Figure 2 - available via license: Creative Commons Attribution 3.0 Unported
Content may be subject to copyright.
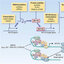
D-4F treatment of stroke in T1DM rats decreases white matter damage. Stroke in T1DM rats significantly decreases
Source publication
D-4F is an apolipoprotein-A1 mimetic peptide that promotes anti-inflammatory effects. MicroRNA-124 is the most abundant brain-specific microRNA and has anti-inflammatory effects. In this study, we investigated the therapeutic efficacy and mechanisms of D-4F treatment of stroke in type one diabetes mellitus (T1DM) rats. Male Wistar rats were induced...
Contexts in source publication
Context 1
... test whether D-4F treatment promotes white matter (WM) remodeling, Bielschowsky silver (BS) and Luxol fast blue (LFB) staining were performed. Stroke in T1DM rats significantly decreases axon (BS, Figure 2a, #p<0.05, n=8/group) and myelin (LFB, Figure 2b, #p<0.05, ...
Context 2
... in T1DM rats significantly decreases axon (BS, Figure 2a, #p<0.05, n=8/group) and myelin (LFB, Figure 2b, #p<0.05, n=8/group) density compared to T1DM- sham control rats. ...
Context 3
... density compared to T1DM- sham control rats. D-4F treatment of stroke in T1DM rats significantly increases axon (BS, Figure 2a, n=8/ group, *p<0.05) and myelin (LFB, Figure 2b, n=8/group, *p<0.05) density in the ischemic border zone (IBZ) compared to PBS treated T1DM stroke rats at 48 hours after stroke. ...
Context 4
... density compared to T1DM- sham control rats. D-4F treatment of stroke in T1DM rats significantly increases axon (BS, Figure 2a, n=8/ group, *p<0.05) and myelin (LFB, Figure 2b, n=8/group, *p<0.05) density in the ischemic border zone (IBZ) compared to PBS treated T1DM stroke rats at 48 hours after stroke. ...
Similar publications
The central nervous system (CNS) parenchyma is enclosed by anatomical interfaces including multilayered meninges, the blood-brain barrier (BBB), the choroid plexuses within ventricles and the glia limitans. These border areas hold distinct functional specializations which control the trafficking of monocyte-derived cells toward the CNS parenchyma,...
Citations
... In another study, apoA-I mimetic peptide L-4F crossed the BBB and was able to reduce neurovascular and white matter damage and improve neurological function in mice with stroke (Zhou et al., 2022). D-4F treatment in stroke rats significantly reduced BBB leakage and white matter damage, decreased neuroinflammation, and improved functional prognosis (Ning et al., 2017). Notably, Swaminathan et al. (2020) found that 4F reduced the accumulation of toxic amyloid beta in a mouse model of AD. ...
CD36 is a highly glycosylated integral membrane protein that belongs to the scavenger receptor class B family and regulates the pathological progress of metabolic diseases. CD36 was recently found to be widely expressed in various cell types in the nervous system, including endothelial cells, pericytes, astrocytes, and microglia. CD36 mediates a number of regulatory processes, such as endothelial dysfunction, oxidative stress, mitochondrial dysfunction, and inflammatory responses, which are involved in many central nervous system diseases, such as stroke, Alzheimer’s disease, Parkinson’s disease, and spinal cord injury. CD36 antagonists can suppress CD36 expression or prevent CD36 binding to its ligand, thereby achieving inhibition of CD36-mediated pathways or functions. Here, we reviewed the mechanisms of action of CD36 antagonists, such as Salvianolic acid B, tanshinone IIA, curcumin, sulfosuccinimidyl oleate, antioxidants, and small-molecule compounds. Moreover, we predicted the structures of binding sites between CD36 and antagonists. These sites can provide targets for more efficient and safer CD36 antagonists for the treatment of central nervous system diseases.
... Under physiological conditions, miR-124 is constitutively and highly expressed in microglia and plays a key role in its quiescence [19,20]. Under pathological conditions, the downregulation of miR-124 has increased neuroinflammation by polarizing activated microglia toward M1 phenotype [21], while the upregulation of miR-124 has reduced neuroinflammation by polarizing activated microglia toward M2 phenotype [22,23]. Interestingly, one previous study had indicated that the expression of miR-124 was decreased by TLR4 activator LPS, and miR-124 overexpression was shown to inhibit LPS-induced microglia activation as well [16]. ...
