Fig 2 - uploaded by Eduardo R S Roldan
Content may be subject to copyright.
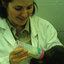
Histological appearance of grafted testicular tissue. (a) Iberian lynx, 28 weeks after grafting; (b) germ cells labelled by protein gene product (PGP) 9.5 immunostaining in Iberian lynx testis graft, 66 weeks after transplantation; (c) Cuvier's gazelle testis graft after 58 weeks; and (d ) Mohor gazelle testis graft 28 weeks after grafting. Arrows indicate spermatogonia; arrowheads indicate round spermatids; asterisks indicate Leydig cells. Scale bars ¼ 100 mm.
Source publication
The use of assisted reproductive techniques for endangered species is a major goal for conservation. One of these techniques, testis tissue xenografting, allows for the development of spermatozoa from animals that die before reaching sexual maturity. To assess the potential use of this technique with endangered species, testis tissue from six Iberi...
Contexts in source publication
Context 1
... one and three grafts were recovered from the fetus and tissue from perinatal cubs, respectively, but none of them con- tained testicular tissue and recipient mouse seminal vesicles weighed ,10 mg, indicating that the grafts did not contain functional Leydig cells (Table 2). Survival of testicular grafts from 6-month-old lynxes was different from that observed in grafts of fetus and perinatal cubs. Grafts were recovered from all mice with tissue from Donor 1 (Tables 1, 2). The percentage of recovered grafts presenting seminiferous tubules was lower after 40 weeks than before 40 weeks post-grafting (P ¼ 0.037), but seminal vesicle weight increased with time (P ¼ 0.049; Table 2). Seminiferous tubules with a small lumen could be observed in grafts, but no differentiated germ cells were found at any time point (Fig. 2a). Six mice hosting testicular tissue from 6-month-old Donor 2 (Tables 1, 2) were kept for more than 40 weeks and grafts with seminiferous tubules were found in two of them. The histological appearance was similar to that of the other 6-month-old donor. PGP 9.5 staining of grafts recovered 28 weeks after grafting showed spermatogonia in 10% of tubules, whereas 66 weeks after transplantation, 15% of tubules contained spermatogonia (Fig. ...
Context 2
... one and three grafts were recovered from the fetus and tissue from perinatal cubs, respectively, but none of them con- tained testicular tissue and recipient mouse seminal vesicles weighed ,10 mg, indicating that the grafts did not contain functional Leydig cells (Table 2). Survival of testicular grafts from 6-month-old lynxes was different from that observed in grafts of fetus and perinatal cubs. Grafts were recovered from all mice with tissue from Donor 1 (Tables 1, 2). The percentage of recovered grafts presenting seminiferous tubules was lower after 40 weeks than before 40 weeks post-grafting (P ¼ 0.037), but seminal vesicle weight increased with time (P ¼ 0.049; Table 2). Seminiferous tubules with a small lumen could be observed in grafts, but no differentiated germ cells were found at any time point (Fig. 2a). Six mice hosting testicular tissue from 6-month-old Donor 2 (Tables 1, 2) were kept for more than 40 weeks and grafts with seminiferous tubules were found in two of them. The histological appearance was similar to that of the other 6-month-old donor. PGP 9.5 staining of grafts recovered 28 weeks after grafting showed spermatogonia in 10% of tubules, whereas 66 weeks after transplantation, 15% of tubules contained spermatogonia (Fig. ...
Context 3
... were recovered before and after 40 weeks from five of 11 mice grafted with cryopreserved Cuvier's gazelle testicular tissue, but seminiferous tubules were not found in any of them. Seminal vesicle weights of these mice were not different from seminal vesicles from castrated mice that received no grafts (P . 0.05; mean (AE s.e.m.) 9.2 AE 0.3 vs 9.3 AE 0.7 mg, respec- tively; n ¼ 11 and 3, respectively). Grafts from Cuvier's gazelle fresh tissue showed no differentiated germ cells when recovered less than 40 weeks after grafting. When grafts were recovered after 40 weeks post-grafting (between 57 and 67 weeks), sper- matocytes were the most advanced germ cells found and they were present in 82% of tubules examined (Fig. 2c). At this time, the size of seminal vesicles from grafted mice had increased (Table ...
