Fig 5 - uploaded by Ekaterina V Raikova
Content may be subject to copyright.
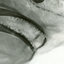
Gametogenesis in ‘male’ gonad (schema) and the corresponding stages of the anatomy of the gonad of Polypodium hydriforme . (A) Endodermal fold within gastric cavity; (B) multiplication of gonocytes; (C) meiotic prophase; (D) first meiotic division, reconstitution of the endodermal envelope of the gonad; (E) second meiotic division, formation of the ectodermal lid of the gonad; (F) binucleate cells, mature gonad. bc, binucleate cell (corresponds to a spermatid); cc, somatic cap cells; ec, ectoderm; ecl, ectodermal lid; en, endoderm; enf, endodermal fold, its flagellated cells contain food inclusions; g, gonad; meiocytes I, II, (correspond to spermatocytes I and II in a classic spermatogenesis); I md, first meiotic division; II md, second meiotic division.
Source publication
Cytomorphological characters of Polypodium hydriforme—a unique cnidarian, adapted to intracellular parasitism inside oocytes of Acipenseriform fish—are reviewed and analysed. Polypodium's unique characters, those shared with the Myxozoa, and the characters shared with bilateral triploblastic animals are listed. The set of unique features has allowe...
Contexts in source publication
Context 1
... In origin, cnidarians are considered either as derivatives of Triploblastica (Boero et al. , 1998), or as sharing a common ancestor with them (Gr ́ ́ger & Schmid, 2001; Piraino et al. , 2003). The idea about ‘bilateriality’ of Cnidaria is by no means new; V. Beklemishev (1964) reported an in-depth analysis of cnidarian symmetry and found vivid examples of bilateral symmetry in each cnidarian group. Recently, the authors debating this problem, have advanced additional arguments based on morphological, molecular development and molecular phylogenetic data (e.g. Martindale, 2005; Seipel & Schmid, 2006). Without data on P. hydriforme , a comparative analysis of cnidarian morphological characters appears obviously incomplete. The present paper analyses the cytomorphological features of this unusual animal on subsequent stages of its life cycle. Polypodium ’s life cycle consists of two phases: a parasitic one and a free-living one (Figure 1). Polypodium ’s embryogenesis takes place inside the developing host oocytes and spans several years (Raikova, 1987). The initial stage of infection looks like a binucleate cell, with one big and one small nucleus (Figure 2A). By means of endocytokinesis, the small nucleus becomes surrounded by a portion of cytoplasm, thus a new smaller cell is formed inside the large cell with the bigger nucleus. This stage is known as the ‘cell-in-a-cell’ stage (Figure 2B). The outer large cell becomes the trophamnion cell, while the internal small cell undergoes cleavage (Figure 2C, D). The internal generative cell and the early blastomeres (down to the 16-cell stage) keep haploid quantity of DNA inherited from the post-meiotic pre-gametes, formed in the free-living Polypodium (Raikova, 1965, 1987). The state of haploidy of the generative nucleus and its derivatives, the blastomeres, lasts for some years. The moment and manner of restoring diploidy, characteristic of free-living P. hydriforme , remains unclear, but fusion of blastomeres is hypothesized. After gastrulation by delamination, a two-layered planuliform larva with inverted position of germ layers is formed (Figure 2E). The larva grows into a long stolon that forms numerous internal tentacles (Figure 2F). All these stages develop in the trophamnion cell’s cavity. The trophamnion is homologous to the polar body of the second meiotic division (Raikova, 1980). Since the ‘cell-in-a-cell’ stage, the trophamnion carries out protective and trophic functions. Its nucleus branches and reaches a high degree of polyploidy—more than 400 C (Raikova, 1965, 1987). At the end of the parasitic phase of the life cycle, the trophamnion shows obvious apoptotic features: a picnotic nucleus with per- ipheral chromatin caps, occurrence of intranuclear cristal- loids, and membrane profiles in cytoplasm (Raikova, 1984). Before the spawning of the host fish, the parasitic stolon inside the infected egg turns inside out, with the ectoderm now facing outwards; the trophamnion cell is destroyed. During the spawning, the stolon with a normal arrangement of germinal layers emerges. Thus, in fresh water, the free- living part of the life cycle begins (Figure 1). In water, the stolon with external tentacles (Figure 3A) consistently fragments, resulting in individual specimens with six tentacles on each side (Figure 3B). Four of the initial six tentacles are sensory and two others are supporting. The free-living benthic specimens usually ‘walk’ on the bottom using the supporting tentacles. They are biradially- symmetric, lack an umbrella, the mouth is turned upwards and not surrounded by tentacles; sense organs are not observed. Twelve-tentacled specimens usually prevail. They divide asexually with a longitudinal fissure starting at the aboral pole. Before each division, in the middle of the aboral surface of the body, two complete sets of new tentacles appear. Thus, a 12-tentacled specimen becomes for some time 24-tentacled. The fissure starts near the new tentacles and continues towards the oral pole; the mouths are separated the last (Figure 3C). After division, each individual receives the complete set of 6 old tentacles on one side, and the complete set of 6 new tentacles on the other side, which slowly grow to the normal size. Thus, the free-living individuals are practically always asymmetric to some extent. They