Fig 3 - uploaded by Matthias Kolberg
Content may be subject to copyright.
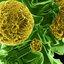
Examples of immunohistochemical staining in malignant peripheral nerve sheath tumors using antibodies against four proteins: p53, CDK4, cyclin D1, and p14 ARF. For each protein, a negative sample, nuclear expression, cytoplasmic expression, and positive control tissue are shown (from left to right). The positive tissues (right column) are colorectal cancer (top right) and malignant melanoma (all others). *For CDK4, there is positive cytoplasmic staining in the sample with negative nuclei.
Source publication
The purpose of this study was to identify new prognostic biomarkers with clinical impact in malignant peripheral nerve sheath tumor (MPNST), a highly aggressive malignancy for which no consensus therapy exists besides surgery. We have used tissue microarrays (TMAs) to assess in situ expression of 14 cell-cycle-regulating proteins in 64 well-charact...
Context in source publication
Context 1
... scoring results from the in situ protein expression analysis of the MPNSTs are illustrated in Fig. 3. The detailed results for nuclear and cytoplasmic staining for each antibody are shown in Table 2, and our staining remarks are presented in Supplementary Table 1. Results for each patient group (with and without NF1) are shown in detail in Table 3. The largest difference between NF1- associated and sporadic MPNSTs was found for ...
Citations
... Review articles, case reports, animal studies, cell line studies, molecular/genetic/cellular markers studies, and duplicated studies were excluded. As in the previous literature [19], six articles [14,[20][21][22][23][24] were combined according to the same first author to form three distinct studies in our meta-analysis, after verifying that there was no duplication. For three further studies, [9,25,26] only the latest published result was included since the prior two studies were incorporated in the last publication. ...
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are malignancies that demonstrate nerve sheath differentiation in the peripheral nervous system. They can occur sporadically or be associated with neurofibromatosis type 1 (NF1), an autosomal dominant neurocutaneous disorder, with up to 13% of patients developing MPNSTs in their lifetimes. Previous studies have suggested conflicting findings regarding the prognosis of NF1 for patients with MPNSTs. The elucidation of NF1 as an independent prognostic factor on mortality has implications for clinical management. We aim to investigate the role of NF1 status as an independent prognostic factor of overall survival (OS) and disease-specific survival (DSS) in MPNSTs.
Methods
An electronic literature search of PubMed and MEDLINE was performed on studies reporting OS or DSS outcomes of MPNSTs with and without NF1. A grey literature search by reviewing bibliographies of included studies and review articles was performed to find pertinent studies. Data was extracted and assessed in accordance with the PRISMA guidelines. A meta-analysis was performed to calculate hazard ratios (HRs) using a random-effects model. The primary and secondary outcomes were all-cause and disease-specific mortality, respectively, with NF1 as an independent prognostic factor of interest.
Results
A total of 59 retrospective studies involving 3602 patients fulfilled the inclusion criteria for OS analysis, and 23 studies involving 704 MPNST patients were included to evaluate DSS outcomes. There was a significant increase in the hazard of all-cause mortality (HR 1.63, 95% CI 1.45 to 1.84) and disease-specific mortality (HR 1.52, 95% CI 1.24 to 1.88) among NF1 as compared to sporadic cases. Subgroup analyses and meta-regression showed that this result was consistent regardless of the quality of the study and year of publication.
Conclusion
NF1 is associated with a substantially higher risk of all-cause and disease-specific mortality. This finding suggests that closer surveillance is required for NF1 patients at risk of developing MPNSTs.
... Activation of the PI3K/AKT/mTOR pathway is a common event, occurring in approximately 50% of MPNSTs, and is associated with poor prognosis (28). Additionally, 25-60% of MPNSTs are p53-de cient (9,10,29), which is associated with signi cantly diminished survival (29)(30)(31) and poor response to neo-adjuvant chemotherapy (32). De ning the role of these pathways in therapeutic response is critical to predicting effective targeted therapies and predictive biomarkers for future MPNST trials. ...