Context 4
... tissue recovered from Mohor gazelle after 12 weeks post-grafting presented no differentiated germ cells, but seminal vesicle weight was twice that recorded for seminal vesicles from castrated mice (Fig. 3). After 16 weeks post- grafting, 62% of grafts were recovered (Table 2) and they showed 7% of tubules with spermatocytes and 1% with round spermatids. Seminal vesicle weight increased 10-fold at this time point (Fig. 3). At 20 weeks after transplantation, round spermatids were not observed but 10% of tubules had spermatocytes. Finally, at 28 weeks after grafting, 8% of seminiferous tubules in graft tissue contained spermatocytes and 1% contained round sperma- tids (Fig. 2d). Seminal vesicles weighed $300 mg (Fig. ...
Similar publications
The corpus luteum (CL) is a transient gland formed in the ovary after ovulation and is the major source of progesterone. In the Iberian and Eurasian lynx, CL physiologically persist after parturition and retain their capacity to produce progesterone, thus suppressing the ovarian activity. This unique reproductive characteristic has a big impact on...
Citations
... Une naissance a été également rapportée après cryoconservation et autogreffe chez le macaque rhésus [84]. Chez les autres espèces sauvages étudiées, la spermatogenèse complète n'a été rapportée après xénogreffe qu'à partir de tissus frais, comme chez le bison [86] ou le pécari [87] ; des spermatides rondes ont été observées après xénogreffe de tissu congelé chez la gazelle de Mhorr [88]. Cette stratégie est particulièrement encourageante pour la conservation post-mortem d'animaux prépubères ou si la mort de l'animal survient en dehors de la saison de reproduction. ...
The extinction rate of animal and plant species has increased sharply in recent decades, leading to a major loss of biodiversity. Data shows that almost a quarter of the species studied are threatened with extinction. Human activities, such as habitat destruction, geopolitical conflicts and pollution, are the main threats for wildlife. In situ conservation, based on habitat protection, represent the ideal solution but is often difficult to achieve in a context of rapid decline of the species. Ex situ conservation, based on preserving species outside their natural habitat, is an alternative when measures are insufficient in nature. Ex situ conservation programs have limits, particularly in terms of maintaining genetic diversity. To improve the success of these programs, the use of biological resource banks (gametes, gonadal tissues and embryos) has been proposed as a complementary approach. This strategy would allows the long-term storage of genetic material in a minimum space, thus avoiding the phenomena of selection, genetic drift and loss of diversity over several centuries. To minimize genetic drift, it is suggested to set up these cryobanks from the start of conservation programs, or even before the numbers of wild populations are decreased too much. This literature review focuses on developments in cryobanking and assisted reproduction techniques approaches which will facilitate the achievement of conservation programs for mammalian species in the future.
... Nevertheless, in 2014, Zuo et al. created interspecies SCNT embryos from Przewalski's gazelle fibroblasts and bovine oocytes; however, significant alterations in mitochondria-related transcriptome profiles were indicative of poor reprogramming success, which correlated with the developmental failure of the interspecies embryos [172]. In the same year, Arregui et al. documented the first successful xenotransplantation of testicular tissue in Cuvier's and Mohor gazelles with round spermatids observed between 7 and 10 months post-transplantation in these species [173]. ...
... The great viability of rounded cells might allow for the design of systems for their in vitro cultivation [20], or for in vivo production following autologous transplantation [7] or even xenotransplantation [6,21]. Functional spermatozoa could then be obtained for use in an in vitro fertilization/ICSI system. ...