have never shown any signs of regeneration. By the middle of the summer, the free-living specimens form endodermal gonads of two kinds. The first to appear are sexual complexes consisting of two glands with gonoducts (Figure 4) opening into the gastric cavity. Gonads of the first type are ana- tomically female (Figure 4), characterized by the presence of highly polyploid (up to 37 C; Raikova, 1965) nurse cells and by gametogenesis similar to oogenesis (Lipin, 1925). However, apparently, ‘female’ gonads do not produce mature eggs and degenerate, displaying apoptotic features (Raikova, 1963, 1994). Subsequently, four ‘male’ gonads are formed (Figure 5) which are endodermal folds protruding into the gastric cavity, but lacking gonoducts (Lipin, 1925). The ‘male’ gonads can appear both in ‘female’ and in asexual specimens—then the former become hermaphrodites and the latter become ‘males’ (Raikova, 1961, 1963). Female, and male individuals differ morphologically; females are usually bigger than males, often have 24 tentacles each, and continue to multiply by means of longitudinal fission. Hermaphrodites are similar to females. Gametogenesis in ‘male’ gonads (Figure 5) is at first similar to spermatogenesis, but then changes to oogenesis (Raikova, 1961, 1994) resulting in formation of binucleate cells filling the gonad (Figure 5). The I meiocytes often have one – two adjoining somatic cap-like cells (Figure 5C) (Raikova, 1961). In the first and in the second meiotic divisions the spindle is shifted to the periphery of the cell (Figure 5D). The first meiotic division results in formation of a II meiocyte and a polar body (Figure 5D). The second meiotic division is acyto- kinetic, therefore all the resulting cells become binucleate (Figure 5F). The nuclei have unequal size, because one of the nuclei, the more central one, becomes polyploid at once (about 3 C; Raikova, 1965). Cytokinesis only occurs after a very long delay, probably lasting several years, after the binucleate cell finally gets within an oocyte of a host-fish (Raikova, 1980, 1987). Owing to the fact that both meiotic divisions in the ‘male’ gonads are unequal and produce polar bodies, and, mainly, because the next generation develops from binucleate cells formed in these gonads, we have come to the conclusion that sex reorientation of male gonads into female ones has occurred in the evolution of Polypodium (Raikova, 1985, 1994). By the end of gametogenesis, the gonad fills with binucleate cells becoming a gametophore; situated at the bottom is an ectodermal layer bearing nematocytes (Figure 5E, F). Free-living specimens of Polypodium were observed actively laying the gametophores on the skin of the future host, the prelarval Acipenser stellatus (Smolyanov & Raikova, 1961). After the laying of gametophores, the free-living Polypodium specimens die. How binucleate cells get inside the oocytes is still unknown. Cnidom of P. hydriforme comprises isorhizas of two cate- gories: atrichous isorhiza situated at the tips of the supporting tentacles and holotrichous isorhiza, with two rows of small spines (Ibragimov & Raikova, 2004), located along the sensory tentacles and around the mouth. Nematocytes of Polypodium are characterized by a unique, radially symmetri- cal cnidocil apparatus, with the cnidocil situated directly above the nematocyst lid (Figure 6A) (Raikova, 1990). Ectodermal cells of P. hydriforme have neither flagella, nor myofibrils (Figure 6B), and are characterized by apical granules containing acid mucopolysaccharides. The granules line the internal cavity of the parasitic stolon (Figure 6B) and later form the external cover of the free-living specimen. Endodermal (gastrodermal) cells are flagellated, but lack any muscle fibres. The pharynx region of the gastrodermis in free-living specimens is composed of collar cells (Figure 6C) (Raikova, 1995). Numerous mucous and proteinaceous gland cells occur in the gastrodermis, especially in the pharyngeal epithelium. A network of nerve fibres underlies the epidermis (Lipin, 1911). In the mesoglea beneath the epidermis, there is a layer of longitudinal smooth muscle cells independent from the epithelial ones (Figure 6B). Muscle cells are formed from i-cells migrating from ectoderm into the mesoglea (Raikova, 1961; Raikova & Napara, 1999; Raikova et al. , 2007). In the mesoglea of the mouth cone, amoebocytes have been observed (Lipin, 1911; Napara & Raikova, 2003). Extracellular microtubules have been detected within the mesoglea of the stolon (Figure 6D) (Raikova & Kameneva, 1996, 1997). Within the ‘male’ gonads, in the space between the gastrodermis and the epidermis (corresponding to the mesoglea), sexual cells develop. Ectodermal cells display septate (Figure 7A) and gap (Figure 7B) junctions; endodermal cells show a septate junction between the apical parts and interdigitations in the basal parts (Raikova, 1984). Junctions between muscular cells (Figure 7C) are similar to those in vertebrate cardiac muscles (Raikova, 1984). Mitosis in Polypodium cells is typical for Metazoa and Cnidaria. In all the cells lacking flagellae (ectodermal, i-cells and their derivatives—muscle cells and nematocytes), a pair of centrioles has been observed in contact with the nucleus, in special pockets formed by the nuclear envelope that has pronounced pores (Figure 7D) (Raikova, 1984). During mitosis, as well as during cnidogenesis, centrioles acquire the usual appear- ance, without surrounding fragments of the nuclear envelope. At all the stages of the life cycle, all Polypodium cells have mitochondria with tubular cristae (Figure 7B) (Seravin & ...
