Figure 4 - available via license: Creative Commons Attribution 4.0 International
Content may be subject to copyright.
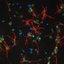
Coronal mouse sections from the Allen Brain Atlas (brain-map.org/api/index.html) showing the anterograde transport of a green fluorescent protein tagged AAV in case #11 (Allen Atlas exp. 159374329). Images a-d show coronal sections from rostral (a) to caudal (d) levels. The injection site (e) involved both the subiculum and postsubiculum, showing features of both sets of efferents. AC: anterior commissure; AD: anterodorsal thalamic nucleus; AM: anteromedial thalamic nucleus; AV: anteroventral thalamic nucleus; fxcol: columns of fornix; LMB: lateral mammillary nucleus; LS: lateral septum; MMB: medial mammillary nucleus; pocfx: postcommissural fornix; PostS: postsubiculum; RSC: retrosplenial cortex. The numbers (bottom left) correspond to the image number in the Atlas. Scale bars = 700 µm. All images are courtesy of the Allen Institute. © 2015 Allen Institute for Brain Science. Allen Brain Atlas API. Case available at: brain-map.org/search/index.html?query=159374329
Source publication
The routes by which the hippocampal formation projects bilaterally to the anterior thalamic nuclei and mammillary bodies were examined in the mouse, rat, and macaque monkey. Despite using different methods and different species, the principal pattern remained the same. For both target areas, the contralateral hippocampal (subiculum) projections aro...
Context in source publication
Context 1
... no label was apparent in the contralateral anteroventral nucleus, while occasional labelled fibres were seen in the interanteromedial nucleus (#12, 13, 14). The only exception was case #11, in which more contralateral label was present in the anteroventral and anteromedial nuclei than any of the other mice in this group, but this was the only case with an appreciable contribution from the subiculum (Figure 4). ...
Citations
... Our data showed no monosynaptic connections from the amygdala to the contralateral ROIs, consistent with previous studies in the literature [82]. However, the amygdala is tightly linked with the ipsilateral hippocampus and PHG [14,26,28], which are connected to the contralateral structures [83][84][85]. Importantly, all studies included in our analysis [5,12,35] used a protocol based on positive autobiographical memory recall to up-regulate the BOLD signal of the left amygdala [11]. Neurofeedback studies using this protocol reported the hippocampus and PHG coactivation during the neurofeedback training task [11,36,37], increased functional connectivity between the left amygdala and left hippocampal/PHG structures [11,12], and increased gray matter volume of hippocampal subfields [55]. ...
Background
fMRI neurofeedback targeting the amygdala is a promising therapeutical tool in psychiatry. It induces resting-state functional connectivity (rsFC) changes between the amygdala and regions of the salience and default mode networks (SN and DMN, respectively). We hypothesize these rsFC changes happen on the amygdala’s underlying anatomical circuits.
Methods
We used the coordinates from regions of interest (ROIs) from studies showing pre-to-post-neurofeedback changes in rsFC with the left amygdala. Using a cross-species brain parcellation, we identified the homologous locations in non-human primates. We injected bidirectional tracers in the amygdala of adult macaques and used bright- and dark-field microscopy to identify cells and axon terminals in each ROI. We also performed additional injections in specific ROIs to validate the results following amygdala injections and delineate potential disynaptic pathways. Finally, we used high-resolution diffusion MRI data from four post-mortem macaque brains and one in vivo human brain to translate our findings to the neuroimaging domain.
Results
The amygdala had significant monosynaptic connections with all the SN and DMN ipsilateral ROIs. Amygdala connections with the DMN contralateral ROIs are disynaptic through the hippocampus and parahippocampal gyrus. Diffusion MRI in both species benefitted from using the ground-truth tracer data to validate its findings, as we identified false-negative ipsilateral and false-positive contralateral connectivity results.
Conclusions
Amygdala neurofeedback modulates the SN and DMN through monosynaptic connections and disynaptic pathways - including hippocampal structures involved in the neurofeedback task. Neurofeedback may be a tool for rapid modulation and reinforcement of these anatomical connections, leading to clinical improvement.
... thalamus midline (4). These findings support previous reports in the literature that documented sporadic bilateral projections from the cortex to the contralateral thalamus, particularly from the hippocampus (5), prefrontal cortex (4,6), and primary motor cortex (4,7). This suggests that TCs may represent a normotypical pathway for interhemispheric connectivity and could be present in other high-order species. ...