Introduction:
Neuroinflammation contributes to secondary injury after traumatic brain injury (TBI), which has been mainly mediated by the microglia. MiR-124 was reported to play an important role in the polarization of microglia by targeting TLR4 signaling pathway. However, the role and mechanism of miR-124 in neuroinflammation mediated by microglia after TBI is unclear. To clarify this, we performed this research.
Methods:
The expression of miR-124 was first measured by RT-PCR in the injured brain at 1/3/7 days post-TBI. Then, miR-124 mimics or inhibitors administration was used to interfere the expression of miR-124 at 24 h post-TBI. Subsequently, the microglia polarization markers were detected by RT-PCR, the expression of inflammatory cytokines was detected by ELISA, the expression of TLR4/MyD88/IRAK1/TRAF6/NF-κB was measured by WB, and the neurological deficit was evaluated by NSS and MWM test. At last, in vitro experiments were performed to explore the exact target molecule of miR-124 on TLR4 signaling pathway.
Results:
Animal research indicated that the expression of miR-124 was downregulated after TBI. Upregulation of miR-124 promoted the M2 polarization of microglia and inhibited the activity of TLR4 pathway, as well as reduced neuroinflammation and neurological deficit after TBI. In vitro experiments indicated that miR-124 promoted the M2 polarization of microglia and reduced neuroinflammation by inhibiting TRAF6.
Conclusion:
This study demonstrated that upregulation of miR-124 promoted the M2 polarization of microglia and reduced neuroinflammation after TBI by inhibiting TRAF6.
... D-4F, an apolipoprotein A-I (APOA-I) peptidomimetic made of D-amino acids, can promote cholesterol efflux in macrophages via the cAMP-PKA-ABCA1 pathway [9]. It can also promote polarization of M2-type macrophages to treat stroke in rats with type one diabetes [10] and pass through the blood-brain barrier for the treatment of Alzheimer's disease [11]. Furthermore, it has been proven to play a role in the treatment of neurogenic inflammation [12], inflammation of cerebral arterioles [13], and other diseases. ...
... The known effects of D-4F on macrophages are as follows: in atherosclerosis models, D-4F can improve HDL-mediated cholesterol efflux in macrophages and reverse cholesterol transport in macrophages, thereby reducing lesions, and its molecular pathway is cAMP-PKA-ABCA1 [9,25]. In rat models of type one diabetes mellitus with stroke, D-4F can increase the expression of microRNA-124 and promote anti-inflammatory effects and M2 macrophage polarization in the brain, which contribute to the improvement of nerve function [10]. Compared with previous studies, our study explored the effect of D-4F on macrophages in SCI models for the first time. ...
After spinal cord injury (SCI), a large number of blood-derived macrophages infiltrate the lesion site and phagocytose myelin debris to become foamy macrophages, which leads to chronic inflammation. The drug D-4F, an apolipoprotein A-I peptidomimetic made of D-amino acids, has been reported to promote the lipid metabolism of foamy macrophages in atherosclerosis. However, the role and mechanism of D-4F in SCI are still unclear. In this study, we found that D-4F can promote the removal of myelin debris, reduce the formation of foamy macrophages in the lesion core and promote neuroprotection and recovery of motor function after SCI. These beneficial functions of D-4F may be related to its ability to upregulate the expression of ATP-binding cassette transporter A1 (ABCA1), the main transporter that mediates lipid efflux in foamy macrophages because inhibiting the activity of ABCA1 can reverse the effect of D-4F in vitro. In conclusion, D-4F may be a promising candidate for treating SCI by promoting the clearance of myelin debris by foamy macrophages via the ABCA1 pathway.