Context 2
... and molecular phylogenetic data (e.g. Martindale, 2005; Seipel & Schmid, 2006). Without data on P. hydriforme , a comparative analysis of cnidarian morphological characters appears obviously incomplete. The present paper analyses the cytomorphological features of this unusual animal on subsequent stages of its life cycle. Polypodium ’s life cycle consists of two phases: a parasitic one and a free-living one (Figure 1). Polypodium ’s embryogenesis takes place inside the developing host oocytes and spans several years (Raikova, 1987). The initial stage of infection looks like a binucleate cell, with one big and one small nucleus (Figure 2A). By means of endocytokinesis, the small nucleus becomes surrounded by a portion of cytoplasm, thus a new smaller cell is formed inside the large cell with the bigger nucleus. This stage is known as the ‘cell-in-a-cell’ stage (Figure 2B). The outer large cell becomes the trophamnion cell, while the internal small cell undergoes cleavage (Figure 2C, D). The internal generative cell and the early blastomeres (down to the 16-cell stage) keep haploid quantity of DNA inherited from the post-meiotic pre-gametes, formed in the free-living Polypodium (Raikova, 1965, 1987). The state of haploidy of the generative nucleus and its derivatives, the blastomeres, lasts for some years. The moment and manner of restoring diploidy, characteristic of free-living P. hydriforme , remains unclear, but fusion of blastomeres is hypothesized. After gastrulation by delamination, a two-layered planuliform larva with inverted position of germ layers is formed (Figure 2E). The larva grows into a long stolon that forms numerous internal tentacles (Figure 2F). All these stages develop in the trophamnion cell’s cavity. The trophamnion is homologous to the polar body of the second meiotic division (Raikova, 1980). Since the ‘cell-in-a-cell’ stage, the trophamnion carries out protective and trophic functions. Its nucleus branches and reaches a high degree of polyploidy—more than 400 C (Raikova, 1965, 1987). At the end of the parasitic phase of the life cycle, the trophamnion shows obvious apoptotic features: a picnotic nucleus with per- ipheral chromatin caps, occurrence of intranuclear cristal- loids, and membrane profiles in cytoplasm (Raikova, 1984). Before the spawning of the host fish, the parasitic stolon inside the infected egg turns inside out, with the ectoderm now facing outwards; the trophamnion cell is destroyed. During the spawning, the stolon with a normal arrangement of germinal layers emerges. Thus, in fresh water, the free- living part of the life cycle begins (Figure 1). In water, the stolon with external tentacles (Figure 3A) consistently fragments, resulting in individual specimens with six tentacles on each side (Figure 3B). Four of the initial six tentacles are sensory and two others are supporting. The free-living benthic specimens usually ‘walk’ on the bottom using the supporting tentacles. They are biradially- symmetric, lack an umbrella, the mouth is turned upwards and not surrounded by tentacles; sense organs are not observed. Twelve-tentacled specimens usually prevail. They divide asexually with a longitudinal fissure starting at the aboral pole. Before each division, in the middle of the aboral surface of the body, two complete sets of new tentacles appear. Thus, a 12-tentacled specimen becomes for some time 24-tentacled. The fissure starts near the new tentacles and continues towards the oral pole; the mouths are separated the last (Figure 3C). After division, each individual receives the complete set of 6 old tentacles on one side, and the complete set of 6 new tentacles on the other side, which slowly grow to the normal size. Thus, the free-living individuals are practically always asymmetric to some extent. They have never shown any signs of regeneration. By the middle of the summer, the free-living specimens form endodermal gonads of two kinds. The first to appear are sexual complexes consisting of two glands with gonoducts (Figure 4) opening into the gastric cavity. Gonads of the first type are ana- tomically female (Figure 4), characterized by the presence of highly polyploid (up to 37 C; Raikova, 1965) nurse cells and by gametogenesis similar to oogenesis (Lipin, 1925). However, apparently, ‘female’ gonads do not produce mature eggs and degenerate, displaying apoptotic features (Raikova, 1963, 1994). Subsequently, four ‘male’ gonads are formed (Figure 5) which are endodermal folds protruding into the gastric cavity, but lacking gonoducts (Lipin, 1925). The ‘male’ gonads can appear both in ‘female’ and in asexual specimens—then the former become hermaphrodites and the latter become ‘males’ (Raikova, 1961, 1963). Female, and male individuals differ morphologically; females are usually bigger than males, often have 24 tentacles each, and continue to multiply by means of longitudinal fission. Hermaphrodites are similar to females. Gametogenesis in ‘male’ gonads (Figure 5) is at first similar to spermatogenesis, but then changes to oogenesis (Raikova, 1961, 1994) resulting in formation of binucleate cells filling the gonad (Figure 5). The I meiocytes often have one – two adjoining somatic cap-like cells (Figure 5C) (Raikova, 1961). In the first and in the second meiotic divisions the spindle is shifted to the periphery of the cell (Figure 5D). The first meiotic division results in formation of a II meiocyte and a polar body (Figure 5D). The second meiotic division is acyto- kinetic, therefore all the resulting cells become binucleate (Figure 5F). The nuclei have unequal size, because one of the nuclei, the more central one, becomes polyploid at once (about 3 C; Raikova, 1965). Cytokinesis only occurs after a very long delay, probably lasting several years, after the binucleate cell finally gets within an oocyte of a host-fish (Raikova, 1980, 1987). Owing to the fact that both meiotic divisions in the ‘male’ gonads are unequal and produce polar bodies, and, mainly, because the next generation develops from binucleate cells formed in these gonads, we have come to the conclusion that sex reorientation of male gonads into female ones has occurred in the evolution of Polypodium (Raikova, 1985, 1994). By the