Figure 4 - uploaded by Sook Wah Yee
Content may be subject to copyright.
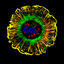
Cellular localization of green fluorescent protein (GFP)-tagged hMATE1 (human multidrug and toxin compound extrusion-1) reference and four nonsynonymous variants. HEK-293 cells were transiently transfected with GFP-labeled hMATE1 reference, hMATE1-G64D, hMATE1-L125F, hMATE1-V338I or hMATE1-V480M. The plasma membrane was stained using red fluorescent AlexaFluor594 wheat germ agglutinin (WGA) and cells were visualized by confocal microscopy using a Zeiss 510 laser scanning microscope. (a) hMATE1 reference, (b) hMATE1-G64D, (c) hMATE1-L125F, (d) hMATE1-V338I and (e) hMATE1-V480M. The colocalization of the GFP-tagged hMATE1 construct with the red-fluorescent stain of the plasma membrane is shown in the third panel of each image.
Source publication
hMATE1 (human multidrug and toxin compound extrusion-1; encoded by SLC47A1) is thought to have an important function in the renal and hepatic elimination of drugs, endogenous compounds and environmental toxins. The goals of this study were to identify genetic variants of hMATE1 and to determine their effects on hMATE1 transport function. We identif...
Contexts in source publication
Context 1
... HEK-293 cells were transiently transfected with green fluorescent protein GFP-labeled hMATE1 reference, hMATE1-G64D, hMATE1-L125F, hMATE1-V338I or hMATE1-V480M. Representative confocal images are shown in Figures 4a e. hMATE1 reference was mainly located on the plasma membrane ( Figure 4a) whereas the reduced function variants hMATE1-L125F and hMATE1-V338I (Figures 4c and d) were located on both plasma membrane and in the intracellular spaces. The nonfunctional variants hMATE1-G64D and hMATE1-V480M appeared to have less protein expression on the plasma membrane compared with hMATE1 reference; instead, the nonfunctional variants were primarily localized intracellularly (Figures 4b and e). ...
Context 2
... confocal images are shown in Figures 4a e. hMATE1 reference was mainly located on the plasma membrane ( Figure 4a) whereas the reduced function variants hMATE1-L125F and hMATE1-V338I (Figures 4c and d) were located on both plasma membrane and in the intracellular spaces. The nonfunctional variants hMATE1-G64D and hMATE1-V480M appeared to have less protein expression on the plasma membrane compared with hMATE1 reference; instead, the nonfunctional variants were primarily localized intracellularly (Figures 4b and e). ...
Context 3
... a substitution of a negatively charged aspartic acid for a neutral amino acid, glycine, in the first extracellular loop of MATE1, represents a large chemical change, which could have contributed to the loss of function. Furthermore, we found that hMATE1-G64D tagged with GFP is expressed mainly in the intracellular space and less on the plasma membrane ( Figure 4b) compared to hMATE1 reference, which may explain its functional loss. ...
Context 4
... further studies with a GFP chimera, we observed that the substitution appeared to change the intracellular localization of the transporter. That is, we found that hMATE1- V480M tagged with GFP was poorly localized to the plasma membrane (Figure 4), which rendered it functionally inert. Though further studies are needed, it is possible that valine at position 480 is critical for trafficking of hMATE1 to the plasma membrane. ...
Context 5
... metformin uptake, in which larger differences in uptake between the hMATE1 reference and the two polymorphic variants (hMATE1-L125F and hMATE1-V338I) were observed, the V max /K m ratios exhibited trends toward significantly lower values in cells expressing the variant transporters in comparison to the reference transporter (P=0.051). Confocal microscopy showed that hMATE1-L125F and hMATE1-V338I variants were located on both plasma membrane and in the intracellular spaces, in contrast to the reference hMATE1, which was predominately distributed to the plasma membrane (Figure 4). These data are consistent with the reduced function of hMATE1-L125F and hMATE1-V338I in comparison to the reference transporter. ...
Citations
... It facilitates the efflux of metformin from renal tubular cells into urine, thereby reducing its plasma concentration. Genetic variations in MATE1 have been associated with altered metformin responses and pharmacokinetics [25]. On the other hand, MATE2K, also expressed in the kidneys, is involved in the reabsorption of metformin from urine back into the renal tubular cells. ...