... We have previously demonstrated that deficiency of ABCA1 in the brain induces worse neurological functional deficits after stroke, increases BBB leakage and aggravates OL loss and WM injury (17,(34)(35)(36). The Apolipoprotein A-I (ApoA-I) mimetic peptide, 4F (D-4F, synthesized from D-amino acids, and L-4F, synthesized from Lamino acids) increases cholesterol efflux (37)(38)(39)(40)(41)(42)(43) and has antiinflammatory effects (44)(45)(46). In our previous study, we have shown that treatment of stroke in type 1 diabetes mellitus (T1DM) rats with D-4F or db/db-T2DM stroke mice treated with L-4F significantly decreases neurovascular and WM damage and improves neurological function in the early stage (4-7 days) after stroke (45,46). ...
... The Apolipoprotein A-I (ApoA-I) mimetic peptide, 4F (D-4F, synthesized from D-amino acids, and L-4F, synthesized from Lamino acids) increases cholesterol efflux (37)(38)(39)(40)(41)(42)(43) and has antiinflammatory effects (44)(45)(46). In our previous study, we have shown that treatment of stroke in type 1 diabetes mellitus (T1DM) rats with D-4F or db/db-T2DM stroke mice treated with L-4F significantly decreases neurovascular and WM damage and improves neurological function in the early stage (4-7 days) after stroke (45,46). However, whether L-4F is capable of crossing the BBB and whether long-term post-stroke treatment with L-4F promotes neurovascular and WM remodeling and improves recovery of neurological function in T2DM, and whether ABCA1 mediates L-4F-induced neurorestoration have not been studied. ...
... We also found previously that in vitro, L-4F treatment does not increase angiogenesis in mouse-brain ECs cultured in high-glucose media, but increases primary artery explant cell migration after stroke-induced injury and enhances neurite and axonal outgrowth in primary cortical neurons subjected to oxygen-glucose deprivation or high glucose (46). D-4F treatment of T1DM-stroke rats also increases tight junction protein expression and decreases BBB leakage, WM damage, and pro-inflammatory factors, while increasing antiinflammatory M2 macrophage polarization in the ischemic brain 7 days after stroke (45). L-4F decreases markers of plasma oxidation and promotes EC migration and EC-healing of carotid arterial injuries in HFD-fed mice (74). ...
Patients with type 2 diabetes mellitus (T2DM) exhibit a distinct and high risk of ischemic stroke with worse post-stroke neurovascular and white matter (WM) prognosis than the non-diabetic population. In the central nervous system, the ATP-binding cassette transporter member A 1 (ABCA1), a reverse cholesterol transporter that efflux cellular cholesterol, plays an important role in high-density lipoprotein (HDL) biogenesis and in maintaining neurovascular stability and WM integrity. Our previous study shows that L-4F, an economical apolipoprotein A member I (ApoA-I) mimetic peptide, has neuroprotective effects via alleviating neurovascular and WM impairments in the brain of db/db-T2DM stroke mice. To further investigate whether L-4F has neurorestorative benefits in the ischemic brain after stroke in T2DM and elucidate the underlying molecular mechanisms, we subjected middle-aged, brain-ABCA1 deficient (ABCA1−B/−B), and ABCA1-floxed (ABCA1fl/fl) T2DM control mice to distal middle cerebral artery occlusion. L-4F (16 mg/kg, subcutaneous) treatment was initiated 24 h after stroke and administered once daily for 21 days. Treatment of T2DM-stroke with L-4F improved neurological functional outcome, and decreased hemorrhage, mortality, and BBB leakage identified by decreased albumin infiltration and increased tight-junction and astrocyte end-feet densities, increased cerebral arteriole diameter and smooth muscle cell number, and increased WM density and oligodendrogenesis in the ischemic brain in both ABCA1−B/−B and ABCA1fl/fl T2DM-stroke mice compared with vehicle-control mice, respectively (p < 0.05, n = 9 or 21/group). The L-4F treatment reduced macrophage infiltration and neuroinflammation identified by decreases in ED-1, monocyte chemoattractant protein-1 (MCP-1), and toll-like receptor 4 (TLR4) expression, and increases in anti-inflammatory factor Insulin-like growth factor 1 (IGF-1) and its receptor IGF-1 receptor β (IGF-1Rβ) in the ischemic brain (p < 0.05, n = 6/group). These results suggest that post-stroke administration of L-4F may provide a restorative strategy for T2DM-stroke by promoting neurovascular and WM remodeling. Reducing neuroinflammation in the injured brain may contribute at least partially to the restorative effects of L-4F independent of the ABCA1 signaling pathway.