end of gametogenesis, the gonad fills with binucleate cells becoming a gametophore; situated at the bottom is an ectodermal layer bearing nematocytes (Figure 5E, F). Free-living specimens of Polypodium were observed actively laying the gametophores on the skin of the future host, the prelarval Acipenser stellatus (Smolyanov & Raikova, 1961). After the laying of gametophores, the free-living Polypodium specimens die. How binucleate cells get inside the oocytes is still unknown. Cnidom of P. hydriforme comprises isorhizas of two cate- gories: atrichous isorhiza situated at the tips of the supporting tentacles and holotrichous isorhiza, with two rows of small spines (Ibragimov & Raikova, 2004), located along the sensory tentacles and around the mouth. Nematocytes of Polypodium are characterized by a unique, radially symmetri- cal cnidocil apparatus, with the cnidocil situated directly above the nematocyst lid (Figure 6A) (Raikova, 1990). Ectodermal cells of P. hydriforme have neither flagella, nor myofibrils (Figure 6B), and are characterized by apical granules containing acid mucopolysaccharides. The granules line the internal cavity of the parasitic stolon (Figure 6B) and later form the external cover of the free-living specimen. Endodermal (gastrodermal) cells are flagellated, but lack any muscle fibres. The pharynx region of the gastrodermis in free-living specimens is composed of collar cells (Figure 6C) (Raikova, 1995). Numerous mucous and proteinaceous gland cells occur in the gastrodermis, especially in the pharyngeal epithelium. A network of nerve fibres underlies the epidermis (Lipin, 1911). In the mesoglea beneath the epidermis, there is a layer of longitudinal smooth muscle cells independent from the epithelial ones (Figure 6B). Muscle cells are formed from i-cells migrating from ectoderm into the mesoglea (Raikova, 1961; Raikova & Napara, 1999; Raikova et al. , 2007). In the mesoglea of the mouth cone, amoebocytes have been observed (Lipin, 1911; Napara & Raikova, 2003). Extracellular microtubules have been detected within the mesoglea of the stolon (Figure 6D) (Raikova & Kameneva, 1996, 1997). Within the ‘male’ gonads, in the space between the gastrodermis and the epidermis (corresponding to the mesoglea), sexual cells develop. Ectodermal cells display septate (Figure 7A) and gap (Figure 7B) junctions; endodermal cells show a septate junction between the apical parts and interdigitations in the basal parts (Raikova, 1984). Junctions between muscular cells (Figure 7C) are similar to those in vertebrate cardiac muscles (Raikova, 1984). Mitosis in Polypodium cells is typical for Metazoa and Cnidaria. In all the cells lacking flagellae (ectodermal, i-cells and their derivatives—muscle cells and nematocytes), a pair of centrioles has been observed in contact with the nucleus, in special pockets formed by the nuclear envelope that has pronounced pores (Figure 7D) (Raikova, 1984). During mitosis, as well as during cnidogenesis, centrioles acquire the usual appear- ance, without surrounding fragments of the nuclear envelope. At all the stages of the life cycle, all Polypodium cells have mitochondria with tubular cristae (Figure 7B) (Seravin & Raikova, ...
Context 3
... characters appears obviously incomplete. The present paper analyses the cytomorphological features of this unusual animal on subsequent stages of its life cycle. Polypodium ’s life cycle consists of two phases: a parasitic one and a free-living one (Figure 1). Polypodium ’s embryogenesis takes place inside the developing host oocytes and spans several years (Raikova, 1987). The initial stage of infection looks like a binucleate cell, with one big and one small nucleus (Figure 2A). By means of endocytokinesis, the small nucleus becomes surrounded by a portion of cytoplasm, thus a new smaller cell is formed inside the large cell with the bigger nucleus. This stage is known as the ‘cell-in-a-cell’ stage (Figure 2B). The outer large cell becomes the trophamnion cell, while the internal small cell undergoes cleavage (Figure 2C, D). The internal generative cell and the early blastomeres (down to the 16-cell stage) keep haploid quantity of DNA inherited from the post-meiotic pre-gametes, formed in the free-living Polypodium (Raikova, 1965, 1987). The state of haploidy of the generative nucleus and its derivatives, the blastomeres, lasts for some years. The moment and manner of restoring diploidy, characteristic of free-living P. hydriforme , remains unclear, but fusion of blastomeres is hypothesized. After gastrulation by delamination, a two-layered planuliform larva with inverted position of germ layers is formed (Figure 2E). The larva grows into a long stolon that forms numerous internal tentacles (Figure 2F). All these stages develop in the trophamnion cell’s cavity. The trophamnion is homologous to the polar body of the second meiotic division (Raikova, 1980). Since the ‘cell-in-a-cell’ stage, the trophamnion carries out protective and trophic functions. Its nucleus branches and reaches a high degree of polyploidy—more than 400 C (Raikova, 1965, 1987). At the end of the parasitic phase of the life cycle, the trophamnion shows obvious apoptotic features: a picnotic nucleus with per- ipheral chromatin caps, occurrence of intranuclear cristal- loids, and membrane profiles in cytoplasm (Raikova, 1984). Before the spawning of the host fish, the parasitic stolon inside the infected egg turns inside out, with the ectoderm now facing outwards; the trophamnion cell is destroyed. During the spawning, the stolon with a normal arrangement of germinal layers emerges. Thus, in fresh water, the free- living part of the life cycle begins (Figure 1). In water, the stolon with external tentacles (Figure 3A) consistently fragments, resulting in individual specimens with six tentacles on each side (Figure 3B). Four of the initial six tentacles are sensory and two others are supporting. The free-living benthic specimens usually ‘walk’ on the bottom using the supporting tentacles. They are biradially- symmetric, lack an umbrella, the mouth is turned upwards and not surrounded by tentacles; sense organs are not observed. Twelve-tentacled specimens usually prevail. They divide asexually with a longitudinal fissure