variant of ERK1 (Aebersold et al., 2004), that is highly expressed and activated during the G2 and M stages of the cell cycle (Shaul and Seger, 2006). ...
Golgi fragmentation is a highly regulated process that allows division of the Golgi apparatus between the two daughter cells. The mitotic reorganization of the Golgi is accompanied by a temporary block in Golgi functioning, as protein transport in and out of the Golgi stops. Our group has previously demonstrated the involvement of the alternatively spliced variants, ERK1c and MEK1b, in mitotic Golgi fragmentation. We also found that ERK1c translocates to the Golgi at G2/M, but the molecular mechanism underlying this recruitment remains unknown. In this study, we narrowed the translocation timing to prophase/prometaphase and elucidated its molecular mechanism. We found that CDK1 phosphorylates Ser343 of ERK1c, thereby allowing the binding of phosphorylated ERK1c to a complex that consists of PI4KIIIβ and 14-3-3γ dimer. The stability of the complex is regulated by PKD phosphorylation of PI4KIIIβ. The complex assembly induces the Golgi shuttling of ERK1c, where it is activated by MEK1b, and induces Golgi fragmentation. Our work shows that protein shuttling to the Golgi is not completely abolished in G2/M, thus integrating several independent Golgi-regulating processes into one coherent pathway.
... Thus, monophosphorylated forms of ERK2 were proposed to possibly represent intermediate activity states which might have distinct biological roles in vivo 12 . Tyrosine-monophosphorylated ERK2 was reported to transiently associate with the Golgi complex in HELA cells during cell cycle G2 and M phases, suggesting a role in the regulation of Golgi structure 14 . ...
The extracellular signal regulated kinases ERK1/2 play important roles in the regulation of diverse cellular functions and have been implicated in several human diseases. In addition to the fully activated, diphosphorylated ERK1/2 protein, monophosphorylated forms of ERK1/2 have been observed, which may have distinct biological functions. We report here on the highly sensitive detection and differentiation of unphosphorylated, threonine-phosphorylated (pT), tyrosine-phosphorylated (pY) and diphosphorylated ERK1 and ERK2 by capillary isoelectric focusing followed by immunological detection (CIEF-immunoassay). Eight different phosphorylated and unphosphorylated forms of ERK1/2 were resolved according to charge. The unequivocal identification and differentiation of ERK1 and ERK2 forms monophosphorylated at either threonine or tyrosine was achieved by competitive blocking with specific phospho-peptides and different phosphorylation-sensitive antibodies. The suitability of the additional pT-ERK1/2 and pY-ERK1/2 differentiation for the time-resolved in-depth study of phospho-form distribution in response to specific stimuli is demonstrated in human neuroblastoma SH-SY5Y and monocytic THP-1 cell lines, and in human peripheral blood mononuclear cells.
... Cell membrane fractions were separated as described previously. 40 In brief, cells were lysed in extracting buffer containing 10 mM Hepes, pH 7.4, 1.5 mM MgCl 2 , 10 mM KCl, 1 mM DTT, 0.2 mM sodium orthovanadate, 1 mM NaF, and 0.5 mM PMSF, followed by incubation on ice for 15 min. Then, cells were passed through a 26-gauge needle 10 times and the homogenate was centrifuged at 14,000 rpm for 1 min to separate the nuclei from the cytosolic and membrane proteins in the cytoplasmic supernatant. ...
Abstract Before a cell enters mitosis, the Golgi apparatus undergoes extensive fragmentation. This is required for the correct partitioning of the Golgi apparatus into daughter cells, and inhibition of this process leads to cell cycle arrest in G2 phase. AMP-activated protein kinase (AMPK) plays critical roles in regulating growth and reprogramming metabolism. Recent studies have suggested that AMPK promotes mitotic progression and Golgi disassembly, and that this seems independent of the cellular energy status. However, the molecular mechanism underlying these events is not well understood. Here, we show that both treatment with compound C and depletion of AMPKα2 (but not AMPKα1) delays the G2/M transition in synchronized HeLa cells, as evidenced by flow cytometry and mitotic index analysis. Furthermore, knockdown of AMPKα2 specifically delays further fragmentation of isolated Golgi stacks. Interestingly, pAMPKα(Thr172) signals transiently appear in the perinuclear region of late G2/early prophase cells, partially co-localizing with the Golgi matrix protein, GM-130. This Golgi pAMPKα(Thr172) signals were also specifically abolished by AMPKα2 knockdown, indicating specific spatio-temporal activation of AMPKα2 at Golgi complex during late G2/early prophases. We also found that the specific CaMKKβ inhibitor, STO-609, reduces the pAMPKα (Thr172) signals in the perinuclear region of G2 phase cells and delays mitotic Golgi fragmentation. Taken together, these data suggest that AMPKα2 is the major catalytic subunit of AMPKα which regulates Golgi fragmentation and G2/M transition, and that the CaMKKβ activates AMPKα2 during late G2 phase.
... In reality, there is a population of molecules present, each molecule has its own phospho-form and these phospho-forms are being dynamically modified by the opposing actions of the cognate kinases and phosphatases. This viewpoint is important because different phosphoforms may have different biological effects-both the numbers and the positions of phosphorylations may be significant-as observed for signalling proteins (Cha and Shapiro, 2001), transcription factors (Pufall et al, 2005), transcriptional coactivators (Wu et al, 2004), ion channels (Park et al, 2006) and circadian clock components (Baker et al, 2009). The realisation of substantial cross-talk between phosphorylation and other PTMs (Hunter, 2007), as found in the histone 'code' (Pesavento et al, 2008;Phanstiel et al, 2008), reinforces the same point in a broader context, while theoretical studies suggest that global patterns of modification can encode substantial amounts of information (Thomson and Gunawardena, 2009). ...
... Because these sites are so close together, a quantitative comparison between western blots, MS and NMR becomes feasible. The MAP kinase cascade is also a reiterated motif found in numerous signalling networks (Lewis et al, 1998), Erk is widely studied as a paradigmatic signalling protein (Yao and Seger, 2009) and evidence suggests that different Erk phosphoforms have different biological effects (Cha and Shapiro, 2001). Despite its apparent simplicity with only two sites, the analysis of Erk illuminated the challenges in quantifying phospho-form distributions. ...
The functional impact of multisite protein phosphorylation can depend on both the numbers and the positions of phosphorylated sites-the global pattern of phosphorylation or 'phospho-form'-giving biological systems profound capabilities for dynamic information processing. A central problem in quantitative systems biology, therefore, is to measure the 'phospho-form distribution': the relative amount of each of the 2(n) phospho-forms of a protein with n-phosphorylation sites. We compared four potential methods-western blots with phospho-specific antibodies, peptide-based liquid chromatography (LC) and mass spectrometry (MS; pepMS), protein-based LC/MS (proMS) and nuclear magnetic resonance spectroscopy (NMR)-on differentially phosphorylated samples of the well-studied mitogen-activated protein kinase Erk2, with two phosphorylation sites. The MS methods were quantitatively consistent with each other and with NMR to within 10%, but western blots, while highly sensitive, showed significant discrepancies with MS. NMR also uncovered two additional phosphorylations, for which a combination of pepMS and proMS yielded an estimate of the 16-member phospho-form distribution. This combined MS strategy provides an optimal mixture of accuracy and coverage for quantifying distributions, but positional isomers remain a challenging problem.