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide. There has been significant progress in understanding the risk factors and epidemiology of HCC during the last few decades, resulting in efficient preventative, diagnostic and treatment strategies. Type 2 diabetes mellitus (T2DM) has been demonstrated to be a major risk factor for developing HCC. Metformin is a widely used hypoglycemic agent for patients with T2DM and has been shown to play a potentially beneficial role in improving the survival of patients with HCC. Experimental and clinical studies evaluating the outcomes of metformin as an antineoplastic drug in the setting of HCC were reviewed. Pre-clinical evidence suggests that metformin may enhance the antitumor effects of immune checkpoint inhibitors (ICIs) and reverse the effector T cells’ exhaustion. However, there is still limited clinical evidence regarding the efficacy of metformin in combination with ICIs for the treatment of HCC. We appraised and analyzed in vitro and animal studies that aimed to elucidate the mechanisms of action of metformin, as well as clinical studies that assessed its impact on the survival of HCC patients.
... In vitro studies have shown that some genomic variants of this gene induced a complete loss of function for the transportation of metformin among other drugs, while others significantly altered the transport function in a substrate dependent way. 307 These alterations in expression of MATE1 could lead to increased systemic metformin and lactic acidosis induced by metformin. 308, 309 Furthermore, the SLC47A2 gene responsible for encoding the MATE2 transporter is highly expressed in renal cells and acts in the renal excretion of metformin together with MATE1 and OCT2. ...
... Chen et al., 2010;Y. Chen et al., 2009;Futatsugi, Masuo, Kawabata, Nakamichi, & Kato, 2016;Kimura, Masuda, Katsura, & Inui, 2009; Lechner et al., 2016) Masuda et al., 2006; Ruan et al., 2021;) (Boxberger, Hagenbuch, & Lampe, 2018;Meyer zu Schwabedissen, Verstuyft, Kroemer, Becquemont, & Kim, 2010)(Nies et al., 2009)(Hendrickx et al., 2013; Nakamichi et al.et al., 2010;Masuda ...
About 30% of all small molecular drugs are organic cations (OCs). If these are more or less hydrophilic, they require membrane transporters to pass through biological membranes. Here, the proton-organic cation (H+ OC) antiporter may play a physiologically most relevant role, particularly concerning passage through the blood-brain barrier. Membrane transport of about 70 OCs is significantly enhanced by this H+ OC antiporter. Surprisingly still today the gene coding for this antiporter was not yet identified. However, the H+ OC antiporter is characterized by concentration- and pH-dependent uptake, antiport with another OC, and susceptibility to inhibition by specific inhibitors. Moreover, in the studied tissues and cell types, transport is not mediated by already well-known organic cation transporters. The review explains the typically used assays to identify potential substrates of the H+ OC antiporter. Thus far, the gene encoding for this transporter has not yet been identified, but a better understanding of this protein may be most relevant because it may affect the pharmacokinetics of up to 10% of all low molecular substances. This review summarizes the known functional characteristics of the H+ OC antiporter, its cell and tissue expression, and its substrate spectrum. Summarizing the features of the substrates of the H+ OC antiporter may even suggest that for OCs, good penetration through the blood-brain barrier is almost synonymous with being a substrate of the H+ OC antiporter. In clinical studies, pharmacokinetics of typical substrates of the antiporter showed outstanding between-subject variability.
... In turn, MATE2 (K m of 1.05 mM) and MATE1 (K m of 0.23 mM) participate in metformin secretion from the tubule cells into the urine. Furthermore, MATE1 is also involved in the secretion of metformin into the bile [17,[19][20][21]. The fate of metformin in the human body is presented in Figure 1. ...
... In turn, MATE2 (Km of 1.05 mM) and MATE1 (Km of 0.23 mM) participate in metformin secretion from the tubule cells into the urine. Furthermore, MATE1 is also involved in the secretion of metformin into the bile [17,[19][20][21]. The fate of metformin in the human body is presented in Figure 1. ...