starting at the aboral pole. Before each division, in the middle of the aboral surface of the body, two complete sets of new tentacles appear. Thus, a 12-tentacled specimen becomes for some time 24-tentacled. The fissure starts near the new tentacles and continues towards the oral pole; the mouths are separated the last (Figure 3C). After division, each individual receives the complete set of 6 old tentacles on one side, and the complete set of 6 new tentacles on the other side, which slowly grow to the normal size. Thus, the free-living individuals are practically always asymmetric to some extent. They have never shown any signs of regeneration. By the middle of the summer, the free-living specimens form endodermal gonads of two kinds. The first to appear are sexual complexes consisting of two glands with gonoducts (Figure 4) opening into the gastric cavity. Gonads of the first type are ana- tomically female (Figure 4), characterized by the presence of highly polyploid (up to 37 C; Raikova, 1965) nurse cells and by gametogenesis similar to oogenesis (Lipin, 1925). However, apparently, ‘female’ gonads do not produce mature eggs and degenerate, displaying apoptotic features (Raikova, 1963, 1994). Subsequently, four ‘male’ gonads are formed (Figure 5) which are endodermal folds protruding into the gastric cavity, but lacking gonoducts (Lipin, 1925). The ‘male’ gonads can appear both in ‘female’ and in asexual specimens—then the former become hermaphrodites and the latter become ‘males’ (Raikova, 1961, 1963). Female, and male individuals differ morphologically; females are usually bigger than males, often have 24 tentacles each, and continue to multiply by means of longitudinal fission. Hermaphrodites are similar to females. Gametogenesis in ‘male’ gonads (Figure 5) is at first similar to spermatogenesis, but then changes to oogenesis (Raikova, 1961, 1994) resulting in formation of binucleate cells filling the gonad (Figure 5). The I meiocytes often have one – two adjoining somatic cap-like cells (Figure 5C) (Raikova, 1961). In the first and in the second meiotic divisions the spindle is shifted to the periphery of the cell (Figure 5D). The first meiotic division results in formation of a II meiocyte and a polar body (Figure 5D). The second meiotic division is acyto- kinetic, therefore all the resulting cells become binucleate (Figure 5F). The nuclei have unequal size, because one of the nuclei, the more central one, becomes polyploid at once (about 3 C; Raikova, 1965). Cytokinesis only occurs after a very long delay, probably lasting several years, after the binucleate cell finally gets within an oocyte of a host-fish (Raikova, 1980, 1987). Owing to the fact that both meiotic divisions in the ‘male’ gonads are unequal and produce polar bodies, and, mainly, because the next generation develops from binucleate cells formed in these gonads, we have come to the conclusion that sex reorientation of male gonads into female ones has occurred in the evolution of Polypodium (Raikova, 1985, 1994). By the end of gametogenesis, the gonad fills with binucleate cells becoming a gametophore; situated at the bottom is an ectodermal layer bearing nematocytes (Figure 5E, F). Free-living specimens of Polypodium were observed actively laying the gametophores on the skin of the future host, the prelarval Acipenser stellatus (Smolyanov & Raikova, 1961). After the laying of gametophores, the free-living Polypodium specimens die. How binucleate cells get inside the oocytes is still unknown. Cnidom of P. hydriforme comprises isorhizas of two cate- gories: atrichous isorhiza situated at the tips of the supporting tentacles and holotrichous isorhiza, with two rows of small spines (Ibragimov & Raikova, 2004), located along the sensory tentacles and around the mouth. Nematocytes of Polypodium are characterized by a unique, radially symmetri- cal cnidocil apparatus, with the cnidocil situated directly above the nematocyst lid (Figure 6A) (Raikova, 1990). Ectodermal cells of P. hydriforme have neither flagella, nor myofibrils (Figure 6B), and are characterized by apical granules containing acid mucopolysaccharides. The granules line the internal cavity of the parasitic stolon (Figure 6B) and later form the external cover of the free-living specimen. Endodermal (gastrodermal) cells are flagellated, but lack any muscle fibres. The pharynx region of the gastrodermis in free-living specimens is composed of collar cells (Figure 6C) (Raikova, 1995). Numerous mucous and proteinaceous gland cells occur in the gastrodermis, especially in the pharyngeal epithelium. A network of nerve fibres underlies the epidermis (Lipin, 1911). In the mesoglea beneath the epidermis, there is a layer of longitudinal smooth muscle cells independent from the epithelial ones (Figure 6B). Muscle cells are formed from i-cells migrating from ectoderm into the mesoglea (Raikova, 1961; Raikova & Napara, 1999; Raikova et al. , 2007). In the mesoglea of the mouth cone, amoebocytes have been observed (Lipin, 1911; Napara & Raikova, 2003). Extracellular microtubules have been detected within the mesoglea of the stolon (Figure 6D) (Raikova & Kameneva, 1996, 1997). Within the ‘male’ gonads, in the space between the gastrodermis and the epidermis (corresponding to the mesoglea), sexual cells develop. Ectodermal cells display septate (Figure 7A) and gap (Figure 7B) junctions; endodermal cells show a septate junction between the apical parts and interdigitations in the basal parts (Raikova, 1984). Junctions between muscular cells (Figure 7C) are similar to those in vertebrate cardiac muscles (Raikova, 1984). Mitosis in Polypodium cells is typical for Metazoa and Cnidaria. In all the cells lacking flagellae (ectodermal, i-cells and their derivatives—muscle cells and nematocytes), a pair of centrioles has been observed in contact with the nucleus, in special pockets formed by the nuclear envelope that has pronounced pores (Figure 7D) (Raikova, 1984). During mitosis, as well as during cnidogenesis, centrioles acquire the usual appear- ance, without surrounding fragments of the nuclear envelope. At all the stages of the life cycle, all Polypodium cells have mitochondria with tubular cristae (Figure 7B) (Seravin & Raikova, ...
