Figure 1 - available via license: Creative Commons Attribution 4.0 International
Content may be subject to copyright.
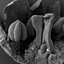
Glyptostrobus pensilis, wild-type seed cones at pollination (A-E), seed ripening (F and G) and seed dehiscence (H and I). (A and F-I) taken using macrophotography; (B, C and E) SEM of dissected cone scales; (D) light micrograph. (A) Entire cone from above; only the bract and micropylar tubes are visible externally. (B) Ovulate cone scale with two ovules; several abaxial teeth are present and significantly shorter than the ovules. (C) Cone scale with a single ovule and several abaxial teeth. (D) Longitudinal section of a fertile cone scale; the bract, ovule and abaxial teeth are each supplied by an individual vascular bundle. (E) Sterile basal cone scale showing only axillary teeth (ovules not developed). (F) Fertilised seed cone in lateral view. (G) Detail of a cone scale; a series of nine axillary teeth (numbered 1-9) form an arc above the bract. (H) Seed cone after release of seeds. (I) Detail of a single cone scale removed from (H); a clear boundary layer is marked as white dotted line between the bract and the distal teeth. Key: B, bract; I, integument; M, mucro; N, nucellus; O, ovule; T, tooth; TL, transitional leaf; VBB, vascular bundle of bract; VBO, vascular bundle of ovule; VBOS, vascular bundle of ovuliferous scale (= teeth). Photo credit: V.M. Dörken. Full-size DOI: 10.7717/peerj.4948/fig-1
Source publication
Both wild-type and teratological seed cones are described in the monoecious conifer Glyptostrobus pensilis and compared with those of other Cupressaceae sensu lato and other conifers. Some Cupressaceae apparently possess a proliferation of axillary structures in their cone scales. In our interpretation, in Glyptostrobus each bract of both typical a...
Contexts in source publication
Context 1
... cones are developed terminally on short lateral shoots and subtended by closely spaced scale-like leaves. At pollination stage, the cone is typically shaped like a spiked ball or mace (Fig. 1A) with a more-or-less plagiotropic orientation, though soon after pollination it changes to an upright position. The cone consists of several helically arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). ...
Context 2
... lateral shoots and subtended by closely spaced scale-like leaves. At pollination stage, the cone is typically shaped like a spiked ball or mace (Fig. 1A) with a more-or-less plagiotropic orientation, though soon after pollination it changes to an upright position. The cone consists of several helically arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of ...
Context 3
... ball or mace (Fig. 1A) with a more-or-less plagiotropic orientation, though soon after pollination it changes to an upright position. The cone consists of several helically arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial ...
Context 4
... pollination it changes to an upright position. The cone consists of several helically arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial teeth does not correlate with the number of ovules. In the material investigated, the ...
Context 5
... cone consists of several helically arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial teeth does not correlate with the number of ovules. In the material investigated, the number of adaxial teeth per bract varied from three to 11. ...
Context 6
... arranged cone scales that overlap each other (Figs. 1A, 1F and 1G). Only the cone scales in the middle of the seed cone are fertile; the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial teeth does not correlate with the number of ovules. In the material investigated, the number of adaxial teeth per bract varied from three to 11. Teeth are also present on ...
Context 7
... the basal and distal ones are sterile (Fig. 1A). Several sterile transitional leaves are present below the basal cone scales (Fig. 1F). There are mostly two ovules per cone scale (Figs. 1A and 1B), or rarely one (Fig. 1C), three were not found. At the stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial teeth does not correlate with the number of ovules. In the material investigated, the number of adaxial teeth per bract varied from three to 11. Teeth are also present on the sterile distal and basal cone scales (Fig. 1F), which were described as semifertile by Takaso & Tomlinson (1990). After pollination, the ...
Context 8
... stage of pollination, several small teeth are present below the ovules in the axial of the bract (Figs. 1B-1D). The number of abaxial teeth does not correlate with the number of ovules. In the material investigated, the number of adaxial teeth per bract varied from three to 11. Teeth are also present on the sterile distal and basal cone scales (Fig. 1F), which were described as semifertile by Takaso & Tomlinson (1990). After pollination, the teeth become strongly elongated by intercalary growth so that they finally exceed the bract in length (Figs. 1F-1I) and take part in closing the cone (Figs. 1F and 1G). At maturity, a series of outer teeth forms an arc above the bract (Figs. ...