Metformin, a cheap and safe biguanide derivative, due to its ability to influence metabolism, is widely used as a first-line drug for type 2 diabetes (T2DM) treatment. Therefore, the aim of this review was to present the updated biochemical and molecular effects exerted by the drug. It has been well explored that metformin suppresses hepatic glucose production in both AMPK-independent and AMPK-dependent manners. Substantial scientific evidence also revealed that its action is related to decreased secretion of lipids from intestinal epithelial cells, as well as strengthened oxidation of fatty acids in adipose tissue and muscles. It was recognized that metformin’s supra-therapeutic doses suppress mitochondrial respiration in intestinal epithelial cells, whereas its therapeutic doses elevate cellular respiration in the liver. The drug is also suggested to improve systemic insulin sensitivity as a result of alteration in gut microbiota composition, maintenance of intestinal barrier integrity, and alleviation of low-grade inflammation.
... Furthermore, PQ is hardly metabolized and its predominant route of excretion is in unmodified form via the kidneys into urine. While glomerular filtration plays a pivotal role in its elimination, PQ clearance exceeds the glomerular filtration rate, indicating an active secretion component which was reported to mainly involve OCT2 and the multidrug and toxic compound extrusion (MATE) transport protein (Chan et al. 1998;Chen et al. 2009;Chen et al. 2007). ...
... Given the cationic nature of PQ the active transporters mainly involved in the renal excretion of PQ are the uptake transporter OCT2 and the multidrug and toxin compound extrusion (MATE) efflux transporter (Chen et al. 2007;George et al. 2017). Several studies reported the activity of OCT2 and MATE for PQ transport, but it remains to be evaluated to what extent OCT2 mediated secretion affects the total plasma clearance in vivo (Chan et al. 1998;Chen et al. 2009;Chen et al. 2007). This is also illustrated by the fact that previous PBK models for PQ ignored this active excretion component deliberately assuming that glomerular filtration of PQ would suffice for the model predictions Lohitnavy et al. 2017;Stevens et al. 2021). ...
... In addressing this difference, diverse pharmacogenetic studies have focused on the solute carrier superfamily (SLC) members that are involved in metformin transport and drug response [7][8][9][10][11]. Most of these studies have been carried out in Asian and Caucasian populations [6,8,[12][13][14][15][16], with just a few including Latin Americans [17][18][19][20]. The pioneering study by Chen et. ...
... rmacogenetic studies have focused on the solute carrier superfamily (SLC) members that are involved in metformin transport and drug response [7][8][9][10][11]. Most of these studies have been carried out in Asian and Caucasian populations [6,8,[12][13][14][15][16], with just a few including Latin Americans [17][18][19][20]. The pioneering study by Chen et. al. in 2009 identified variants in the SLC47A1 gene, which encodes multidrug and toxin extrusion 1 (MATE1) protein, in ethnically diverse individuals [17]. Interestingly, a non-synonymous variant (MATE1 rs77474263C>T), a leucine to phenylalanine change at amino acid residue 125 p. [L125F], was present in 5.1% of Mexican Americans and almost absent ...
... The pioneering study by Chen et. al. in 2009 identified variants in the SLC47A1 gene, which encodes multidrug and toxin extrusion 1 (MATE1) protein, in ethnically diverse individuals [17]. Interestingly, a non-synonymous variant (MATE1 rs77474263C>T), a leucine to phenylalanine change at amino acid residue 125 p. [L125F], was present in 5.1% of Mexican Americans and almost absent in the other populations. ...
Genetic factors that affect variability in metformin response have been poorly studied in the Latin American population, despite its being the initial drug therapy for type 2 diabetes, one of the most prevalent diseases in that region. Metformin pharmacokinetics is carried out by members of the membrane transporters superfamily (SLCs), being the multidrug and toxin extrusion protein 1 (MATE1), one of the most studied. Some genetic variants in MATE1 have been associated with reduced in vitro metformin transport. They include rs77474263 p.[L125F], a variant present at a frequency of 13.8% in Latin Americans, but rare worldwide (less than 1%). Using exome sequence data and TaqMan genotyping, we revealed that the Mexican population has the highest frequency of this variant: 16% in Mestizos and 27% in Amerindians, suggesting a possible Amerindian origin. To elucidate the metformin pharmacogenetics, a children cohort was genotyped, allowing us to describe, for the first time, a MATE1 rs77474263 TT homozygous individual. An additive effect of the L125F variant was observed on blood metformin accumulation, revealing the highest metformin and lactate serum levels in the TT homozygote, and intermediate metformin values in the heterozygotes. Moreover, a molecular dynamics analysis suggested that the genetic variant effect on metformin efflux could be due to a decreased protein permeability. We conclude that pharmacogenetics could be useful in enhancing metformin pharmacovigilance in populations having a high frequency of the risk genotype, especially considering that these populations also have a higher susceptibility to the diseases for which metformin is the first-choice drug.