Similar publications
The Myxozoa are oligo-cellular parasites with alternate hosts—fish and annelid worms— and some myxozoan species harm farmed fish. The phylum Myxozoa, comprising 2,100 species, was difficult to position in the tree of life, due to its fast evolutionary rate. Recent phylogenomic studies utilizing an extensive number of nuclear-encoded genes have conf...
It is reported the parasitization of Kudoa sp. (Myxozoa, Multivalvulida) within the somatic muscles of the fish Odontesthes bonariensis (Valenciennes, 1835), Micropogonias furnieri (Desmarest, 1823) and Mugil liza Valenciennes, 1836, captured off Rio Grande, Rio Grande do Sul State, Brazil. Other species of fish caught at the same place were not in...
Members of Myxozoa, a parasitic metazoan taxon, have considerable detrimental effects on fish hosts and also have been associated
with human food-borne illness. Little is known about their biology and metabolism. Analysis of the genome of Thelohanellus kitauei and comparative analysis with genomes of its two free-living cnidarian relatives revealed...
Citations
... The compelling association between P. hydriforme and sturgeon eggs has spurred investigations into the far-reaching consequences of this parasitic interaction. Beyond its impact on the reproductive success of the host sturgeon, the presence of P. hydriforme has the potential to reverberate throughout the broader aquatic ecosystem [6]. ...
Polypodium hydriforme is the only known parasite adapted to intra-cellular parasitism of sturgeon oocytes, thus affecting the delicate life balance of these endangered species. Aquatic ecosystems are often shaped by intricate interactions between various organisms, each playing a distinct role in the overall health and sustainability of the environment, parasites on the other hand pose no beneficial roles and are a threat especially for fragile or declining species. Sturgeons, which are endangered and ecologically important fish species, serve as hosts for this parasite during their crucial reproductive phase. The presence of Polypodium hydriforme in sturgeon eggs has prompted investigations into its effects on both the host’s reproductive success and the broader aquatic ecosystem. Stating the intricacies of this parasitic interaction is essential not only for unravelling its evolutive adaptations but also for ensuring the conservation of sturgeon populations and the preservation of aquatic biodiversity as a whole.
... The stolons fragment into individual buds that take up benthic life, actively feeding and undergoing growth and fission during summer months ( Fig. 4.1). Reproductively mature individuals produce a specialised multicellular stage derived from gonadal tissue (Raikova 1994(Raikova , 2008 reproductively mature, at which stage the development of Polypodium within fish eggs has been characterised (Raikova 1994). Larvae and budding stolons inside the eggs have inverted germ layers, an inner ectoderm and outer endoderm-a condition that is reversed prior to emerging from fish eggs. ...
... Fusion may occur within spores that develop in myxozoan invertebrate hosts (resulting in self-fertilisation) or in fish hosts after cells released from spores invade and proliferate within fish (also potentially involving self-fertilisation). In Polypodium fusion has been inferred to happen at some time after fish hosts are invaded and to involve self-fertilisation of 'blastomeres' that develop within a nurse cell (the trophamnion) which becomes polyploid (Raikova 2008). This trophamnionblastomere complex arises from the original binucleate cells that invade fish hosts. ...
... Present (Raikova 2008) Absent (Lom 1990;Canning et al. 2000;Feist et al. 2015) Gametes ...
Parasitism has evolved in cnidarians on multiple occasions but only one clade—the Myxozoa—has undergone substantial radiation. We briefly review minor parasitic clades that exploit pelagic hosts and then focus on the comparative biology and evolution of the highly speciose Myxozoa and its monotypic sister taxon, Polypodium hydriforme, which collectively form the Endocnidozoa. Cnidarian features that may have facilitated the evolution of endoparasitism are highlighted before considering endocnidozoan origins, life cycle evolution and potential early hosts. We review the fossil evidence and evaluate existing inferences based on molecular clock and cophylogenetic analyses. Finally, we consider patterns of adaptation and diversification and stress how poor sampling might preclude adequate understanding of endocnidozoan diversity.
... The 'stolons' fragment into individual buds that take up benthic life, actively feeding and undergoing growth and fission during summer months ( Figure 1). Reproductively mature individuals produce a specialised multicellular stage derived from gonadal tissue (Raikova 1994(Raikova , 2008) that enables infection following direct contact with larval fish. Post-invasion infection dynamics are unknown until fish become reproductively mature, at which stage the development of Polypodium within fish eggs has been characterised (Raikova 1994). ...
... Fusion may occur within spores that develop in myxozoan invertebrate hosts (resulting in self-fertilisation) or in fish hosts after cells released from spores invade and proliferate within fish (also potentially involving self-fertilisation). In Polypodium fusion has been inferred to happen at some time after fish hosts are invaded and to involve self-fertilisation of 'blastomeres' that develop within a nurse cell (the trophamnion) which becomes polyploid (Raikova 2008). This trophamnionblastomere complex arises from the original binucleate cells that invade fish hosts. ...
... The free-living Polypodium body plan is relatively consistent with that of other free-living cnidarians with an external ectoderm and internal endoderm (gastrodermis). We postulate that the independent sub-epidermal muscles may have evolved to facilitate unrestricted reversal of Absent (Lom 1990;Feist et al. 2015) Centrioles Present (Raikova 2008) Absent (Lom 1990;Canning et al. 2000;Feist et al. 2015) ...