Malignant peripheral nerve sheath tumors (MPNSTs) are chemotherapy resistant sarcomas that are a leading cause of death in neurofibromatosis type 1 (NF1). Although NF1-related MPNSTs derive from neural crest cell origin, they also exhibit intratumoral heterogeneity. TP53 mutations are associated with significantly decreased survival in MPNSTs, however the mechanisms underlying TP53- mediated therapy responses are unclear in the context of NF1 -deficiency. We evaluated the role of two commonly altered genes, MET and TP53 , in kinome reprograming and cellular differentiation in preclinical MPNST mouse models. We previously showed that MET amplification occurs early in human MPNST progression and that Trp53 loss abrogated MET-addiction resulting in MET inhibitor resistance. Here we demonstrate a novel mechanism of therapy resistance whereby p53 alters MET stability, localization, and downstream signaling leading to kinome reprogramming and lineage plasticity. Trp53 loss also resulted in a shift from RAS/ERK to AKT signaling and enhanced sensitivity to MEK and mTOR inhibition. In response to MET, MEK and mTOR inhibition, we observed broad and heterogeneous activation of key differentiation genes in Trp53 -deficient lines suggesting Trp53 loss also impacts lineage plasticity in MPNSTs. These results demonstrate the mechanisms by which p53 loss alters MET dependency and therapy resistance in MPNSTS through kinome reprogramming and phenotypic flexibility.
... Activation of the PI3K/AKT/mTOR pathway is a common event, occurring in approximately 50% of MPNSTs, and is associated with poor prognosis (28). Additionally, 25-60% of MPNSTs are p53-deficient (9,10,29), which is associated with significantly diminished survival (29)(30)(31) and poor response to neo-adjuvant chemotherapy (32). Defining the role of these pathways in therapeutic response is critical to predicting effective targeted therapies and predictive biomarkers for future MPNST trials. ...
Malignant peripheral nerve sheath tumors (MPNSTs) are chemotherapy resistant sarcomas that are a leading cause of death in neurofibromatosis type 1 (NF1). Although NF1-related MPNSTs derive from neural crest cell origin, they also exhibit intratumoral heterogeneity. TP53 mutations are associated with significantly decreased survival in MPNSTs, however the mechanisms underlying TP53-mediated therapy responses are unclear in the context of NF1-deficiency. We evaluated the role of two commonly altered genes, MET and TP53, in kinome reprograming and cellular differentiation in preclinical MPNST mouse models. We previously showed that MET amplification occurs early in human MPNST progression and that Trp53 loss abrogated MET-addiction resulting in MET inhibitor resistance. Here we demonstrate a novel mechanism of therapy resistance whereby p53 alters MET stability, localization, and downstream signaling leading to kinome reprogramming and lineage plasticity. Trp53 loss also resulted in a shift from RAS/ERK to AKT signaling and enhanced sensitivity to MEK and mTOR inhibition. In response to MET, MEK and mTOR inhibition, we observed broad and heterogeneous activation of key differentiation genes in Trp53-deficient lines suggesting Trp53 loss also impacts lineage plasticity in MPNSTs. These results demonstrate the mechanisms by which p53 loss alters MET dependency and therapy resistance in MPNSTS through kinome reprogramming and phenotypic flexibility.
... 20 Brekke, et al. reported that p53-positive MPNST patients are a high-risk group and they are candidates for adjuvant treatment. 21 Chemotherapy for soft-tissue sarcoma is limited in benefit and in variety. Chemotherapy options that include vincristine, doxorubicin, ifosfamide, and etoposide have a positive effect among metastatic MPNST patients, but not in non-metastatic patients. ...
Objective: To investigate and report the clinical profiles, treatment patterns, and oncologic outcomes in MPNST patients, and to identify the prognostic factors that significantly affect survival.Materials and Methods: Patients diagnosed with and treated for histologically confirmed MPNST at our institute during the January 1997 to June 2018 study period were included. Patient medical records and surgical specimens were reviewed, and study-related data was extracted and analyzed.Results: There were 27 males and 32 females with a mean age of 44 years. Most patients presented with mass and most patients were AJCC stage III. Twenty-nine percent of patients had MPNST that was associated with NF-1. At a median follow-up time, 18 patients (30.51%) suffered from local disease recurrence. Two-year and 5-year overall survival was 72% and 46%, respectively. In univariate analysis, chemotherapy treatment and positive tumor margin were adverse prognostic factors for disease-free survival. In multivariate analysis, chemotherapy treatment (hazard ratio (HR): 3.415, 95% CI: 1.367-16.021; p=0.013) and positive tumor margin (HR: 4.680, 95% CI 1.828-10.314; p=0.014) were found to be independent prognostic factors for disease-free.Conclusion: Chemotherapy treatment and positive tumor margin were identified as independent adverse prognostic factors for disease-free and overall survival, respectively. Accordingly, early detection and appropriate treatment are essential for improved patient outcome.