... MATE1, encoded by SLC47A1 gene, has been identified as a major efflux transporter involved in the renal excretion of many drugs including chloroquine. 91,[111][112][113][114][115] Pharmacogenomics Informing Chloroquine Malaria Pharmacotherapy Individual variation in drug response is a critical challenge in effective drug pharmacotherapy. Both the nature of the drug, as well as the dose of the drug, are subjected to vary on an individual basis. ...
... 113 Other SNPs may also alter transport activity of MATE1 and lead to changed elimination of the corresponding drugs. 114,115 Genetic polymorphisms of MATE1 can affect renal elimination of CQ and HCQ and, therefore, may require dose adjustment based on pharmacogenomic profiles of COVID-19 patients. ...
Background
A new coronavirus SARS-CoV-2 has been identified as the etiological agent of the severe acute respiratory syndrome, COVID-19, the source and cause of the 2019–20 coronavirus pandemic. Hydroxychloroquine and chloroquine have gathered extraordinary attention as therapeutic candidates against SARS-CoV-2 infections. While there is growing scientific data on the therapeutic effect, there is also concern for toxicity of the medications. The therapy of COVID-19 by hydroxychloroquine and chloroquine is off-label. Studies to analyze the personalized effect and safety are lacking.
Methods
A review of the literature was performed using Medline/PubMed/Embase database. A variety of keywords were employed in keyword/title/abstract searches. The electronic search was followed by extensive hand searching using reference lists from the identified articles.
Results
A total of 126 results were obtained after screening all sources. Mechanisms underlying variability in drug concentrations and therapeutic response with chloroquine and hydroxychloroquine in mediating beneficial and adverse effects of chloroquine and hydroxychloroquine were reviewed and analyzed. Pharmacogenomic studies from various disease states were evaluated to elucidate the role of genetic variation in drug response and toxicity.
Conclusion
Knowledge of the pharmacokinetics and pharmacogenomics of chloroquine and hydroxychloroquine is necessary for effective and safe dosing and to avoid treatment failure and severe complications.
... Owing to the hydrophilic and cationic nature of metformin at physiological pH, its cellular absorption, distribution, and elimination requires membrane-bound transporter molecules. While the organic cation transporters 1, 2, and 3 (OCT1, OCT2, and OCT3) support metformin transport into the cell, the plasma membrane monoamine transporter (PMAT) and multidrug and toxin extrusion protein 1 and 2 (MATE1 and MATE2) are involved in the cellular extrusion of metformin [60][61][62][63][64][65][66][67]. Reports also suggest that the intestinal absorption and renal reabsorption of metformin are dependent on the presence of the thiamine transporter 2 (THTR2), while the hepatic uptake of metformin depends on OCT1 [68,69]. ...
... Reports also suggest that the intestinal absorption and renal reabsorption of metformin are dependent on the presence of the thiamine transporter 2 (THTR2), while the hepatic uptake of metformin depends on OCT1 [68,69]. Consequently, the levels of expression of the various transporters (OCTs, PMAT, and MATEs) on cancer cells would determine their sensitivity to metformin and the extent to which metformin can exert its inhibitory effect on cancer cell proliferation, invasion, and metastasis while inducing apoptotic cell death [60][61][62][63][64][65][66][67]69,70]. The overexpression of OCT3 in MCF7, BT20, and MDA-MB-468 breast cancer cells support the accumulation, and hence, the anti-proliferative effects of metformin in these cells [71]. ...