Parasitism has evolved in cnidarians on multiple occasions but only one clade – the Myxozoa – has undergone substantial radiation. We briefly review minor parasitic clades that exploit pelagic hosts and then focus on the comparative biology and evolution of the highly speciose Myxozoa and its monotypic sister taxon, Polypodium hydriforme, which collectively form the Endocnidozoa. Cnidarian features that may have facilitated the evolution of endoparasitism are highlighted before considering endocnidozoan origins, life cycle evolution and potential early hosts. We review the fossil evidence and evaluate existing inferences based on molecular clock and co-phylogenetic analyses. Finally, we consider patterns of adaptation and diversification and stress how poor sampling might preclude adequate understanding of endocnidozoan diversity.
... Parasitic and free living P. hydriforme have been studied with light and electron microscope. The summarized results permitted us to define characters that were presumably determined by the parasitiс way of life and the unique ones not connected to parasitism (Raikova, 2008). For instance, due to the inversion of germ layers at its parasitic stages, P. hydriforme became famous among coelenterates as an "Enantiozoon". ...
Polypodium hydriforme, the only species in Polypodiozoa, which is currently considered a class of Cnidaria, and likely a sister group to Medusozoa (together with Myxozoa), is a cnidarian adapted to intracellular parasitism inside sturgeon oocytes. Free-living P. hydriforme lives on river bottoms; it walks on supporting tentacles and uses sensory tentacles to capture food and bring it to the mouth. The nervous system of free-living P. hydriforme was studied by confocal microscopy and immunohistochemistry using antibodies to FMRF-amide and α-tubulin combined with phalloidin-staining of F-actin fibres. A sensory FMRF-amide immunoreactive (IR) nerve net and an α-tubulin IR nerve net have been identified. The FMRF-amide IR nerve net underlies the epidermis along the tentacles and around the mouth; it consists of neurites emanating from epidermal sensory cells and basiepidermal ganglion cells, and it connects with cnidocytes. A deeper-lying α-tubulin IR nerve net occurs only in tentacles and looks like chains of different-sized beads crossing the mesoglea and entwining muscles. Anti-α-tubulin staining also reveals microtubules in muscle cells following the longitudinal muscle fibres or the thin circular F-actin fibres of the tentacles. Cnidocytes in the tentacles are embedded in a regular hexagonal non-neural network formed by the tubulin IR cytoskeleton of epidermal cells. Cnidocils of the cnidocytes around the mouth and in walking tentacles are identical, but those in sensory tentacles differ in length and width. The possible homology of the tubulin IR nerve net with motor nerve nets of cnidarians is discussed. The absence of a classic nerve ring around the mouth and the lack of specialised sense organs are considered to be plesiomorphic characters for Cnidaria.
... nurse cells within oocytes and the incorporation of algal symbionts (see Morris 2012 for further discussion). The cell-within-cell organisation is also achieved by the larval parasitic stage of Polypodium hydriforme in which a haploid cell is contained within a nurse cell (Raikova 2008). ...
Now that we have strong evidence for the phylogenetic placement of Myxozoa within the Cnidaria it is of great interest to explore their evolutionary history. In particular, what cnidarian features may have facilitated the transition to an endoparasitic lifestyle and can we identify a potential cnidarian sister group? In this chapter we summarise evidence for characters linking myxozoans to cnidarians and identify cnidarian traits that may have promoted endoparasitism including: their diploblastic condition, their capacity for regeneration, transdifferentiation, and dormancy, the production of novel propagative stages, cell-within-cell development, and asexual reproduction. Equating the basic cnidarian life cycle (benthic polyps and planktonic medusae) with the complex myxozoan life cycle is problematic because of great plasticity in cnidarian development, which can entail the loss of stages and associated transfer of function. The sexual phase of myxozoans involves the production of isogametes but divergent views on their subsequent fusion lead to questions about whether sexual reproduction involves selfing or outcrossing and if it may result in the development of multicellular chimaeras. The apical structures of myxozoan polar capsules closely resemble those of medusozoan but not those of anthozoan nematocysts, thus supporting a medusozoan affinity for Myxozoa.
... Some basic features present in Polypodium that are absent in myxozoans include centrioles, flagellated gastrodermal cells, a cnidocil (a cilium-derived structure associated with nematocysts), gonads and a network of nerve fibres underlying the epidermis (see Raikova 2008 for review). Features shared by Polypodium and myxozoans include parasitism of fish, infection via nematocysts, a similar type of nematocyst (putatively atrichous isorhiza), longitudinally arranged and mesodermal-like muscle cells, cellwithin-cell stages (see Chap. 8 for review of endogeny processes in myxozoans) and mitochondria with tubular cristae (Raikova 2008). Many of these shared features are, however, also found in other cnidarians. ...
There is now strong evidence that myxozoans have evolved from free-living cnidarians but until recently their higher level relationships have been the subject of considerable controversy. This chapter reviews the morphological and molecular evidence that has contributed to problems in placement and how further collective support has finally resolved their cnidarian affinity. We then consider the inherently difficult but fascinating topic of how myxozoans may have evolved as endoparasitic cnidarians. We first explore how a close association of free-living precursors could have led to the evolution of myxozoans with simple life cycles and the nature of the first myxozoan hosts. We propose that either freshwater bryozoans or fish (or their precursors) were ancestral hosts (in view of the more derived nature of myxozoans that infect annelids and the fact that fish are hosts for most members of all major myxozoan clades) and suggest that the morphological complexity of myxozoans in freshwater bryozoans renders a scenario of fish as first hosts less likely. We then discuss how new hosts may have been adopted subsequently, resulting in the complex life cycles involving invertebrate and vertebrate hosts that now characterise all myxozoans. Cnidarian traits, including life cycle plasticity and a capacity to evolve novel propagative stages, ultimately support many different scenarios regarding the route to endoparasitism.