... It is important to note that unstimulated microglia express more miR-124 than microglia in co-culture with astrocytes or neurons [99], and in addition to our findings in which the inflammatory state of the donor cell does not affect the antitumor activity of sEVs (Figure 1), this further supports our choice of using sEVs derived from unstimulated microglia for the treatment of glioma-bearing mice. Of note, the lack of effects of the microglia-derived sEVs on microglia phenotype is in line with the observation that in pathological conditions, miR-124 determines a switch of microglia towards an anti-inflammatory phenotype and its inhibition favors a pro-inflammatory phenotype [100][101][102][103]. The partial effect observed by sEVs released by BV2 transfected with the miR-124 inhibitor may be due to other components of vesicle cargos. ...
Brain homeostasis needs continuous exchange of intercellular information among neurons, glial cells, and immune cells, namely microglial cells. Extracellular vesicles (EVs) are active players of this process. All the cells of the body, including the brain, release at least two subtypes of EVs, the medium/large EVs (m/lEVs) and small EVs (sEVs). sEVs released by microglia play an important role in brain patrolling in physio-pathological processes. One of the most common and malignant forms of brain cancer is glioblastoma. Altered intercellular communications constitute a base for the onset and the development of the disease. In this work, we used microglia-derived sEVs to assay their effects in vitro on murine glioma cells and in vivo in a glioma model on C57BL6/N mice. Our findings indicated that sEVs carry messages to cancer cells that modify glioma cell metabolism, reducing lactate, nitric oxide (NO), and glutamate (Glu) release. sEVs affect Glu homeostasis, increasing the expression of Glu transporter Glt-1 on astrocytes. We demonstrated that these effects are mediated by miR-124 contained in microglia-released sEVs. The in vivo benefit of microglia-derived sEVs results in a significantly reduced tumor mass and an increased survival of glioma-bearing mice, depending on miR-124.
... Furthermore, EV miR-124-3p showed high sensitivity and specificity for the diagnosis of AIS compared to serum miR-124-3p. miR-124 has been identified as a negative regulator of inflammation after stroke (Hamzei Taj et al., 2016a;Ning et al., 2017). In the present study, we investigated the antiinflammatory effect of miR-124-3p on LPS-induced BV2 microglia. ...
Background: A delay in the diagnosis of acute ischemic stroke (AIS) reduces the eligibility and outcome of patients for thrombolytic therapy. Therefore, early diagnosis and treatment of AIS are crucial. The present study evaluated the sensitivity and accuracy of serum extracellular vesicle (EV)-derived miR-124-3p in the diagnosis and prediction of AIS.
Methods: An miRNA expression profile was downloaded from Gene Expression Omnibus (GEO) database and analyzed by R software. EVs were harvested from the serum of AIS patients using a total exosome isolation kit and characterized by Western blotting, a transmission electron microscope, and the nanoparticle tracking analysis. BV2 microglia were pre-stimulated with lipopolysaccharide (LPS), followed by miR-124-3p treatment for 24 h and subsequent analysis of viability, apoptosis, and migration (scratch assay), and Western blotting. The relative expression of the selected genes was assessed by qRT-PCR. The phosphorylation of Erk1/2, PI3K/Akt, and p38MAPK in BV2 microglia cells was evaluated by Western blotting, while the luciferase reporter gene assay detected the correlation between key genes involved in the pro-inflammatory signaling pathways and miR-124-3p .
Results: hsa-miR-124-3p was downregulated in AIS serum compared to the non-AIS serum ( p < 0.05), and the gene expression of has-miR-124-3p in EVs was negatively correlated with serum pro-inflammatory cytokines and the NIHSS ( p < 0.05). In addition, miR-124-3p promoted the viability and inhibited the apoptosis of LPS-induced BV2 microglia. Furthermore, miR-124-3p reduced the phosphorylation of Erk1/2, PI3K/Akt, and p38MAPK, and promoted the migration in LPS-induced BV2 microglia ( p < 0.05).