Sperm cryopreservation is the most common procedure used to establish germplasm banks for endangered species - but sometimes sperm cells cannot be obtained. In such cases, freezing testicular tissue may be the only option. The testes contains germ cells at different stages of differentiation, including spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids, and spermatozoa, among which differences in cryoresistance might be expected. The present work compares the viability and DNA integrity of ‘rounded’ cells, and of elongated spermatids and spermatozoa, from the dog and wild boar, following the cryopreservation of testicular tissue by slow freezing or vitrification. Cell viability was analyzed by PI/SYBR14 staining, and DNA integrity via the TUNEL technique. For wild boar, no significant differences were seen between the two methods with respect to the percentage of viable cells, nor in the percentage of cells with DNA damage. In the dog, the percentage of viable rounded germ cells (65.0 ± 2.4%) was higher (P < 0.05) after vitrification than after slow freezing (45.1 ± 6.7%). No difference was found between the two methods in terms of the viability of elongated cells. For rounded cells, the percentage of intact DNA was greater (P < 0.05) after vitrification (90.5 ± 2.1%) than after slow freezing (42.6 ± 11.0%), while for elongated spermatids and spermatozoa it was higher (P < 0.05) after slow freezing (66.9 ± 6.1%) than after vitrification (50.7 ± 4.5%). Thus, the response to cryopreservation is cell type-, cryopreservation type-, and species-dependent. Vitrification would appear to be the most appropriate method for preserving dog testicular tissue given the associated high cell viability and low degree of DNA fragmentation, while in wild boar, either method might be used.
... Autologous/Allogenic transplantation Mouse Honaramooz et al. [36] Schlatt et al. [157] Shinohara et al. [37] Schlatt et al. [35] Geens et al. [158] Yu et al. [159] Goossens et al. [160] Neonatal tissues Fresh-dorsal skin [35,36,[157][158][159][160] Cryopreserved-dorsal skin [36,157,160]; scrotum [37] • Spermatids: [157] • Spermatozoa: [158][159][160] • Embryos: [36] • Offspring: [35,37] Monkey Luetjens et al. [161] Fayomi et al. [40] Prepubertal tissues Fresh-dorsal skin [40,161]; scrotum [40,161] Cryopreserved-dorsal skin [40,161]; scrotum [40,161] Adult tissues Fresh-dorsal skin [161]; scrotum [161] Cryopreserved-dorsal skin [161] • Degenerated tubules: fresh adult grafts under dorsal skin [53] • Spermatogonia: fresh prepubertal grafts under dorsal skin [53] • Spermatocytes: fresh and cryopreserved prepubertal grafts under dorsal skin [53] • Spermatozoa: fresh prepubertal grafts under dorsal skin and in the scrotum [161]; all grafts [40] • Offspring [40] (Continued) [157] Cryopreserved-dorsal skin [157] • Spermatozoa: [157] Cat Snedaker et al. [162] Kim et al. [163] Arregui et al. [164] Fetal tissues Cryopreserved-dorsal skin [164] Neonatal tissues Cryopreserved-dorsal skin [164] Prepubertal tissues Fresh-dorsal skin [162,163] Adult tissues Fresh-dorsal skin [163,164] • Degenerated: ≥ 8-month-old tissues [163], cryopresereved perinatal grafts [164] • Spermatogonia: fresh adult grafts [164] • Elongating spermatids: 7-month-old grafts [163] • Spermatozoa: prepubertal grafts [162]; 8-to 16-week-old grafts ...
... Autologous/Allogenic transplantation Mouse Honaramooz et al. [36] Schlatt et al. [157] Shinohara et al. [37] Schlatt et al. [35] Geens et al. [158] Yu et al. [159] Goossens et al. [160] Neonatal tissues Fresh-dorsal skin [35,36,[157][158][159][160] Cryopreserved-dorsal skin [36,157,160]; scrotum [37] • Spermatids: [157] • Spermatozoa: [158][159][160] • Embryos: [36] • Offspring: [35,37] Monkey Luetjens et al. [161] Fayomi et al. [40] Prepubertal tissues Fresh-dorsal skin [40,161]; scrotum [40,161] Cryopreserved-dorsal skin [40,161]; scrotum [40,161] Adult tissues Fresh-dorsal skin [161]; scrotum [161] Cryopreserved-dorsal skin [161] • Degenerated tubules: fresh adult grafts under dorsal skin [53] • Spermatogonia: fresh prepubertal grafts under dorsal skin [53] • Spermatocytes: fresh and cryopreserved prepubertal grafts under dorsal skin [53] • Spermatozoa: fresh prepubertal grafts under dorsal skin and in the scrotum [161]; all grafts [40] • Offspring [40] (Continued) [157] Cryopreserved-dorsal skin [157] • Spermatozoa: [157] Cat Snedaker et al. [162] Kim et al. [163] Arregui et al. [164] Fetal tissues Cryopreserved-dorsal skin [164] Neonatal tissues Cryopreserved-dorsal skin [164] Prepubertal tissues Fresh-dorsal skin [162,163] Adult tissues Fresh-dorsal skin [163,164] • Degenerated: ≥ 8-month-old tissues [163], cryopresereved perinatal grafts [164] • Spermatogonia: fresh adult grafts [164] • Elongating spermatids: 7-month-old grafts [163] • Spermatozoa: prepubertal grafts [162]; 8-to 16-week-old grafts ...