... RABL6A was found to function, at least in part, by activating oncogenic CDK4/6 through inhibition of p27, thereby disabling RB1-mediated tumor suppression in tumor cells. Those findings, and the fact that p27 loss and RB1 inactivation are hallmark events in MPNSTs associated with worse prognosis [30,31], predicted RABL6A may be dysregulated in patient tumors. Indeed, RABL6A protein expression is dramatically increased in MPNSTs compared to patient-matched, benign neurofibromas [18]. ...
Background: Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive sarcomas that display complex molecular and genetic alterations. Powerful tumor suppressors CDKN2A and TP53 are commonly disrupted in these lesions along with NF1, a gene that encodes a negative regulator of Ras. Many additional factors have been implicated in MPNST pathogenesis. A greater understanding of critical drivers of the disease is needed to guide more informed targeted therapies for patients. RABL6A is a newly identified driver of MPNST cell survival and proliferation whose in vivo role in the disease is unknown.
Methods: Using CRISPR-Cas9 targeting of Nf1+Cdkn2a or Nf1+Tp53 in the sciatic nerve to form de novo MPNSTs, we investigated the biological significance of RABL6A in MPNST development. Molecular evaluation of terminal tumors (western blot, qRT-PCR, immunohistochemistry) yielded several insights.
Results: Mice lacking Rabl6 displayed slower tumor growth and extended survival relative to wildtype animals in both genetic contexts. YAP oncogenic activity was selectively downregulated in RABL6A-null, Nf1+Cdkn2a lesions but not in RABL6A-null, Nf1+Tp53 tumors. Regardless of genetic context, loss of RABL6A caused upregulation of the CDK inhibitor, p27 in tumors. Paradoxically, both models displayed elevated Myc protein expression and Ki67 staining in terminal tumors lacking RABL6A.
Conclusions: These findings demonstrate RABL6A is required for optimal tumor progression of NF1 mutant MPNSTs in vivo in both Cdkn2a and p53 inactivated settings. However, sustained RABL6A loss may provide selective pressure for molecular alterations, such as Myc upregulation, that ultimately promote an unwanted, hyper-proliferative tumor phenotype akin to drug resistant lesions.
... Sixteen immunohistochemical markers of cell cycle regulation were evaluated, most commonly p53 ( Table 2) (4,24,26,28,29,33,40,42,43,46,47,51,53,56,60,61,63). Low p14, p16, checkpoint with forkhead-associated domain and ring finger (CHFR), and increase in p53, p14, cyclin D1, p27, and forkhead box protein M1 (FOXM1) staining were associated with worse survival in univariable analysis (4,26,28,40,42,43,51,56,61 2) were also independently associated with worse survival in one study each (28,30,43,61). ...
... TP53 is one of the few recurrently mutated genes found in MPNST. TP53 mutations and high p53 staining were independently associated with survival or DFS in five different studies (4,24,34,43,56). This may indicate that aberrations in this gene may indeed be of clinical importance. ...
Background
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive soft tissue sarcomas with dismal prognosis. Pathological and genetic markers may predict more aggressive behavior in MPNSTs but have uncommonly been investigated, and few are used in daily practice. This study reviews the prognostic value of immunohistochemical markers and genetic alterations in MPNST.
Methods
A systematic search was performed in PubMed and Embase databases according to the PRISMA guidelines. Search terms related to ‘MPNST’ and ‘prognostic’ were used. Studies investigating the association of immunohistochemical markers or genetic alterations with prognosis were included. Qualitative synthesis was performed on all studies. A distinction was made between univariable and multivariable associations.
Results
Forty-six studies were included after full-text screening. Sixty-seven different immunohistochemical markers were investigated. Absence of S100 and H3K27me3 and high Ki67 and p53 staining was most commonly independently associated with worse survival and disease-free survival. Several genetic alterations were investigated as well with varying association to survival. TP53, CDK4, RASSF1A alterations were independently associated with worse survival, as well as changes in chromosomal length in Xp, 10q, and 16p.