... There are numerous data on cytological character istics of various Polypodium cells in both body layers at the sequential stages of the life cycle (Raikova, 2008). Preliminary data on its nerve cells obtained by modern methods have been reported at conferences (Raikova and Napara, 1997;Napara, 1997;Raikova et al., 2003), but no papers have been published on the sub ject so far. ...
The nervous system of intracellular parasitic cnidarian Polypodium hydriforme at various stages of its life cycle has been studied by the immunocytochemical method using antibodies to FMRF-amide and by electron microscopy. Neurosecretory, sensory, and ganglion cells have been identified both at the parasitic stage (planula and stolon stages, when body layers are inverted) and in free-living animals. These cells are characterized by the presence of round neurosecretory granules about 80–120 nm in diameter. Gap junctions have been detected between nerve cells. Most of the neurosecretory and sensory cells have been observed in the epidermis of sensory tentacles of free-living animals. Sensory cells possess immobile flagella. The chains of ganglion cells are located under the epidermis and penetrate mesoglea. A centriole encircled by a fragment of nuclear envelope, which is a marker of ectodermal lineage cells in Polypodium, has been described in the cytoplasm of the sensory cells, thus proving the ectodermal nature of the nervous system. Like in most cnidarians, the nervous system of Polypodium hydriforme is a network containing FMRF-amide-like neuropeptides. Neither sense organs, nor ring-shaped nerve concentrations have been observed.
... This cnidarian has both a free living and parasitic stage. The parasitic phase infects fish eggs, and shares a number of features with myxozoans, including the cell within cell phenomenon (Raikova, 2008). Phylogenetic analysis of P. hydriforme while confirming placement within the Cnidaria, has remained problematic with regards to Myxozoa, with inclusion of this group influencing the relative placement of P. hydriforme within the Eumetazoa (Evans et al., 2008(Evans et al., , 2010. ...
... Phylogenetic analysis of P. hydriforme while confirming placement within the Cnidaria, has remained problematic with regards to Myxozoa, with inclusion of this group influencing the relative placement of P. hydriforme within the Eumetazoa (Evans et al., 2008(Evans et al., , 2010. It is interesting to note that the cell within cell stage of P. hydriforme is represented by a haploid cell contained within a polyploid 'nurse' cell (trophamnion) (Raikova, 2008). The conclusion that a major part of the myxosporean life cycle exists as a syncytial 'nurse' cell surrounding an oocyte(s), therefore suggests an intriguing similarity with P. hydriforme. ...
The phylum Myxozoa is composed of endoparasitic species that have predominately been recorded within aquatic vertebrates. The simple body form of a trophic cell containing other cells within it, as observed within these hosts, has provided few clues to relationships with other organisms. In addition, the placement of the group using molecular phylogenies has proved very difficult, although the majority of analyses now suggest that they are cnidarians. There have been relatively few studies of myxozoan stages within invertebrate hosts, even though these exhibit multicellular and sexual stages that may provide clues to myxozoan evolution. Therefore an ultrastructural examination of a myxozoan infection of a freshwater oligochaete was conducted, to reassess and formulate a model for myxozoan development in these hosts. This deemed that meiosis occurs within the oligochaete, but that fertilisation is not immediate. Rather, the resultant haploid germ cell (oocyte) is engulfed by a diploid sporogonic cell (nurse cell) to form a sporoplasm. It is this sporoplasm that infects the fish, resulting in the multicellular stages observed. Fertilisation occurs after the parasites leave the fish and enter the oligochaete host. The nurse cell/oocyte model explains previously conflicting evidence in the literature regarding myxosporean biology, and aligns phenomena considered distinctive to the Myxozoa, such as endogenous budding and cell within cell development, with processes recorded in cnidarians. Finally, the evolutionary origin of the Myxozoa as cnidarian parasites of ova is hypothesised.
... In Leptomedusae the discovery and description of rare morphotypes leads to an understanding and reconstruction of the morphogenetic evolutionary scenario (Kosevich, 2008). The cytomorphological study of Polypodium, a unique cnidarian parasite of fish eggs, suggests that this species is not aberrant as previously thought but is a relic of a major hydrozoan group (Raikova, 2008). Study of the organization of soft tissue in hydroids has shown that their structural complexity, comprising something more than two epithelial layers, does not contravene limits to the ground plan of this group of Hydrozoa (Pyataeva & Kosevich, 2008). ...
Polypodium hydriforme is a parasitic cnidarian that develops within the eggs of acipenseriform fish in the Old and New Worlds. Currently regarded as monotypic, P. hydriforme has been studied largely in the context of caviar production in Russian sturgeon species. We report the first robust epidemiological study of P. hydriforme in North American acipenseriform fish. We sampled infection prevalences (in 2017 and 2018) and intensities (in 2017) during annual surveys of American Paddlefish, Polyodon spathula, caught during spawning migration in north‐eastern Oklahoma. Egg masses were characterized for the presence and intensity of P. hydriforme infection. Prevalences were similar in 2017 and 2018 (49% and 45%, respectively). Generally, a small number of eggs were infected per egg mass, but a few were heavily infected. Longer, heavier and older fish are more likely to be infected and to harbour more severe infections. In addition, infection is linked to decreases in roe fat weight independently of fish length, weight, age or roe weight. Infection thus diminishes Paddlefish energy reserves (roe fat) which could in turn impact host fitness. Our results raise questions about the impacts of infection on caviar production and Paddlefish conservation and suggest insights on infection dynamics and parasite strategies.