... Autologous/Allogenic transplantation Mouse Honaramooz et al. [36] Schlatt et al. [157] Shinohara et al. [37] Schlatt et al. [35] Geens et al. [158] Yu et al. [159] Goossens et al. [160] Neonatal tissues Fresh-dorsal skin [35,36,[157][158][159][160] Cryopreserved-dorsal skin [36,157,160]; scrotum [37] • Spermatids: [157] • Spermatozoa: [158][159][160] • Embryos: [36] • Offspring: [35,37] Monkey Luetjens et al. [161] Fayomi et al. [40] Prepubertal tissues Fresh-dorsal skin [40,161]; scrotum [40,161] Cryopreserved-dorsal skin [40,161]; scrotum [40,161] Adult tissues Fresh-dorsal skin [161]; scrotum [161] Cryopreserved-dorsal skin [161] • Degenerated tubules: fresh adult grafts under dorsal skin [53] • Spermatogonia: fresh prepubertal grafts under dorsal skin [53] • Spermatocytes: fresh and cryopreserved prepubertal grafts under dorsal skin [53] • Spermatozoa: fresh prepubertal grafts under dorsal skin and in the scrotum [161]; all grafts [40] • Offspring [40] (Continued) [157] Cryopreserved-dorsal skin [157] • Spermatozoa: [157] Cat Snedaker et al. [162] Kim et al. [163] Arregui et al. [164] Fetal tissues Cryopreserved-dorsal skin [164] Neonatal tissues Cryopreserved-dorsal skin [164] Prepubertal tissues Fresh-dorsal skin [162,163] Adult tissues Fresh-dorsal skin [163,164] • Degenerated: ≥ 8-month-old tissues [163], cryopresereved perinatal grafts [164] • Spermatogonia: fresh adult grafts [164] • Elongating spermatids: 7-month-old grafts [163] • Spermatozoa: prepubertal grafts [162]; 8-to 16-week-old grafts ...
Medical treatments for cancers or other conditions can lead to permanent infertility. Infertility is an insidious disease that impacts not only the ability to have a biological child, but also the emotional well-being of the infertile individuals, relationships, finances, and overall health. Therefore, all patients should be educated about the effects of their medical treatments on future fertility and about fertility preservation options. The standard fertility preservation option for adolescent and adult men is sperm cryopreservation. Sperm can be frozen and stored for long periods of time, thawed at a later date and, used to achieve pregnancy with existing assisted reproductive technologies. However, sperm cryopreservation is not applicable for prepubertal patients who do not yet produce sperm. The only fertility preservation option available to prepubertal boys is testicular tissue cryopreservation. Next-generation technologies are being developed to mature those testicular cells or tissues to produce fertilization-competent sperm. When sperm and testicular tissues are not available for fertility preservation, induce pluripotent stem cells (iPSCs) derived from somatic cells such as blood or skin may provide an alternative path to produce sperm through a process call in vitro gametogenesis. This review describes standard and experimental options to preserve male fertility as well as experimental options to produce functional spermatids or sperm from immature cryopreserved testicular tissues or somatic cells.
... Compared with immature testicular tissue, the adult testicular tissue transplant usually showed poor outcomes due to its sensitivity to ischemia and hypoxia during the grafting procedure [13]. Different prepubertal donor ages were also proven to affect graft outcome, for example, testicular tissue from a 6-month-old lynx survived better than those from perinatal and 2-year-old lynx after xenografting [14]; therefore, it is still necessary to understand whether prepubertal testes of different stages may exhibit different results after transplantation. Generally, castrated immunodeficient mice were chosen as the transplantation host; however, in some cases, it was found that castration of mice before the transplantation did not modify the outcome of pig testis xenografts [15], and spermatogenic arrest was observed in buffalo testis tissue grafts [16]. ...