... The stereotaxic coordinates were obtained from the MBM atlas (34). These specific brain regions were chosen because they are known to make corticothalamic interhemispheric connections in different mammalian species (4)(5)(6)(7). Four weeks after the injection, the animals were euthanized and perfused with PBS 0.1M followed by PFA 4% in PBS, and their brains were extracted and placed in 30% sucrose in 0.1M PBS for cryoprotection for a week. ...
Cortical neurons of eutherian mammals project to the contralateral hemisphere, crossing the midline primarily via the corpus callosum and the anterior, posterior, and hippocampal commissures. We recently reported an additional commissural pathway in rodents, termed the thalamic commissures (TCs), as another interhemispheric axonal fiber pathway that connects cortex to the contralateral thalamus. Here, we demonstrate that TCs also exist in primates and characterize the connectivity of these pathways with high-resolution diffusion-weighted magnetic resonance imaging, viral axonal tracing, and functional MRI. We present evidence of TCs in both New World ( Callithrix jacchus and Cebus apella ) and Old World primates ( Macaca mulatta ). Further, like rodents, we show that the TCs in primates develop during the embryonic period, forming anatomical and functionally active connections of the cortex with the contralateral thalamus. We also searched for TCs in the human brain, showing their presence in humans with brain malformations, although we could not identify TCs in healthy subjects. These results pose the TCs as an important fiber pathway in the primate brain, allowing for more robust interhemispheric connectivity and synchrony and serving as an alternative commissural route in developmental brain malformations.
Significance statement
Brain connectivity is a central topic in neuroscience. Understanding how brain areas can communicate allows for the comprehension of brain structure and function. We have described in rodents a new commissure pathway that connects the cortex to the contralateral thalamus. Here, we investigate whether this pathway exists in non-human primates and humans. The presence of these commissures poses the TCs as an important fiber pathway in the primate brain, allowing for more robust interhemispheric connectivity and synchrony and serving as an alternative commissural route in developmental brain malformations.
... Related findings have linked increased white matter fibre complexity to better scene memory (Tavor et al., 2014) (see also Giacosa et al., 2016 for links between reduced fibre coherence and acquired expertise in adults). It may be that fornix complexity reflects individual differences in the extent to which fornix axons disperse to reach target sites (Mathiasen et al., 2019;Poletti & Creswell, 1977), although other factors, such as an increase in the presence of glial cells, could contribute (Gagnon et al., 2022). ...
Converging evidence from studies of human and nonhuman animals suggests that the hippocampus contributes to sequence learning by using temporal context to bind sequentially occurring items. The fornix is a white matter pathway containing the major input and output pathways of the hippocampus, including projections from medial septum, and to diencephalon, striatum, and prefrontal cortex. If the fornix meaningfully contributes to hippocampal function, then individual differences in fornix microstructure might predict sequence memory. Here, we tested this prediction by performing tractography in 51 healthy adults who had undertaken a sequence memory task. Microstructure properties of the fornix were compared with those of tracts connecting medial temporal lobe regions, but not predominantly the hippocampus: the Parahippocampal Cingulum bundle (PHC) (conveying retrosplenial projections to parahippocampal cortex) and the Inferior Longitudinal Fasciculus (ILF) (conveying occipital projections to perirhinal cortex). Using principal components analysis, we combined Diffusion Tensor Imaging and Neurite Orientation Dispersion and Density Imaging measures obtained from multi-shell diffusion MRI into two informative indices, the first (PC1) capturing axonal packing/myelin, the second (PC2) capturing microstructural complexity. We found a significant correlation between fornix PC2 and implicit reaction-time indices of sequence memory, indicating that greater fornix microstructural complexity is associated with better sequence memory. No such relationship was found with measures from the PHC and ILF. This study highlights the importance of the fornix in aiding memory for objects within a temporal context, potentially reflecting a role in mediating network communication within an extended hippocampal system.
... Within 2 weeks of perfusion fixation, brain diffusion-weighted imaging (DWI) and 3D-T2-WI scans were acquired using a 9.4 T animal MRI system (Biospec 94/30 MRI; Bruker BioSpin, Ettlingen, Germany) solenoid type coil with an inner diameter of 28 mm (Takashima seisakusho Co., Ltd., Japan). Whole-DWI were acquired using spin-echo planar imaging with the following parameters: repetition time, 320 ms; echo time, 26 [22]. The major commissures (AC, CC, VHC, DHC, and PC) were manually isolated by a neuroradiologist blinded to the genotypes of the mice using a median sagittal slice of B0 images. ...