Context 9
... cone scales (Fig. 1F), which were described as semifertile by Takaso & Tomlinson (1990). After pollination, the teeth become strongly elongated by intercalary growth so that they finally exceed the bract in length (Figs. 1F-1I) and take part in closing the cone (Figs. 1F and 1G). At maturity, a series of outer teeth forms an arc above the bract (Figs. ...
Context 10
... cone scale, axillary teeth and ovules are each supplied by an individual vascular bundle that enters the stem bundle of the cone axis as a separate strand (Fig. 1D). Stomata are irregularly but densely scattered on both sides of the bract and axillary teeth (Figs. 1B, 1C, 1E and ...
Context 11
... seed cones are pyriform to obovate in shape (Fig. 1F). The apex of the cone axis is used up in forming the distal cone scales. The teeth are adnate to the bract for half their length (Figs. 1F-1I) and also basally connate (Figs. 2A-2C) so that only their distal tips remain free ( Fig. 2A). There is no distinct boundary between the bract and the axillary teeth (Fig. 2D), but the tissue of ...
Context 12
... its tip becomes shifted to the back of the bract; the tip is visible at maturity as a small backward-pointing mucro (Figs. 1F, 1G, 1I, 2A, 2B and 2D). The seed cones mature in the year of pollination and dry out to release the winged seeds. Shrinking of the cone axis and cone scales causes the cones to open and the cone scales to spread apart ( Fig. 1H). At maturity, the bracts and teeth remain fused to each other ( Fig. ...
Context 13
... maturity as a small backward-pointing mucro (Figs. 1F, 1G, 1I, 2A, 2B and 2D). The seed cones mature in the year of pollination and dry out to release the winged seeds. Shrinking of the cone axis and cone scales causes the cones to open and the cone scales to spread apart ( Fig. 1H). At maturity, the bracts and teeth remain fused to each other ( Fig. ...
Context 14
... and the tongue-like structures of Athrotaxoideae and Cunninghamioideae. In all three genera of Taxodioideae (including Glyptostrobus), the teeth start to develop relatively late, significantly after ovule formation, so that they are shorter than the ovules at pollination (Takaso & Tomlinson, 1989, 1990Jagel, 2002;Jagel & Dörken, 2014; this paper, Fig. 1). During maturation they become fused to each other and further growth occurs basal to the teeth in an intercalary fashion. Only their distal tips remain free and distinctly exceed the bract in length, so that at maturity an arc is visible above each bract, formed by a series of teeth. Thus, the teeth participate in closure of the seed ...
Context 15
... vasculature of the wild-type Glyptostrobus seed cones investigated here provides further evidence for the presence of descending accessory shoots. Three distinct vascular bundle strands are present in a typical fertile bract/scale complex (Aase, 1915; this paper, Fig. 1D). The bract, the sterile region of the seed scale (i.e. the teeth) and the fertile region of the seed scale (which is reduced to its ovules) are each supplied with their own vascular bundle. The vascular bundles are not fused to each other and enter the concentric bundle of the cone axis as separate strands. This vascular branching ...
Context 16
... arose from the end of the seed-scale bundle (Florin, 1951;Sporne, 1965;Stewart & Rothwell, 1993). In contrast, in Glyptostrobus each ovule is supplied by a distinct vascular bundle strand that enters the stem vasculature separately and does not fuse with the bundle strands of the lower teeth and the bract before diverging from the cone axis (Fig. 1D). The Glyptostrobus ovules are not supplied by a vascular strand derived from the seed-scale strand, a critical difference from ...
Citations
... Therefore, protecting and expanding its remaining habitat and increasing its population size are the most important measures to protect G. pensilis [29]. At present, there are few studies on G. pensilis, most of which focus on population dynamics, physiological ecology, reproductive biology and genetic diversity [27][28][29][30][31][32][33][34][35][36][38][39][40]. However, none of the above studies has carried out an in-depth study on the relationship between climate factors and the distribution pattern of G. pensilis. ...