In the past two decades, testicular tissue grafting and xenografting have been well established, with the production of fertilization-competent sperm in some studies. However, few studies have been carried out to observe the development of grafted prepubertal testicular tissue of rats and compare the biological differences between in situ testis and grafted testis. In this study, we established the prepubertal testicular tissue xenografting model using a 22-day-old rat and evaluated certain parameters, including testicular histology, testosterone production, and ultrastructure of the grafted testes. We also assessed gene expression of cell proliferation markers, testicular cell markers, and antioxidative defense system. Our results showed that 47 days after transplantation, intratesticular testosterone concentration was not significantly altered; however, cell proliferation, spermatogenesis, and Sertoli cell markers in the transplanted testes were significantly disrupted compared with the control group, accompanied by aggravated apoptosis and oxidative damage. Moreover, the transplanted testes showed smaller tubular diameter and disrupted spermatogenic epithelium with apparent vacuoles, distorted and degenerated germ cells with obscure nuclear margin, and no spermatids in the center of the tubules. Although testis xenografting has been extensively tested and attained great achievement in other species, the prepubertal rat testicular tissue xenografting to immunodeficient mice exhibited obvious spermatogenesis arrest and oxidative damage. The protocol still needs further optimization, and there are still some unknown factors in prepubertal rat testes transplantation.
... Support from this idea comes from studies of xenografting in which testicular tissue fragments were transplanted subcutaneously to immunodeficient mice (11,188). Under these conditions communication between the endocrine system and the donor testis become functional and this leads to full spermatogenesis and competent sperm cells in grafts from species that are distantly related from mice, such as primates (180,339), carnivores (1, 386) and ungulates (12,181,337,338). ...
The spermatozoon is a highly differentiated and polarized cell, with two main structures: the head, containing a haploid nucleus and the acrosomal exocytotic granule, and the flagellum, which generates energy and propels the cell; both structures are connected by the neck. The sperm's main aim is to participate in fertilization, thus activating development. Despite this common bauplan and function there is an enormous diversity in structure and performance of sperm cells. For example, mammalian spermatozoa may exhibit several head patterns and overall sperm lengths ranging from ~30 to 350 µm. Mechanisms of transport in the female tract, preparation for fertilization, and recognition and interaction with the oocyte also show considerable variation. There has been much interest in understanding the origin of this diversity, both in evolutionary terms and in relation to mechanisms underlying sperm differentiation in the testis. Here, relationships between sperm bauplan and function are examined at two levels. First, analyzing the selective forces that drive changes in sperm structure and physiology to understand the adaptive values of this variation and impact on male reproductive success. Second, examining cellular and molecular mechanisms of sperm formation in the testis that may explain how differentiation can give rise to such a wide array of sperm forms and functions.
Open access: https://doi.org/10.1152/physrev.00009.2020
... Lessons learned from transplantation of fresh or cryopreserved animal testicular tissue (excluding NHPs) Testicular tissue transplantation has been reported with different results depending on the goal of the research teams. During the last decade, most studies have used tissue transplantation as a tool to evaluate cryopreservation protocols or techniques (Milazzo et al., 2010;Abrishami et al., 2010b;Baert et al., 2012;Mota et al., 2012;Kaneko et al., 2013;Yildiz et al., 2013;Arregui et al., 2014;Pothana et al., 2015;Pukazhenthi et al., 2015;Yamini et al., 2016;Benvenutti et al., 2018;Kirpatovskii et al., 2018) and to study the impact of tissue cooling at 4 C on tissue survival and function. Maintenance of immature porcine testis tissue integrity, survival and proliferative potential after storage at 4 C for 72 h (Abrishami et al., 2010b), graft development of bison ITT after storage overnight at 4 C (Abbasi and Honaramooz, 2011) and spermatogenic potential of cat ITT preserved on ice for up to 5 days (Mota et al., 2012) represent relevant observations when developing FP networks where testicular tissue transport before cryopreservation may be required. ...