... The DHC projects to the parahippocampal region, which plays an important role in learning and memory [25]. Studies using DW-MRI and white matter tractography of the DHC in both humans and nonhuman primates demonstrated a clear consistency between the known anatomy and DHC tract reconstructions [26]. ...
During brain development, neural circuits are formed through cellular differentiation, cell migration, axon guidance, and synaptogenic processes by the coordinated actions of many genes. Abnormalities in neural development, especially connectivity defects, can result in psychiatric disorders, such as schizophrenia and autism. Recent advances in diffusion tensor imaging have enabled us to examine the brain's macroscopic nerve trajectories. In this study, we investigated the abnormalities of the commissural fibers that connect the left and right cerebral hemispheres in mice lacking heparan sulfate 6-O endosulfatases, Sulf1 and Sulf2 (Sulf1/2), which are extracellular enzymes that remove 6-O sulfate from heparan sulfate and thereby modulate the function of axon guidance factors. We previously demonstrated that Sulf1/2 double knockout (DKO) mouse embryos harbored defects in their corticospinal tract and that some of these DKO mice experienced corpus callosum agenesis. However, abnormalities of the commissural fibers in the adult DKO brain have not been systematically assessed. In this study, we investigated commissural fiber abnormalities in these mice by the combined use of radiological and histological analyses. First, we acquired diffusion-weighted images and three-dimensional-T2 weighted images of adult brains using a 9.4 T animal magnetic resonance imaging system and found that Sulf1/2 DKO mice had a smaller corpus callosum and dorsal hippocampal commissure. Next, we performed myelin staining and anterograde tracing, revealing that the dorsal hippocampal commissure was elongated in a rostral direction. These results suggest that Sulf1/2 play an important role in the formation of commissural tracts and that diffusion tensor imaging associated with microscopic analysis is a powerful tool to clarify nerve tract abnormalities.
... It is important to note that we did not observe EYFP-positive cells bodies outside of the hippocampus in our mice even when EYFP expression was amplified using immunohistochemistry, suggesting that the extrahippocampal fibers and terminals were those belonging to hippocampal neurons infected with the virus. Upon close examination, hippocampal efferents were observed in areas previously shown to receive projections from the hippocampus, including the RSCg, with fibers also visible in the columns of the fornix in the septum, lateral hypothalamus, and mammillary bodies (Irle and Markowitsch, 1982;Mathiasen et al., 2019). ...
... Despite the reduced forniceal fibers on the sclerotic side, there was no significant decrease in terminals in the LMN, a projection field of the postcommissural fornix, perhaps suggesting that fiber loss is principally precommissural. The relatively lesser impact on the fornix may also be attributed to the existence of contralateral projections from the hippocampus to the MB suggested by a tracer study using data from the Allen Brain Institute mouse connectome (Mathiasen et al., 2019). Nevertheless, a reduction in efferent fibers was not observed in mice injected with saline, suggesting that the changes we observe are due to the damage caused by kainic acid. ...
Unilateral intrahippocampal injection of kainic acid is used as a model of medial temporal lobe epilepsy and provides a platform to study the mechanisms of epilepsy. Here, we used an AAV-9 EYFP-tagged viral vector as an anterograde tracer, injected into the dorsal and ventral hippocampus after kainic acid injection, to map out the efferent hippocampal projections after the development of spontaneous seizures in this model. The purpose of the study was to identify the extent of changes in hippocampal efferent system in several brain regions that receive significant inputs from the hippocampus. Loss of efferent hippocampal fibers was greatest in the retrosplenial cortex where neuronal loss was also observed. Loss of fibers was also observed in the fornix without any specific effect in the lateral mammillary nuclei. Although expected, these observations provide further evidence of the broader network effects as a result of hippocampal cell loss.
... In addition to utilizing the main brain commissures, interhemispheric axonal fibers may also cross the midline along alternative crossing points. For example, there are reports of brain regions such as the pre-and infra-limbic cortex (Vertes 2002), the hippocampus (Mathiasen et al. 2019), and the primary motor (MOp) cortex (Hoerder-Suabedissen et al. 2018) projecting to the contralateral hemisphere through the thalamus. However, the specific patterns of these projections, their connectivity, and the prevalence in the healthy brain are still unknown. ...