... In such a scenario, higher/repeated doses for longer durations may be required to initiate and sustain a positive remedial response in cancers [290][291][292]. As mentioned earlier in this article, owing to the hydrophilic and cationic nature of metformin at physiological pH, the efficiency of metformin depends on the membrane-bound transporter molecules for its cellular absorption (OCT1, OCT2, OCT3), distribution within the cells, and elimination (MATE1 and PMAT) from cells [60][61][62][63][64][65][66][67]. Metformin will be useful in suppressing cell proliferation and inducing apoptosis in cancer cells and CSCs that express OCTs, which support the intracellular accumulation of metformin, while, cancer cells, and CSCs that overexpress metformin extrusion transporters (MATE1 and PMAT) will confer resistance to metformin treatment [71][72][73][74]. ...
Despite the leaps and bounds in achieving success in the management and treatment of
breast cancers through surgery, chemotherapy, and radiotherapy, breast cancer remains the most frequently occurring cancer in women and the most common cause of cancer-related deaths among women. Systemic therapeutic approaches, such as chemotherapy, although beneficial in treating and curing breast cancer subjects with localized breast tumors, tend to fail in metastatic cases of the disease due to (a) an acquired resistance to the chemotherapeutic drug and (b) the development of intrinsic resistance to therapy. The existence of cancer stem cells (CSCs) plays a crucial role in both acquired and intrinsic chemoresistance. CSCs are less abundant than terminally differentiated cancer cells and confer chemoresistance through a unique altered metabolism and capability to evade the immune response system. Furthermore, CSCs possess active DNA repair systems,
transporters that support multidrug resistance (MDR), advanced detoxification processes, and the ability to self-renew and differentiate into tumor progenitor cells, thereby supporting cancer invasion, metastasis, and recurrence/relapse. Hence, current research is focusing on targeting CSCs to overcome resistance and improve the efficacy of the treatment and management of breast cancer. Studies revealed that metformin (1, 1-dimethylbiguanide), a widely used anti-hyperglycemic agent, sensitizes tumor response to various chemotherapeutic drugs. Metformin selectively targets CSCs and improves the hypoxic microenvironment, suppresses the tumor metastasis and inflammation, as well as regulates the metabolic programming, induces apoptosis, and reverses epithelial–
mesenchymal transition and MDR. Here, we discuss cancer (breast cancer) and chemoresistance, the molecular mechanisms of chemoresistance in breast cancers, and metformin as a chemosensitizing/re-sensitizing agent, with a particular focus on breast CSCs as a critical contributing factor to acquired and intrinsic chemoresistance. The review outlines the prospects and directions for a better understanding and re-purposing of metformin as an anti-cancer/chemo-sensitizing drug in the treatment of breast cancer. It intends to provide a rationale for the use of metformin as a combinatory therapy in a clinical setting.
... Hence, it is evident that metformin requires the presence and support of transporter molecules for its absorption, distribution, and elimination to exert its biological function. In this regard, the organic cation transporters 1, 2, and 3 (OCT1, OCT2, and OCT3), the plasma membrane monoamine transporter (PMAT), and multidrug and toxin extrusion protein 1 and 2 (MATE1 and MATE2) transporters are reported to play key roles in transporting metformin into and out of the cell in the intestine, liver, and kidney [50][51][52][53][54][55][56][57]. The thiamine transporter 2 (THTR2) also plays a role in intestinal absorption and renal re-absorption of metformin [58]. ...
... Althought several of the studies reported metformin treatment-associated inhibition of cancer cell growth and proilferation, activation of cancer cell death, inhibition of invasion and metstasis, and tumor regression, these beneficial effects of metformin were observed at only significantly high concentrations (>5 mM), at least 100-fold higher than with the peak plasma concentration of metformin when administered orally for the treatment of type 2 diabetes [48,49]. As discussed earlier, the selective accumulation of metformin and therefore the sensitivity of cancer cells to metformin should depend on the levels of expression of the various transporters (OCTs, PMAT, and MATEs) in the cells [50][51][52][53][54][55][56][57]59,60]. ...