... Indeed, while the ability of young testis tissue to reinitiate spermatogenesis when grafted has been well demonstrated, transplantation of adult mammal testicular tissue has not resulted in germ cell differentiation and tissue degeneration was often observed (Schlatt et al., 2002;Geens et al., 2006;Rathi et al., 2006;Schlatt et al., 2006;Kim et al., 2007;Arregui et al., 2008;Abrishami et al., 2010a). Differences in graft outcome were also observed for different prepubertal donor ages, e.g. 6 months old lynx ITT survived better than perinatal and 2 years old ITT after xenografting (Arregui et al., 2014). It may thus be anticipated that graft outcomes of ITT from prepubertal boys of different ages will also lead to different results, but further studies are needed to answer this question. ...
BACKGROUND
Childhood cancer incidence and survivorship are both on the rise. However, many lifesaving treatments threaten the prepubertal testis. Cryopreservation of immature testicular tissue (ITT), containing spermatogonial stem cells (SSCs), as a fertility preservation (FP) option for this population is increasingly proposed worldwide. Recent achievements notably the birth of non-human primate (NHP) progeny using sperm developed in frozen-thawed ITT autografts has given proof of principle of the reproductive potential of banked ITT. Outlining the current state of the art on FP for prepubertal boys is crucial as some of the boys who have cryopreserved ITT since the early 2000s are now in their reproductive age and are already seeking answers with regards to their fertility.
OBJECTIVE AND RATIONALE
In the light of past decade achievements and observations, this review aims to provide insight into relevant questions for clinicians involved in FP programmes. Have the indications for FP for prepubertal boys changed over time? What is key for patient counselling and ITT sampling based on the latest achievements in animals and research performed with human ITT? How far are we from clinical application of methods to restore reproductive capacity with cryostored ITT?
SEARCH METHODS
An extensive search for articles published in English or French since January 2010 to June 2020 using keywords relevant to the topic of FP for prepubertal boys was made in the MEDLINE database through PubMed. Original articles on fertility preservation with emphasis on those involving prepubertal testicular tissue, as well as comprehensive and systematic reviews were included. Papers with redundancy of information or with an absence of a relevant link for future clinical application were excluded. Papers on alternative sources of stem cells besides SSCs were excluded.
OUTCOMES
Preliminary follow-up data indicate that around 27% of boys who have undergone testicular sampling as an FP measure have proved azoospermic and must therefore solely rely on their cryostored ITT to ensure biologic parenthood. Auto-transplantation of ITT appears to be the first technique that could enter pilot clinical trials but should be restricted to tissue free of malignant cells. While in vitro spermatogenesis circumvents the risk linked to cancer cell contamination and has led to offspring in mice, complete spermatogenesis has not been achieved with human ITT. However, generation of haploid germ cells paves the way to further studies aimed at completing the final maturation of germ cells and increasing the efficiency of the processes.
WIDER IMPLICATIONS
Despite all the research done to date, FP for prepubertal boys remains a relatively young field and is often challenging to healthcare providers, patients and parents. As cryopreservation of ITT is now likely to expand further, it is important not only to acknowledge some of the research questions raised on the topic, e.g. the epigenetic and genetic integrity of gametes derived from strategies to restore fertility with banked ITT but also to provide healthcare professionals worldwide with updated knowledge to launch proper multicollaborative care pathways in the field and address clinical issues that will come-up when aiming for the child’s best interest.
... This concept has also been supported through the use of testis tissue cryopreservation and TTX using domestic mammals (e.g., sheep, goat, cattle, horse, pig, alpaca, cat, dog) as a model for endangered counterparts (e.g., ungulate herbivores, equid, suid, camelid, felid, canid) (Abrishami et al. 2010a, b;Elzawam et al. 2013;Oatley et al. 2005;Rathi et al. 2006;Snedaker et al. 2004). We and others have also obtained promising results and even donor-derived sperm by using TTX from donors of some of the target wildlife species such as the Javan banteng, Iberian lynx, Cuvier gazelle, Mohor gazelle, white-tailed deer, and bison Honaramooz 2011, 2012;Arregui et al. 2013;Honaramooz et al. 2005). ...