Conclusion: Serum EV-derived miR-124-3p serves as a diagnostic and predictive marker for early-stage AIS.
... Furthermore, D-4F also reduced MMP-9 level, prevented the loss of TJ proteins, decreased NVU damage, and attenuated neuronal damage in brain. Additionally, treatment of stroke with diabetic rats decrease BBB disruption and white matter damage thereby improving functional outcome [66]. These provide concrete evidence that D-4F is a promising neuroprotectant for stroke with diabetes. ...
Ischemic stroke caused by occlusion of cerebral artery is responsible for the majority of stroke that increases the morbidity and mortality worldwide. Diabetes mellitus (DM) is a crucial risk factor for ischemic stroke. Prolonged DM causes various microvascular and macrovascular changes, and blood-brain barrier (BBB) permeability that facilitates inflammatory response following stroke. In the acute phase following stroke, BBB disruption has been considered the initial step that induces neurological deficit and functional disabilities. Stroke outcomes are significantly worse among DM. In this article, we review stroke with diabetes-induce BBB damage, as well as underlying mechanism and possible therapeutic targets for stroke with diabetes.
... The chosen doses matched with our earlier studies and were used by others as well. 5,12,13,[17][18][19][20][21] Previously, we demonstrated that the injection of isotonic saline does not elicit hyperalgesia, infiltration with leukocytes or increased production of proalgesic mediators like prostaglandins or cytokines. 22 ...
High doses of capsaicin are recommended for the treatment of neuropathic pain. However, low doses evoke mechanical hypersensitivity. Activation of the capsaicin chemosensor transient receptor potential vanilloid 1 (TRPV1) induces neurogenic inflammation. In addition to the release of pro-inflammatory mediators, reactive oxygen species are produced. These highly reactive molecules generate oxidised phospholipids and 4-hydroxynonenal (4-HNE) which then directly activate TRP ankyrin 1 (TRPA1). The apolipoprotein A-I mimetic peptide D-4F neutralises oxidised phospholipids. Here, we asked whether D-4F ameliorates neurogenic hypersensitivity in rodents by targeting reactive oxygen species and 4-HNE in the capsaicin-evoked pain model.
... An initial inflammatory event in ischemic stroke is the activation of microglia cells, the first line of the innate immune response in CNS and is activated a few minutes after ischemic stroke onset [70]. At the early stage of ischemic stroke, microglia cells possess anti-inflammatory effects (M2 phenotype), while at the later stage, microglia cells with the pro-inflammatory M1 phenotype secrete inflammatory cytokines, such as necrosis factor-α (TNF-α), toll-like receptor 4, and matrix metalloproteinase 9 [71,72], which are involved in the leakage of the blood-brain barrier. Therefore, suppressing the over-reaction of microglia and microglia-mediated neuroinflammation is deemed to be a therapeutic strategy for cerebral ischemia-induced damage. ...
... Hamzei et al. found that the intracerebral injection of miR-124 suppressed development of inflammation by skewing the microglia into the M2 anti-inflammation phenotype after ischemic stroke [13], which could release anti-inflammatory factors such as interleukin (IL)-10, IL-4, and IL-13, transforming growth factor β, and activating T regulatory cells [73], subsequently improving tissue repair and long-term neurological outcomes after ischemic stroke. Ning et al. reported that miR-124 was at least partially contributing to the apolipoprotein-A1 mimetic peptide, D-4F, induced M2 macrophage polarization and anti-inflammatory effects in type one diabetes mellitus (T1DM) ischemic stroke rats, reducing the expression of inflammatory factors in the ischemic brain, and improving neurological functional outcomes after ischemic stroke [72]. Additionally, activated microglia can release cytokines and cytotoxic factors that accelerate the occurrence or deterioration of neurodegenerative diseases [74], and promote the proliferation of astrocytes, glial fibrillary acidic protein, and some other cytokines that contribute to the enhancement of the glia scar, which has major effects on neuronal excitability and secondary pathologies, such as epilepsy [75,76]. ...