Conclusions
MPNSTs harbor complex and heterogeneous biology. Immunohistochemical markers and genetic alterations have variable prognostic value. Absence of S100 and H3K27me3 and increased Ki67 can be of prognostic value. Alterations in TP53 or increase in p53 staining may distinguish MPNSTs with worse outcomes. Genetic alterations and staining of other cell cycle regulatory and Ras pathway proteins may also help stratifying patients with worse outcomes. A combination of markers can increase the prognostic value.
... These data point to an adaptive mechanism involving RTK signaling for both malignant transformation and clonal selection in MPNSTs [6]. To advance our understanding of the MPNST therapeutic response and resistance to RAS pathway inhibition, we developed diverse preclinical NF1-related MPNST models, including an "MET-addicted" model of NF1-related MPNSTs (NF1-MET), an Nf1/Trp53-deficient model (NF1-P53), and an NF1 model (P53 WT , Hgf -amplified) [7][8][9]. Using these MPNST models, we determined that P53 deficiency significantly exacerbates resistance to MEK inhibition; however, combined MEK and MET inhibition overcame therapy resistance [6]. Importantly, these results demonstrated that NF1-related MPNSTs maintain multiple signaling dependencies beyond RAS, and that genomic determinants, such as P53 and RTK genomic alterations, profoundly influence the therapy response. ...
Neurofibromatosis Type 1 (NF1)-related Malignant Peripheral Nerve Sheath Tumors (MPNST) are highly resistant sarcomas that account for significant mortality. The mechanisms of therapy resistance are not well-understood in MPNSTs, particularly with respect to kinase inhibition strategies. In this study, we aimed to quantify the impact of both the genomic context and targeted therapy on MPNST resistance using reverse phase phosphoproteome array (RPPA) analysis. We treated tumorgrafts from three genetically engineered mouse models using MET (capmatinib) and MEK (trametinib) inhibitors and doxorubicin, and assessed phosphosignaling at 4 h, 2 days, and 21 days. Baseline kinase signaling in our mouse models recapitulated an MET-addicted state (NF1-MET), P53 mutation (NF1-P53), and HGF overexpression (NF1). Following perturbation with the drug, we observed broad and redundant kinome adaptations that extended well beyond canonical RAS/ERK or PI3K/AKT/mTOR signaling. MET and MEK inhibition were both associated with an initial inflammatory response mediated by kinases in the JAK/STAT pathway and NFkB. Growth signaling predominated at the 2-day and 21-day time points as a result of broad RTK and intracellular kinase activation. Interestingly, AXL and NFkB were strongly activated at the 2-day and 21-day time points, and tightly correlated, regardless of the treatment type or genomic context. The degree of kinome adaptation observed in innately resistant tumors was significantly less than the surviving fractions of responsive tumors that exhibited a latency period before reinitiating growth. Lastly, doxorubicin resistance was associated with kinome adaptations that strongly favored growth and survival signaling. These observations confirm that MPNSTs are capable of profound signaling plasticity in the face of kinase inhibition or DNA damaging agent administration. It is possible that by targeting AXL or NFkB, therapy resistance can be mitigated.
... Fig. 1A-C). The induction of RABL6A coincided with loss of nuclear p27 ( Fig. 1D and E), an event associated with poor survival in patients with MPNST (15,37). Downregulation of p27 during the neurofibroma-to-MPNST transition can be caused by reduced transcription as well as mislocalization of p27 from the nucleus to the cytoplasm (15,38). ...
Purpose:
Malignant peripheral nerve sheath tumors (MPNSTs) are deadly sarcomas that lack effective therapies. In most MPNSTs, the retinoblastoma (RB1) tumor suppressor is disabled by hyperactivation of cyclin dependent kinases (CDKs), commonly through loss of CDK inhibitory proteins such as p27(Kip1). RABL6A is an inhibitor of RB1 whose role in MPNSTs is unknown. To gain insight into MPNST development and establish new treatment options, we investigated RABL6A-RB1 signaling and CDK inhibitor-based therapy in MPNSTs.