Glyptostrobus pensilis is a critically endangered living fossil plant species of the Mesozoic era, with high scientific research and economic value. The aim of this study was to assess the impact of climate change on the potential habitat area of G. pensilis in East Asia. The MaxEnt (maximum entropy) model optimized by the ENMeval data package was used to simulate the potential distribution habitats of G. pensilis since the last interglacial period (LIG, 120–140 ka). The results showed that the optimized MaxEnt model has a high prediction accuracy with the area under the receiver operating characteristic curve (AUC) of 0.9843 ± 0.005. The current highly suitable habitats were found in the northeast of Jiangxi, the east of Fujian; the main climatic factors affecting the geographic distribution of G. pensilis are temperature and precipitation, with precipitation as the dominant factor. The minimum temperature of coldest month (Bio6) may be the key factor restricting the northward distribution of G. pensilis; during the LIG, it contracted greatly in the highly suitable habitat area. In the 2070s, the suitable distribution area will move northward and the area of highly suitable habitat will increase. Seasonal changes in temperature and precipitation may be important climatic factors causing the changes in geographic distribution. The results will provide a theoretical basis for the management and resource protection of G. pensilis.
... In some cases, character coding or scoring was altered from the original matrix to accommodate modified character definitions and to eliminate discrepancies between similar characters shared by different matrices. Additional data on the anatomy and morphology of extant and fossil taxa were obtained from the literature (Radais 1894;Carothers 1907;Owens and Blake 1983;Mapes and Rothwell 1984;Takaso and Tomlinson 1989, 1992Tomlinson and Takaso 1989;Tomlinson 1992;Rothwell 1993;Owens et al. 1995Owens et al. , 1998Runions et al. 1995;Takaso and Owens 1995;Mill et al. 2001;Farjon andGarcia 2002, 2003;Schulz and Stützel 2007;Hernandez-Castillo et al. 2009;Escapa et al. 2012;Jagel and Dörken 2014, 2015a, 2015bDörken and Nimsch 2015;Farjon 2017;Dörken and Rudall 2018). More generally, the matrix was designed to accommodate expanded taxon sampling of both extant and fossil seed plants in future analyses. ...
... In Podocarpaceae, the ovuliferous scale is modified into an epimatium surrounding the seed, which is subtended by a bract that is sometimes fused with the axis and other bracts to form a fleshy receptacle (Mill et al. 2001), an interpretation supported by developmental morphology Tomlinson 1992) and genetics (Englund et al. 2011). In most Cupressaceae, developmental studies indicate that the ovuliferous scale is reduced down to either just the ovules or a small pad of tissue, and seeds are instead protected by the bract (Groth et al. 2011;Jagel andDörken 2014, 2015a;Dörken and Rudall 2018). However, some taxodiaceous Cupressaceae, such as Cryptomeria and Cunninghamia, have a prominent lobed structure (sometimes referred to as "teeth") between the ovules and MATSUNAGA ET AL.-OVULATE CONES OF SCHIZOLEPIDOPSIS EDIAE bract that is often interpreted as the ovuliferous scale (e.g., Schulz and Stützel 2007). ...
... The so-called "voltzialean" or "transition" conifers of the Permian and Triassic, (see Clement-Westerhof 1987;Looy 2007;Taylor et al. 2009;Pacyna et al. 2017), produced ovuliferous scales with lobes that are typically interpreted as the remains of leaves (Fig. 4). Extant clades emerged in the Late Triassic (Taylor et al. 2009;Leslie et al. 2012), and their ovuliferous scales typically lack any trace of appendages ( Fig. 4; although this series of events may be more complex and lineage-specific in reality; see Schulz et al. 2014;Dörken and Rudall 2018). Shifts in developmental timing have been invoked to explain some of these changes (see Rothwell et al. 2011;Spencer et al. 2015), and initiating ovules earlier in fertile shoot ontogeny would result in mature ovuliferous scales with either partially-formed leafy appendages (i.e., lobes) or none at all, depending on the extent to which they completed the ancestral pattern of development (Fig. 4). ...
Biologists often study morphological evolution through form and function relationships. But biological structures can perform multiple functional roles, complicating efforts to understand the evolutionary significance of any one relationship. Plant reproductive organs perform multiple roles in a sequence, however, which provides a unique opportunity to understand how structures evolve to meet multiple functional demands. Using conifers as a study group, we discuss how a shared developmental trajectory links the performance of sequential functional roles. Variation in development among lineages can underlie morphological diversity; pollination-stage seed cones in Pinaceae conifers function similarly but show diverse forms reflecting differences in developmental rate. As cones develop further, the morphologies that they use to perform later functional roles is influenced by the specific developmental patterns used to meet earlier demands, which may ultimately limit morphological diversity. However, we also show how selective pressures relating to the final functional stage (seed dispersal) may influence cone anatomy and morphology over all previous stages, highlighting their complex linkages among form, function, and development. We end by discussing the potential relationships between functional ontogeny and morphological disparity in plant reproductive structures more broadly, suggesting that the complex functional roles associated with seed plant reproduction probably underlies the high disparity in this group.