Spermatogonial stem cells (SSCs) are a rare group of cells in the testis that undergo self-renewal and complex sequences of differentiation to initiate and sustain spermatogenesis, to ensure the continuity of sperm production throughout adulthood. The difficulty of unequivocal identification of SSCs and complexity of replicating their differentiation properties in vitro have prompted the introduction of novel in vivo models such as germ cell transplantation (GCT), testis tissue xenografting (TTX), and testis cell aggregate implantation (TCAI). Owing to these unique animal models, our ability to study and manipulate SSCs has dramatically increased, which complements the availability of other advanced assisted reproductive technologies and various genome editing tools. These animal models can advance our knowledge of SSCs, testis tissue morphogenesis and development, germ-somatic cell interactions, and mechanisms that control spermatogenesis. Equally important, these animal models can have a wide range of experimental and potential clinical applications in fertility preservation of prepubertal cancer patients, and genetic conservation of endangered species. Moreover, these models allow experimentations that are otherwise difficult or impossible to be performed directly in the target species. Examples include proof-of-principle manipulation of germ cells for correction of genetic disorders or investigation of potential toxicants or new drugs on human testis formation or function. The primary focus of this review is to highlight the importance, methodology, current and potential future applications, as well as limitations of using these novel animal models in the study and manipulation of male germline stem cells.
... antibody (1:100; Z511601-2-DAKO, Denmark, overnight at 8˚C). This antibody labels all gonocytes, spermatogonia and pre-leptotene germ cells from the domestic cat [18,19]. For nonspecific labeling controls, sections were incubated in blocking solution without primary antibody (negative control) or with rabbit IgG (I5006, Sigma-Aldrich; unspecific IgG labeling control). ...
The reduced number of animals in most wild felid populations implies a loss of genetic diversity. The death of juveniles, prior to the production of mature sperm, represents a loss of potential genetic contribution to future populations. Since 2011 mouse testicular organ culture has introduced an alternative mechanism to produce sperm in vitro from immature tissue. However, extension of this technology to other species has remained limited. We have used the domestic cat (Felis catus) as a model for wild felids to investigate spermatogenesis initiation and regulation, with the mouse serving as a control species. Testicular tissue fragments were cultured in control medium or medium supplemented with knockout serum replacement (KSR), AlbuMax, beta-estradiol or AlbuMax plus beta-estradiol. Contrary to expectations, and unlike results obtained in mouse controls, no germ cell differentiation could be detected. The only germ cells observed after six weeks of culture were spermatogonia regardless of the initial stage of tubule development in the donor tissue. Moreover, the number of spermatogonia decreased with time in culture in all media tested, especially in the medium supplemented with KSR, while AlbuMax had a slight protective effect. The combination of AlbuMax and beta-estradiol led to an increase in the area occupied by seminiferous tubules, and thus to an increase in total number of spermatogonial cells. Considering all the media combinations tested the stimulus for felid germ cell differentiation in this type of system seems to be different from the mouse. Studies using other triggers of differentiation and tissue survival factors should be performed to pursue this technology for the genetic diversity preservation in wild felids.
... Biobanking of ovarian and testicular tissues from Iberian lynx killed in road accidents or died within the breeding programme was conducted from the very beginning of the conservation breeding programme. Tissue can be cryopreserved for future use in xenotransplantation (Arregui, Dobrinski, & Roldan, 2014). ...
Contents
Assisted reproductive technology ( ART ) has great potential for conservation, but its successful application in captive breeding programmes of endangered species is often compromised by limited background on species' biology. Although carnivore species benefit from knowledge obtained in domesticated species (dogs, cats and ferrets), the focus of research is different. In pet animals, research in reproduction has mainly been focused on ovarian function and contraception, although substantial progress has also been made in the field of in vitro embryo production, transgenic embryos and cloning to aid relevant medical models. In endangered species, however, research should focus on characterizing reproductive traits (cyclicity and seasonality) to unravel species‐specific endocrine principles of reproduction physiology. Based on this knowledge, it is crucial to enhance the ability to manipulate female reproductive cycles, especially those of embryo recipients. Furthermore, research conducted on molecular and cellular mechanisms of gamete and embryo development, as well as on cryopreservation protocols of gametes and embryos, is required for successful implementation of advanced ART to wild carnivores. This review will provide a summary on the state of the art with focus on ART contributing to conservation breeding of endangered carnivores.