Experimental design:
We examined patient-matched MPNSTs and precursor lesions by RNA-Seq and IHC. Molecular and biological effects of silencing RABL6A and/or p27 in MPNST lines and normal human Schwann cells were determined. Tumor suppressive effects of CDK inhibitors were measured in MPNST cells and orthotopic tumors.
Results:
RABL6A was dramatically upregulated in human MPNSTs compared to precursor lesions, which correlated inversely with p27 levels. Silencing RABL6A caused MPNST cell death and G1 arrest that coincided with p27 upregulation, CDK downregulation and RB1 activation. The growth suppressive effects of RABL6A loss, and its regulation of RB1, were largely rescued by p27 depletion. Importantly, reactivation of RB1 using a CDK4/6 inhibitor (palbociclib) killed MPNST cells in vitro in a RABL6A-dependent manner and suppressed MPNST growth in vivo. Low-dose combination of drugs targeting multiple RB1 kinases (CDK4/6, CDK2) had enhanced anti-tumorigenic activity associated with potential MPNST cell re-differentiation.
Conclusions:
RABL6A is a new driver of MPNST pathogenesis that acts in part through p27-RB1 inactivation. Our results suggest RB1 targeted therapy with multiple pathway drugs may effectively treat MPNSTs.
... In NF1-related MPNST, the loss or mutation in the TP53 gene has been associated with an increased proliferative potential, formation of metastases and particularly poor prognosis (Upadhyaya et al. 1997). Brekke et al. have shown that patients with NF1-associated MPNST, in whom the accumulation of nuclear p53 was found, form a high-risk subgroup requiring adjuvant treatment, even when in complete remission (Brekke et al. 2009). In our study, 8 of 10 patients with low tumor p53 expression responded well to naCHT, while 9 of 11 children with high p53 immunoreactivity responded poor. ...
Purpose:
Selected cell-cycle regulators and extracellular matrix proteins were found to play roles in malignant peripheral nerve sheath tumor (MPNST) biology. We aimed to analyze whether initial tumor tissue expressions of survivin, p53, cyclin D1, osteopontin (OPN) and fibronectin (FN) correlate with the response to neo-adjuvant CHT (naCHT) in children with advanced inoperable MPNST.
Methods:
The study included 26 children with MPNST (M/F 14/12, median age 130 months) treated in Polish centers of pediatric oncology between 1992 and 2013. Tissue expression of markers was studied immunohistochemically in the manually performed tissue microarrays and assessed semi-quantitatively as low and high, based on the rate of positive cells and staining intensity.
Results:
Good response to naCHT was noted in 47.6%, while poor-in 52.4% of patients. The response to naCHT was influenced negatively by the presence of neurofibromatosis NF1 and high initial tumor tissue expression of OPN, survivin, p53 and cyclin D1. Patients with high tumor expression of either OPN, survivin or p53 and those with simultaneous high expression of ≥ 3 of the markers, responded significantly worse to naCHT, than patients, in whom expression of ≤ 2 markers were detected at diagnosis. Nearly, 85% of patients expressing ≥ 3 markers, responded poor to CHT; while 87.5% of children, expressing ≤ 2 markers, were good responders.
Conclusion:
The initial tumor tissue expression of OPN, survivin, p53 and cyclin D1 may serve as markers to predict response to naCHT in pediatric advanced MPNST. Future studies in more numerous group of patients are needed to confirm these preliminary results.
... 141 143 Studies of MPNSTs at all sites have suggested that elevated Ki67 expression (>25%) is associated with decreased survival. 141 144 Several studies have revealed that TP53 and MDM2 may promote metastasis development and thereby contribute to malignant progression in MPNST, 145 with recently published data in the USA showing that nuclear TP53 expression is significantly associated with a worse MPNST-specific survival outcome 141 (table 2). ...
Neural lesions occur uncommonly in the gastroenteropancreaticobiliary tract. However, due to the growing number of screening colonoscopy procedures, polypoid neural lesions of the colon are being recognised increasingly and range from benign tumours to high-grade malignant neoplasms. Morphological variability of neural tumours can be wide, although some entities share pathological features, and, as such, these lesions can be diagnostically challenging. We review the spectrum of pathology of neural tumours in the gastroenteropancreaticobiliary tract, with the goal of providing a practical approach for practising surgical pathologists.