... In this work, we have found that cortical regions ORBl and MOp that were known only to establish communication across brain hemispheres through the CC also present other, previously unnoticed, interhemispheric pathways that involve the thalamus and the AC, respectively. These connections are in agreement with previous literature that shows other frontal regions projecting through the thalamus to the contralateral cortex (Vertes 2002;Hoerder-Suabedissen et al. 2018;Mathiasen et al. 2019). We identified projections from the MOp area via the AC to the contralateral hemisphere and corticothalamic interhemispheric fiber bundles to the contralateral thalamus. ...
... The examples of interhemispheric corticothalamic pathways shown in Figures 1 and 2 were extracted from the Allen Brain Connectivity Atlas (Oh et al. 2014). We are confident that this new pathway is generally present in the normal brain because we could identify the TC in 2 entirely independent data sets, in agreement with previous reports describing individual interhemispheric corticothalamic fiber bundles (Vertes 2002;Hoerder-Suabedissen et al. 2018;Mathiasen et al. 2019). ...
The corpus callosum (CC), the anterior (AC), and the posterior (PC) commissures are the principal axonal fiber bundle pathways that allow bidirectional communication between the brain hemispheres. Here, we used the Allen mouse brain connectivity atlas and high-resolution diffusion-weighted MRI (DWI) to investigate interhemispheric fiber bundles in C57bl6/J mice, the most commonly used wild-type mouse model in biomedical research. We identified 1) commissural projections from the primary motor area through the AC to the contralateral hemisphere; and 2) intrathalamic interhemispheric fiber bundles from multiple regions in the frontal cortex to the contralateral thalamus. This is the first description of direct interhemispheric corticothalamic connectivity from the orbital cortex. We named these newly identified crossing points thalamic commissures. We also analyzed interhemispheric connectivity in the Balb/c mouse model of dysgenesis of the corpus callosum (CCD). Relative to C57bl6/J, Balb/c presented an atypical and smaller AC and weaker interhemispheric corticothalamic communication. These results redefine our understanding of interhemispheric brain communication. Specifically, they establish the thalamus as a regular hub for interhemispheric connectivity and encourage us to reinterpret brain plasticity in CCD as an altered balance between axonal reinforcement and pruning.
... The fimbria-fornix, a white matter tract bundle containing hippocampal afferent and efferent connections [29,30], has shown abnormalities in individuals with TLE [14,35,36] and in animal models of chronic TLE [9,25]. In our current study, this structure showed the largest diffusivity abnormalities following SE, namely reduced FA due to reduced D ║ and increased D ┴ . ...
Purpose
Temporal lobe epilepsy is associated with tissue abnormalities of several gray and white matter structures that are reproduced in animal models. Few longitudinal studies have focused on the identification of structural differences during epileptogenesis. The diffusion tensor model is a useful tool for evaluating cell death, gliosis, and axonal plasticity in epileptic subjects. This study aimed to evaluate temporal tissue changes after experimental status epilepticus in an animal model of chronic temporal lobe epilepsy.
Methods
Systemic pilocarpine-induced status epilepticus in adult Sprague-Dawley rats. Animals were scanned using diffusion tensor imaging (DTI) at three time points: prior to status epilepticus, and 24 and 64 days post-induction (early and late chronic, respectively). Fractional anisotropy, apparent diffusion coefficient, axial diffusivity (D║), and radial diffusivity (D┴) were evaluated in white (fimbria, cingulum, corpus callosum, and internal capsule) and gray (dorsal hippocampus, dentate gyrus, and CA3) matter regions for the three time points. Histological assessment of neurodegeneration in Klüver-Barrera preparations from the same animals was performed.
Results
Significantly reduced volume of dorsal hippocampus and fimbria of the epileptic animals was observed already at 24 days post-status epilepticus. Progressive changes of DTI parameters in both the white and gray matter structures of the experimental group were also observed. Stained sections confirmed such alterations.
Conclusion
Our study revealed time-dependent diffusion changes in gray and white matter structures after pilocarpine-induced status epilepticus. The characterization of these alterations over time may be potential imaging markers for epileptogenesis.
... The postcommissural fornix, the major output from the hippocampal subiculum, innervates the anterior hypothalamus through the medial-cortico hypothalamic tract and terminates at the posterior hypothalamus in the mammillary body 55,56 . We utilized the same approach described above to quantitatively characterize the postcommissural fornix in control and GOF brains (Fig. 9, Supplementary Fig. 9). ...