... Since the publication of Goethe's illustrations of the rose (Goethe, 1790), it has been 168 known that for some teratological conditions the floral apex can persist and continue to grow 169 as a shoot. The terata that transition from determinate shoots to indeterminate shoots are 170 found primarily in gymnosperms (Rudall et al., 2011;Dörken & Rudall, 2018). In fact, in conifers, and Gnetales, they are determinate. ...
There are two competing hypotheses for the origin of flowers. The traditional hypothesis
is phyllosporous origin which regards a conduplicate carpel as an ancestral form that is the result of longitudinal folding of a leaf bearing ovules along its margins. Alternatively, the carpel formation is the result of a fusion between an ovule-bearing branch and its subtending leaf-like structure; if true, the ovule would originally occur at the middle of the leaf-like structure. For the majority of the angiosperms, the carpel is a single leaf-like structure with the ovule developing later on the lamina. Illicium is a member of the Austrobaileyales, which are one of the three ANA lines that diverged before all other extant angiosperms. This genus with apocarpous gynoecium has various ancestral morphological characteristics in terms of carpel, ovule, and floral apex. Although various aspects of Illicium morphology have been previously investigated, many evolutionary characteristics remain unclear. A more detailed examination of the carpel, ovule, and floral apex of Illicium is needed. In this study, the development of carpel, ovule, and floral apex of I. lanceolatum was studied using LM and SEM. The results showed that the ovule primordium originates at the point where the carpel touches the floral axis. Based on the comparison with other taxa, the evolutionary implication of a persistent floral apex is an apical meristem remnant of a shoot. So the carpel of Illicium is a leaf-like structure that encircles the ovule. This kind of carpel fits the hypothesis that the carpel is a fusion of two parts, ovule-bearing branch and its subtending leaf-like structure.
Different types of pollen cone teratologies in Sequoia sempervirens and Widdringtonia nodiflora were investigated. While wild type pollen cones are uniaxial and hyposporangiate, some teratological polyaxial pollen cones and also perisporangiate microsporangiophores were found. The teratological compound pollen cones in Sequoia seem to represent just random artifacts caused by a spitting of the pollen cone apex, maybe due to a pathogenic event in early developmental stages. In Widdringtonia it remains open, if the compound male reproductive units represent a dense distal cluster of pollen cones or a compound, polyaxial inflorescence comparable to other conifers, e.g. Cephalotaxus (Taxaceae). The perisporangiate microsporangiophores found in some of the teratological pollen cones in Widdringtonia are remarkable, because among extant conifers this type of microsporangiophores is developed only in Taxaceae. Previous studies showed that the perisporangiate type of microsporangiophores represents a radial synangium consisting of several fused hyposporangiate microsporangiophores. However, the perisporangiate microsporangiophores in Taxaceae and Widdringtonia show some distinct structural differences excluding the perisporangiate microsporangiophores in Widdringtonia as representing a radial synangium.
Reproductive structures of both genders of Wollemia nobilis were investigated, including both wild-type and teratological cones. Typically, both pollen cones and seed cones in this species are terminal on first order branches. At maturity, wild-type pollen cones are pendulous and cylindrical; wild-type seed cones are broad and ellipsoidal in shape. The teratological structures consisted of a basal region that resembled a typical fertile seed cone, and an apical proliferation that terminated in a well-developed pollen cone, resulting in a ‘bisexual’ unit. The proximal seed cone and the distal pollen cone were separated by a sterile region that represents an elongation of the cone axis. Of a total of 14 anomalous bisexual units investigated, all had the same bauplan. Such an arrangement of basal ovulate and distal staminate reproductive structures in a teratological conifer cone has previously not been reported for Wollemia. This topology is 'inside-out' with respect to most other reported anomalous bisexual conifer cones, which possess proximal staminate and distal ovulate structures. We discuss these spontaneous abnormalities in the broader context of understanding the homologies of seed-plant reproductive structures. The patterning of conifer cones is apparently highly labile, perhaps related to the extended cone axis and relatively long developmental duration.