Cerebral ischemia injury, the leading cause of morbidity and mortality worldwide, initiates sequential molecular and cellular pathologies that underlie ischemic encephalopathy (IE), such as ischemic stroke, Alzheimer disease (AD), Parkinson’s disease (PD), epilepsy, etc. Targeted therapeutic treatments are urgently needed to tackle the pathological processes implicated in these neurological diseases. Recently, accumulating studies demonstrate that microRNA-124 (miR-124), the most abundant miRNA in brain tissue, is aberrant in peripheral blood and brain vascular endothelial cells following cerebral ischemia. Importantly, miR-124 regulates a variety of pathophysiological processes that are involved in the pathogenesis of age-related IE. However, the role of miR-124 has not been systematically illustrated. Paradoxically, miR-124 exerts beneficial effects in the age-related IE via regulating autophagy, neuroinflammation, oxidative stress, neuronal excitability, neurodifferentiation, Aβ deposition, and hyperphosphorylation of tau protein, while it may play a dual role via regulating apoptosis and exerts detrimental effects on synaptic plasticity and axonal growth. In the present review, we thus focus on the paradoxical roles of miR-124 in age-related IE, as well as the underlying mechanisms. A great understanding of the effects of miR-124 on the hypoxic–ischemic brain will open new avenues for therapeutic approaches to protect against cerebral ischemia injury.
... Neuroinflammation plays a critical role in WM damage, axonal degeneration and myelin breakdown (75). Microglia are activated within minutes after stroke onset and stimulate the production of inflammatory cytokines and promote leukocyte infiltration which exacerbate brain damage (76). Our data show that BDMPs increase the infiltration of leukocytes, microglia/macrophages and neutrophils, and the expression of immune factors IL1β, IL6, and TNF-α in the IBZ of stroke mice. ...
Microparticles (MPs, ~size between 0.1 and 1 mm) are lipid encased containers derived from intact cells which contain antigen from the parent cells. MPs are involved in intercellular communication and regulate inflammation. Stroke increases secretion of brain derived MP (BDMP) which activate macrophages/microglia and induce neuroinflammation. Lactadherin (Milk fat globule–EGF factor-8) binds to anionic phospholipids and extracellular matrices, promotes apoptotic cell clearance and limits pathogenic antigen cross presentation. In this study, we investigate whether BDMP affects stroke-induced neuroinflammation and whether Lactadherin treatment reduces stroke initiated BDMP-induced neuroinflammation, thereby improving functional outcome after stroke. Middle aged (8–9 months old) male C57BL/6J mice were subjected to distal middle cerebral artery occlusion (dMCAo) stroke, and BDMPs were extracted from ischemic brain 24 h after dMCAo by ultracentrifugation. Adult male C57BL/6J mice were subjected to dMCAo and treated via tail vein injection at 3 h after stroke with: (A) +PBS (n = 5/group); (B) +BDMPs (1.5 × 10⁸, n = 6/group); (C) +Lactadherin (400 μg/kg, n = 5/group); (D) +BDMP+Lactadherin (n = 6/group). A battery of neurological function tests were performed and mice sacrificed for immunostaining at 14 days after stroke. Blood plasma was used for Western blot assay. Our data indicate: (1) treatment of Stroke with BDMP significantly increases lesion volume, neurological deficits, blood brain barrier (BBB) leakage, microglial activation, inflammatory cell infiltration (CD45, microglia/macrophages, and neutrophils) into brain, inflammatory factor (TNFα, IL6, and IL1β) expression in brain, increases axon/white matter (WM) damage identified by decreased axon and myelin density, and increases inflammatory factor expression in the plasma when compared to PBS treated stroke mice; (2) when compared to PBS and BDMP treated stroke mice, Lactadherin and BDMP+Lactadherin treatment significantly improves neurological outcome, and decreases lesion volume, BBB leakage, axon/WM injury, inflammatory cell infiltration and inflammatory factor expression in the ischemic brain, respectively. Lactadherin treatment significantly increases anti-inflammatory factor (IL10) expression in ischemic brain and decreases IL1β expression in plasma compared to PBS and BDMP treated stroke mice, respectively. BDMP increases neuroinflammation and aggravates ischemic brain damage after stroke. Thus, Lactadherin exerts anti-inflammatory effects and improves the clearance of MPs to reduce stroke and BDMP induced neurological deficits.