Interest has grown in studying the possible use of well-known anti-diabetic drugs as anti-cancer agents individually or in combination with, frequently used, chemotherapeutic agents and/or radiation, owing to the fact that diabetes heightens the risk, incidence, and rapid progression of cancers, including breast cancer, in an individual. In this regard, metformin (1, 1-dimethylbiguanide), well known as ‘Glucophage’ among diabetics, was reported to be cancer preventive while also being a potent anti-proliferative and anti-cancer agent. While meta-analysis studies reported a lower risk and incidence of breast cancer among diabetic individuals on a metformin treatment regimen, several in vitro, pre-clinical, and clinical studies reported the efficacy of using metformin individually as an anti-cancer/anti-tumor agent or in combination with chemotherapeutic drugs or radiation in the treatment of different forms of breast cancer. However, unanswered questions remain with regards to areas such as cancer treatment specific therapeutic dosing of metformin, specificity to cancer cells at high concentrations, resistance to metformin therapy, efficacy of combinatory therapeutic approaches, post-therapeutic relapse of the disease, and efficacy in cancer prevention in non-diabetic individuals. In the current article, we discuss the biology of metformin and its molecular mechanism of action, the existing cellular, pre-clinical, and clinical studies that have tested the anti-tumor potential of metformin as a potential anti-cancer/anti-tumor agent in breast cancer therapy, and outline the future prospects and directions for a better understanding and re-purposing of metformin as an anti-cancer drug in the treatment of breast cancer.
... Bien que peu étudiées, plusieurs mutations génétiques ont été identifiées pour MATE1 et MATE2-K (Kajiwara et al., 2009 ;Chen et al., 2009 (Juliano et Ling, 1976). Elle est constituée de deux domaines intracellulaires permettant la liaison à l'ATP (domaines NBD) et de deux domaines transmembranaires comprenant chacun six hélices a qui s'assemblent pour former un pore pour le passage des composés (Schinkel et Jonker, 2003) ( Figure 24). ...
La prescription des AOD en remplacement des AVK est de plus en plus importante, du fait d'une utilisation moins contraignante pour le patient et d'une plus faible variabilité intra- et interindividuelle. Contrairement aux AVK, les AOD sont des substrats des pompes d'efflux. Ces transporteurs sont impliqués dans la pharmacocinétique des AOD, particulièrement dans leur absorption et dans leur élimination biliaire et rénale. L'objectif de ce travail de thèse a été d'évaluer in vitro le rôle des transporteurs P-gp et BCRP dans la variabilité pharmacocinétique du rivaroxaban, un inhibiteur direct du facteur Xa, La première étude a été axée sur l'étude d'une interaction entre le riociguat et deux AOD, l'apixaban et le rivaroxaban. L'estimation des IC50 a révélé la capacité des modèles cellulaires MDCK transfectés à détecter une inhibition de l'efflux lié à BCRP des deux AOD par le riociguat, bien que cette inhibition ne soit pas assez puissante pour être cliniquement pertinente. La seconde étude était centrée sur l'étude des facteurs pouvant faire varier les résultats des études de transport du rivaroxaban obtenus in vitro, en particulier la quantité des transporteurs ABC. Un analyse en spectrométrie de masse a révélé une expression plus élevée de P-gp et BCRP dans les cellules MDCK transfectées mais aussi une différence d'expression des transporteurs entre deux lignées Caco-2 issues d'une banque cellulaire différente. Les ratios d'efflux du rivaroxaban et les IC50 des inhibiteurs spécifiques étaient corrélés à la quantité de transporteurs mesurée dans les différents modèles. La troisième étude était basée sur la mise en place d'un modèle d'étude in vitro du transport hépatique, grâce à la lignée HepaRG. Ces cellules présentent des caractéristiques semblables aux hépatocytes in vivo, en particulier l'expression de transporteurs d'efflux fonctionnels au pôle canaliculaire, impliqués dans l'efflux biliaire du rivaroxaban. Des études d'accumulation ont démontré une prise en charge du rivaroxaban par les transporteurs d'efflux hépatiques, ainsi qu'une diminution de l'efflux dans les canalicules biliaires en présence d'inhibiteurs de P-gp et BCRP. Finalement, les modèles cellulaires in vitro démontrent que le rivaroxaban est pris en charge par deux transporteurs d'efflux, P-gp et BCRP, au niveau intestinal, rénal et hépatique, et que ce transport peut contribuer à la variabilité pharmacocinétique du rivaroxaban.