Abnormal levels of fibroblast growth factors (FGFs) and FGF receptors (FGFRs) have been detected in various neurological disorders. The potent impact of FGF-FGFR in multiple embryonic developmental processes makes it challenging to elucidate their roles in postmitotic neurons. Taking an alternative approach to examine the impact of aberrant FGFR function on glutamatergic neurons, we generated a FGFR gain-of-function (GOF) transgenic mouse, which expresses constitutively activated FGFR3 (FGFR3 K650E ) in postmitotic glutamatergic neurons. We found that GOF disrupts mitosis of radial-glia neural progenitors (RGCs), inside-out radial migration of post-mitotic glutamatergic neurons, and axonal tract projections. In particular, late-born CUX1-positive neurons are widely dispersed throughout the GOF cortex. Such a cortical migration deficit is likely caused, at least in part, by a significant reduction of the radial processes projecting from RGCs. RNA-sequencing analysis of the GOF embryonic cortex reveals significant alterations in several pathways involved in cell cycle regulation and axonal pathfinding. Collectively, our data suggest that FGFR3 GOF in postmitotic neurons not only alters axonal growth of postmitotic neurons but also impairs RGC neurogenesis and radial glia processes.
... Unfortunately, such disconnections fail to reveal the anatomic direction of any behavioral effects, nor can contributions from indirect disconnections be excluded. The presence of many crossed hippocampal projections to the anterior thalamus (Mathiasen et al., 2019) adds further complications. Furthermore, permanent lesions in both sites result in retrosplenial cortex dysfunctions (Albasser et al., 2007;Garden et al., 2009) that could contribute to any observed behavioral deficits. ...
The hippocampus is essential for normal memory but does not act in isolation. The anterior thalamic nuclei may represent one vital partner. Using DREADDs, the behavioral consequences of transiently disrupting anterior thalamic function were examined, followed by inactivation of the dorsal subiculum. Next, the anterograde transport of an adeno-associated virus expressing DREADDs was paired with localized intracerebral infusions of a ligand to target specific input pathways. In this way, the direct projections from the anterior thalamic nuclei to the dorsal hippocampal formation were inhibited, followed by separate inhibition of the dorsal subiculum projections to the anterior thalamic nuclei. To assay spatial working memory, all animals performed a reinforced T-maze alternation task, then a more challenging version that nullifies intramaze cues. Across all four experiments, deficits emerged on the spatial alternation task that precluded the use of intramaze cues. Inhibiting dorsal subiculum projections to the anterior thalamic nuclei produced the severest spatial working memory deficit. This deficit revealed the key contribution of dorsal subiculum projections to the anteromedial and anteroventral thalamic nuclei for the processing of allocentric information, projections not associated with head-direction information. The overall pattern of results provides consistent causal evidence of the two-way functional significance of direct hippocampal-anterior thalamic interactions for spatial processing. At the same time, these findings are consistent with hypotheses that these same, reciprocal interactions underlie the common core symptoms of temporal lobe and diencephalic anterograde amnesia.
... The postcommissural fornix, the major output from the hippocampal subiculum, innervates the anterior hypothalamus through the medial-corticohypothalamic tract and terminates at the posterior hypothalamus in the mammillary body 51,52 . We utilized the same approach described above to quantitatively characterize the postcommissural fornix in ctrl and GOF brains ( There is no significant difference in total volume occupied by tdTomato + axonal projections (p=0.1407, were decreased in GOF cortices (Fig. 9B). ...
Abnormal levels of fibroblast growth factors (FGFs) and FGF receptors (FGFRs) have been detected in various neurological disorders. The potent impact of FGF-FGFR in multiple embryonic developmental processes makes it challenging to elucidate their roles in post-mitotic neurons. Taking an alternative approach, we directly examined the impact of aberrant FGFR function after neurogenesis by generating a FGFR gain-of-function (GOF) transgenic mouse which expresses constitutively activated FGFR3 (FGFR3K650E) in post-mitotic glutamatergic neurons. We found that enhanced FGFR activity in glutamatergic neurons results in abnormal radial migration and axonal miswiring. Regarding the lamination phenotype in GOF brains, we found later-born Cux1-positive neurons are dispersed throughout the GOF cortex. Such a cortical migration deficit is likely caused, at least in part, by a significant reduction of the radial processes normally projecting from the radial glia cells (RGCs). In addition, FGFR3 GOF also results in the misrouting of several long-range axonal projections, including the corpus callosum, anterior commissure, and postcommissural fornix. RNA-sequencing analysis of the GOF embryonic cortex reveals significant alterations in several pathways involved in cell cycle regulation and axonal pathfinding. Collectively, our results suggest that FGFR hyperfunction in post-mitotic neurons at the late embryonic stage result in cortical dysplasia and circuit miswiring.